Outcomes following liver transplantation in young infants: Data from the SPLIT registry
Funding information
AKJ received funding from the National Institutes of Health (Grants K08DK098623 and R03EB015955-01) as principal investigator. Additional funding was provided to AKJ via the Saint Louis University internal grant mechanism. SL received funding from the National Institutes of Health (grant K23NR017652).
Abstract
Liver transplantation (LT) in young patients is being performed with greater frequency. We hypothesized that objective analysis of pre-, intra-, and postoperative events would help understand contributors to successful outcomes and guide transplant decision processes. We queried SPLIT registry for pediatric transplants between 2011 and 2018. Outcomes were compared for age groups: 0-<3, 3-<6, 6-<12 months, and 1-<3 years (Groups A, B, C, D respectively) and by weight categories: <5, 5-10, >10 kg; 1033 patients were available for analysis. Cholestatic disease and fulminant failure were highest in group A and those <5 kg; and biliary atresia in group C (72.8%). Group A had significantly higher life support dependence (34.6%; P < .001), listing as United Network for Organ Sharing status 1a/1b (70.4%; P < .001), and shortest wait times (P < .001). The median (interquartile range) for international normalized ratio and bilirubin were highest in group A (3.0 [2.1-3.9] and 16.7 [6.8-29.7] mg/dL) and those <5 kg (2.6 [1.8-3.4] and 13.5 [3.0-28.4] mg/dL). A pediatric end -stage liver disease score ≥40, postoperative hospital stays, rejection, and nonanastomotic biliary strictures were highest in group A with lowest survival at 93.1%. Infants 0 to <3 months and those <5 kg need more intensive care with lower survival and higher complications. Importantly, potential LT before reaching status 1a/1b and aggressive postoperative management may positively influence their outcomes.
1 INTRODUCTION
Over the past decade, there has been a significant worldwide increase in liver transplantation (LT) in young infants.1, 2 Major advances in pre-, intra-, and postoperative care; surgical techniques; infection control measures; and immunosuppressive monitoring and treatment have improved patient and graft survival. However, young infants represent a unique population segment with greater comorbidities and a disease spectrum much different from those undergoing LT at older ages.3, 4
Although LT remains lifesaving in this cohort of patients,5, 6 they are known to have a higher incidence of complications as well as a need for prolonged intensive care underscoring the importance of age and weight at the time of transplant as a determinant of outcomes.7-9 Selection of such patients for LT is thus predicated on answers to questions about the morbidity and the final outcomes of the procedure.
These neonates and younger infants are medically fragile with complex needs.10, 11 However, we hypothesized that an objective analysis of the pre-, intra-, and postoperative events would yield key information in our understanding of the factors that contribute to successful outcomes and guide us in the transplant decision-making process. This manuscript thus aimed to understand and evaluate the variations in the postoperative course, patient and graft survival and the potential pretransplant conditions driving these outcomes.
In order to comprehensively answer these questions, we utilized the largest multicenter data repository for pediatric liver transplantation in North America—Society of Pediatric Liver Transplantation12, 13 (SPLIT) database to query pre-, intra-, and posttransplant objective data stratified by age and weight to ascertain our objectives.
2 METHODS
We utilized the SPLIT registry, which was conceived in 1995 as a repository for data, prospectively gathered on pediatric liver transplant recipients around the world. SPLIT has 43 active centers, including 37 US sites, 3 Canadian sites, and 3 non-North American sites.12 Each participating center obtained an institutional review board approval and with continued monitoring as mandated by the SPLIT protocol.13
We analyzed the SPLIT data for all pediatric liver transplants performed between 2011 to 2018 from all the US and Canadian sites. As with most registries collecting prospective data the SPLIT registry was overhauled in 2010 to address current and future scientific questions of interest to the community. The registry was modified to eliminate the collection of subjective data elements and streamline time individuals spent in data entry. This was a significant factor in choosing the time frame starting from 2011 for our current study.
Data collected by each transplant center underwent quality control procedures at the central SPLIT data coordinating center (Emmes) to objectively map key pre- and posttransplant measures.
