MDSCs paralyze T cells via one-way intercellular transfer of a toxic metabolite
Abstract
Myeloid-derived suppressor cells employ the reactive metabolite methylglyoxal to suppress T cell effector functions.
Summary and Analysis
, , , et al. Regulatory myeloid cells paralyze T cells through cell–cell transfer of the metabolite methylglyoxal. Nat Immunol. 2020; 21: 555–566
Immune cells with suppressive properties are key mediators of immune tolerance, including transplant tolerance, but they can also be exploited by tumors to evade immune attacks. Thus, a better understanding of how suppressive immune cells develop and by what mechanisms they suppress T effector cells has important clinical implications.
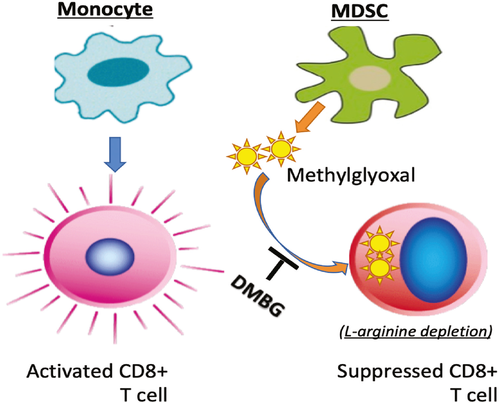
Myeloid-derived suppressor cells (MDSCs) are potent immune-suppressive cells. They are best studied in solid tumors, where they often accumulate in large numbers. Under certain tolerizing conditions, however, MDSCs also congregate in organ transplants to promote graft survival. But how MDSCs function in those situations remains unresolved. A recent study by Baumann et al. has demonstrated that MDSCs possess unique metabolic features in that they selectively produce an abundant reactive metabolite methylglyoxal (MG), which is then transferred into T cells in a cell contact-dependent fashion. The authors have further shown that MG shuts off T effector cells by depleting cytosolic L-arginine (a target of MG), an essential amino acid that is critical to T cell activities.
The authors started by performing transcriptome profiling on human monocytes and MDSCs and found that, in contrast to monocytes, MDSCs showed a markedly reduced expression of genes that encode glycolysis-related enzymes. MDSCs also exhibited reduced glucose uptake, repressed glycolysis, and a much lower rate of mitochondrial respiration than monocytes. Similar metabolic features were also observed in MDSCs isolated from a human hepatocellular carcinoma. These findings led the authors to conclude that MDSCs are in fact in a state of metabolic dormancy. To explore the underlying mechanisms, the authors carried out a series of metabolite labeling and tracing experiments and identified MG as the metabolite that is selectively produced by MDSCs (not by monocytes and Foxp3+ Tregs). MG belongs to the family of dicarbonyls, molecules that have glycation activities: they attach amino and guanidine groups to lysine and arginine (also proteins with such residues) and render them nonfunctional. However, their glycation activities can be neutralized by the chemical dimethylbiguanide (DMBG). Indeed, pretreatment of MDSCs with DMBG reversed their metabolic dormancy and restored glucose uptake, glycolysis and mitochondrial respiration in the treated MDSCs to a level that was comparable to that in monocytes. These findings then led the authors to examine whether MG is involved in MDSC-mediated suppression of T cells.
In a series of detailed experiments, the authors showed that activation of CD8+ T cells consistently led to a burst of glucose uptake and robust glycolysis, responses that are critical to meet the metabolic demand of T cells in their proliferation and effector differentiation. Strikingly, co-culture of T cells with MDSCs, but not with monocytes, completely inhibited T cell proliferation and effector cytokine expression. Interestingly, a brief contact with MDSCs rendered T cells completely unable to uptake glucose and undergo glycolysis, thus showing the same features of metabolic dormancy as MDSCs and suggesting that MDSCs can transfer metabolic dormancy to T cells. The authors further showed that the cytosolic contents, but not the membrane constituents, could be readily transferred from MDSCs to T cells. Additional MG tracing experiments revealed that within minutes of cell–cell contact with MDSCs, MG was detected in CD8+ T cells, which was prevented by pretreatment of MDSCs with DMBG. MG was not observed in T cells co-cultured with monocytes. Moreover, in experiments where CD8+ T cells were co-cultured with DMBG-pretreated MDSCs, CD8+ T cells showed enhanced glucose uptake and vigorous glycolysis. They also produced high levels of effector cytokines and granzyme B, responses that are markedly different from those of the untreated MDSCs. Finally, in a mouse model of metastatic melanoma, the authors showed that blocking MG synergizes with checkpoint inhibitors in promoting T cell–mediated tumor regression. Taken together, these findings demonstrate that MDSCs suppress T cells via intercellular transfer of the metabolite MG.
Clearly, the findings reported in this study highlight the importance of MG as a key metabolite in the suppression of T cells and a biomarker for functional MDSCs. This discovery expands suppression mechanisms to include reactive metabolites (in addition to inhibitory cytokines and inhibitory receptors) and will open the door for targeted manipulations of MDSCs in transplantation, cancer and autoimmune diseases.
Xian C. Li, MD, PhD is professor and director at the Immunobiology and Transplant Science Center and Department of Surgery at Houston Methodist Hospital in Texas.