Our assessed primary outcomes were patient and graft survival post liver transplantation in children aged 0 to <3, 3 to <6, 6 months to <1 year, and 1 to 3 years (Groups A, B, C, D respectively) as well as patient weight at the time of transplant <5, 5-10, >10 kg.
Many prior studies have noted variable outcomes from liver transplantation in pediatric patients based on weight.14-16 Poor weight gain, stunting, and growth failure are also quite prevalent in pediatric patients14, 17, 18 on the transplant waitlist and thus reliance only on age is not appropriate. Given these unique nuances pertinent to pediatric transplant patients, in addition to using age we included weight for our analysis.
2.1 Statistical analysis
All data were summarized descriptively with median and interquartile range (IQR) for continuous data and frequency and percentage for categorical data. The Kaplan-Meier estimate was used to estimate probability of patient and graft survival and the log-rank test was used to compare the patient and graft survival between age and weight groups. Other outcomes were compared between age and weight groups using the Pearson chi-square test for categorical variables and the Kruskal-Wallis test for continuous variable. Because of the nature of the registry-based study, there are some missing data, and imputation methods were not used in the analyses. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). All reported P values are 2 sided and P < .05 was considered statistically significant.
3 RESULTS
3.1 Demographics
A total of 1033 children underwent LT under 3 years of age. Among this cohort, 30 patients were under 3 months of age (Group A). While the majority of patients (488) were between 1-<3 years old (Group D), 98 and 417 patients were 3-<6 months (Group B) and 6 months to <1 year old (Group C) respectively. The median age of LT among the groups A-D was 1.5, 5.1, 8.6, and 20.3 months respectively as detailed in Table 1. We also categorized patients by weight. Out of the 1033 children weight was available for 976 participants. 44 children weighed <5 kg. The largest number of patients (n = 618) had a weight between 5-10 kg. A total of 314 patients weighed more than 10 kg (Table 1).
| Age group | Total (N = 1033)a | P value | ||||
|---|---|---|---|---|---|---|
| 0-<3 mo (N = 30)a | 3-<6 mo (N = 98)a | 6 mo-<1 y (N = 417)a | 1-<3 y (N = 488)a | |||
| Age at transplant, mo | ||||||
| N | 30 | 98 | 417 | 488 | 1033 | |
| Median (IQR) | 1.5 (0.7, 2.1) | 5.1 (4.3, 5.6) | 8.6 (7.4, 10.2) | 20.3 (15.2, 26.5) | 11.5 (7.6, 19.4) | b |
| Gender | ||||||
| Male | 21 (70.0%) | 53 (54.1%) | 187 (44.8%) | 238 (48.8%) | 499 (48.3%) | .029 |
| Female | 9 (30.0%) | 45 (45.9%) | 230 (55.2%) | 250 (51.2%) | 534 (51.7%) | |
| Ethnicity | ||||||
| Hispanic or Latino | 3 (10.0%) | 15 (15.3%) | 84 (20.1%) | 103 (21.1%) | 205 (19.8%) | .164 |
| Not Hispanic or Latino | 25 (83.3%) | 79 (80.6%) | 308 (73.9%) | 341 (69.9%) | 753 (72.9%) | |
| Not reported | 2 (6.7%) | 4 (4.1%) | 25 (6.0%) | 44 (9.0%) | 75 (7.3%) | |
| Race | ||||||
| White | 14 (46.7%) | 60 (61.2%) | 255 (61.2%) | 268 (54.9%) | 597 (57.8%) | .075 |
| Black | 4 (13.3%) | 13 (13.3%) | 55 (13.2%) | 68 (13.9%) | 140 (13.6%) | |
| Other | 6 (20.0%) | 19 (19.4%) | 74 (17.7%) | 82 (16.8%) | 181 (17.5%) | |
| Not reported/unknown | 6 (20.0%) | 6 (6.1%) | 33 (7.9%) | 70 (14.3%) | 115 (11.1%) | |
| Blood type | ||||||
| A | 4 (13.3%) | 32 (32.7%) | 139 (33.7%) | 159 (32.8%) | 334 (32.6%) | .624 |
| B | 5 (16.7%) | 15 (15.3%) | 54 (13.1%) | 71 (14.6%) | 145 (14.1%) | |
| AB | 1 (3.3%) | 2 (2.0%) | 17 (4.1%) | 20 (4.1%) | 40 (3.9%) | |
| O | 20 (66.7%) | 49 (50.0%) | 203 (49.2%) | 235 (48.5%) | 507 (49.4%) | |
| Weight kg | ||||||
| <5 kg | 22 (78.6%) | 11 (12.0%) | 10 (2.6%) | 1 (0.2%) | 44 (4.5%) | b |
| 5-10 kg | 6 (21.4%) | 77 (83.7%) | 357 (91.1%) | 178 (38.4%) | 618 (63.3%) | |
| >10 kg | 0 | 4 (4.3%) | 25 (6.4%) | 285 (61.4%) | 314 (32.2%) | |
| Height, cm | ||||||
| N | 25 | 79 | 357 | 439 | 900 | |
| Median (IQR) | 53.0 (50.0, 56.0) | 61.0 (58.0, 65.0) | 66.0 (63.5, 69.0) | 78.2 (73.4, 86.0) | 70.0 (65.0, 79.0) | b |
| Weight Z-score | ||||||
| N | 26 | 89 | 387 | 462 | 964 | |
| Median (IQR) | −2.1 (−2.7, −0.3) | −1.0 (−2.0, −0.2) | −1.6 (−2.7, −0.5) | −0.8 (−2.0, 0.2) | −1.1 (−2.4, −0.1) | b |
| BMI, kg/m2 | ||||||
| N | 25 | 78 | 354 | 436 | 893 | |
| Median (IQR) | 14.1 (12.8, 16.3) | 16.9 (15.8, 18.8) | 17.0 (15.6, 18.6) | 17.2 (16.0, 18.5) | 17.1 (15.8, 18.5) | b |
| Primary diagnosis | ||||||
| Biliary atresia | 3 (10.0%) | 48 (49.5%) | 302 (72.8%) | 210 (43.1%) | 563 (54.7%) | <.001 |
| Other cholestatic | 5 (16.7%) | 2 (2.1%) | 16 (3.9%) | 42 (8.6%) | 65 (6.3%) | |
| Fulminant L. failure | 13 (43.3%) | 9 (9.3%) | 10 (2.4%) | 35 (7.2%) | 67 (6.5%) | |
| Metabolic | 2 (6.7%) | 20 (20.6%) | 52 (12.5%) | 67 (13.8%) | 141 (13.7%) | |
| Tumor | 0 | 5 (5.2%) | 17 (4.1%) | 97 (19.9%) | 119 (11.6%) | |
| Other | 7 (23.3%) | 13 (13.4%) | 18 (4.3%) | 36 (7.4%) | 74 (7.2%) | |
| Biliary atresia | ||||||
| No | 27 (90.0%) | 49 (50.5%) | 113 (27.2%) | 277 (56.9%) | 466 (45.3%) | <.001 |
| Yes | 3 (10.0%) | 48 (49.5%) | 302 (72.8%) | 210 (43.1%) | 563 (54.7%) | |
| Tumor | ||||||
| No | 30 (100.0%) | 92 (94.8%) | 399 (95.9%) | 390 (80.1%) | 911 (88.4%) | <.0001 |
| Hepatocellular carcinoma | 0 | 0 | 0 | 5 (1.0%) | 5 (0.5%) | |
| Hepatoblastoma | 0 | 5 (5.2%) | 17 (4.1%) | 90 (18.5%) | 112 (10.9%) | |
| Other tumor | 0 | 0 | 0 | 2 (0.4%) | 2 (0.2%) | |
- IQR, interquartile range.
- a The number of participants across categories may not sum to the total number of participants because of missing data.
- b Statistical comparison was not performed.
There were no sex differences in the total number of male (48.3%) and female (51.7%) patients undergoing LT, however there were a significantly higher number of males in group A (P = .03). No differences in ethnicity, race or blood type were noted among the groups.
In order to evaluate potential differences relating to social characteristics as a surrogate driver of LT we evaluated the primary care giver's marital status as well as the actual household primary care provider and their insurance type. However, no differences were noted (Table S1). When stratified by weight, significant differences in gender (P = .003) and ethnicity (P = .016) were noted.
3.2 Indications for LT
We next evaluated the indication of LT stratified by age. While overall biliary atresia (BA) contributed to the largest number of LT (54.7%), there was significant variability in the indication of transplantation among the groups (P < .001). Incidence of BA was significantly lower in group A with only 3 out of 30 patients (10.0%) under 3 months of age receiving LT for BA. The incidence was highest in group C (72.8%).
A primary diagnosis of fulminant liver failure (43.3%) and cholestatic liver disease (16.7%) was highest in group A and those weighing less than 5 kg (27.3% and 9.1%, respectively) (Table 1 and Table S2). We also noted a significantly higher percentage of patients (20.6%) with a primary diagnosis of metabolic disease in group B. Significant differences were also noted in the indication of transplant based on the weight at transplantation (P < .001). A majority of patients with biliary atresia (66.9%) weighed between 5-10 kg. Among those weighing <5 kg biliary atresia or fulminant liver failure was noted in the majority of the patients (Table S2).
Liver tumors are not very common in children19, 20 and in line with these results 88.4% children did not report a tumor. However, both hepatocellular carcinoma (1%) and hepatoblastoma (18.5%) were highest in group D (Table 1).
3.3 Comorbidities, participant status, and donor characteristics
Younger patients tended to be sicker as compared to older children. While a significantly higher, 19 out of 27 (70.4%) patients in group A were listed as United Network for Organ Sharing (UNOS) status 1a/1b (P < .001), overall, 30.3% patients constituted this category (Table 2). Out of those listed as UNOS status 1a/1b 7%, 48%, and 45% belonged to weight groups <5, 5-10, and >10 kg respectively. Significant differences in the pediatric end-stage liver disease (PELD) score were noted for both age groups and weight cohorts (P < .001). A PELD score ≥40 was highest in group A (57.9%) and those <5 kg weight (34.3%). Similarly, PELD exceptions were highest in group A and those <5 kg in weight (P < .001), further attesting to their sicker status.
| Age group | Total (N = 1033)a | P value | ||||
|---|---|---|---|---|---|---|
| 0 to <3 mo (N = 30)a | 3 to <6 mo (N = 98)a | 6 mo to <1 y (N = 417)a | 1 to <3 y (N = 488)a | |||
| Participant status at transplant | ||||||
| Time on the waiting list, mo | ||||||
| N | 28 | 97 | 401 | 480 | 1006 | |
| Median (IQR) | 0.26 (0.11, 0.62) | 0.76 (0.33, 1.58) | 1.91 (0.92, 3.45) | 2.89 (0.87, 7.15) | 1.89 (0.69, 4.24) | <.001 |
| UNOS status 1a/1b at transplant | ||||||
| No | 8 (29.6%) | 70 (72.2%) | 317 (79.6%) | 303 (63.3%) | 698 (69.7%) | <.001 |
| Yes | 19 (70.4%) | 27 (27.8%) | 81 (20.4%) | 176 (36.7%) | 303 (30.3%) | |
| PELD Exception Score | ||||||
| Total | 2 (100.0%) | 27 (100.0%) | 117 (100.0%) | 126 (100.0%) | 272 (100.0%) | |
| <0 | 0 | 0 | 1 (0.9%) | 3 (2.4%) | 4 (1.5%) | .25 |
| 0-<10 | 0 | 1 (3.7%) | 2 (1.7%) | 2 (1.6%) | 5 (1.8%) | |
| 10-<20 | 0 | 1 (3.7%) | 5 (4.3%) | 5 (4.0%) | 11 (4.0%) | |
| 20-<30 | 0 | 4 (14.8%) | 15 (12.8%) | 35 (27.8%) | 54 (19.9%) | |
| 30-<40 | 1 (50.0%) | 8 (29.6%) | 38 (32.5%) | 47 (37.3%) | 94 (34.6%) | |
| ≥40 | 1 (50.0%) | 13 (48.1%) | 56 (47.9%) | 34 (27.0%) | 104 (38.2%) | |
| PELD calculated scoreb for all participants | ||||||
| Total | 19 (100.0%) | 77 (100.0%) | 358 (100.0%) | 440 (100.0%) | 894 (100.0%) | |
| <0 | 0 | 11 (14.3%) | 31 (8.7%) | 156 (35.5%) | 198 (22.1%) | <.001 |
| 0-<10 | 1 (5.3%) | 8 (10.4%) | 39 (10.9%) | 98 (22.3%) | 146 (16.3%) | |
| 10-<20 | 0 | 13 (16.9%) | 91 (25.4%) | 95 (21.6%) | 199 (22.3%) | |
| 20-<30 | 4 (21.1%) | 23 (29.9%) | 119 (33.2%) | 60 (13.6%) | 206 (23.0%) | |
| 30-<40 | 3 (15.8%) | 16 (20.8%) | 61 (17.0%) | 25 (5.7%) | 105 (11.7%) | |
| ≥40 | 11 (57.9%) | 6 (7.8%) | 17 (4.7%) | 6 (1.4%) | 40 (4.5%) | |
| Hospital status at transplant | ||||||
| ICU | 21 (80.8%) | 31 (32.0%) | 90 (22.2%) | 101 (21.0%) | 243 (24.1%) | <.001 |
| Hospitalized, not in ICU | 3 (11.5%) | 34 (35.1%) | 148 (36.5%) | 93 (19.3%) | 278 (27.5%) | |
| Not hospitalized | 2 (7.7%) | 32 (33.0%) | 168 (41.4%) | 287 (59.7%) | 489 (48.4%) | |
| On life support at transplant | ||||||
| No | 17 (65.4%) | 84 (90.3%) | 361 (89.1%) | 434 (90.8%) | 896 (89.4%) | <.001 |
| Yes | 9 (34.6%) | 9 (9.7%) | 44 (10.9%) | 44 (9.2%) | 106 (10.6%) | |
| Intubated prior to being taken to the OR for transplant | ||||||
| No | 16 (61.5%) | 79 (85.9%) | 363 (89.4%) | 440 (91.7%) | 898 (89.4%) | <.001 |
| Yes | 10 (38.5%) | 13 (14.1%) | 43 (10.6%) | 40 (8.3%) | 106 (10.6%) | |
| Chemistries and hematology | ||||||
| Sodium, mEq/L | ||||||
| N | 28 | 90 | 403 | 478 | 999 | |
| Median (IQR) | 139 (135, 148) | 138 (134, 141) | 138 (134, 141) | 138 (136, 141) | 138 (135, 141) | .016 |
| Total bilirubin, mg/dL | ||||||
| N | 24 | 89 | 391 | 470 | 974 | |
| Median (IQR) | 16.7 (6.8, 29.7) | 8.3 (1.6, 19.9) | 10.1 (2.3, 19.8) | 2.2 (0.5, 12.0) | 5.6 (0.8, 17.0) | <.001 |
| Albumin, g/dL | ||||||
| N | 27 | 90 | 391 | 474 | 982 | |
| Median (IQR) | 2.7 (2.4, 3.1) | 2.9 (2.5, 3.5) | 3.0 (2.5, 3.5) | 3.3 (2.7, 3.9) | 3.1 (2.6, 3.7) | <.001 |
| International normalized ratio (INR) | ||||||
| N | 25 | 90 | 385 | 461 | 961 | |
| Median (IQR) | 3.0 (2.1, 3.9) | 1.7 (1.2, 2.6) | 1.6 (1.2, 2.3) | 1.3 (1.1, 1.9) | 1.5 (1.1, 2.2) | <.001 |
| Serum creatinine, mg/dL | ||||||
| N | 28 | 92 | 398 | 474 | 992 | |
| Median (IQR) | 0.2 (0.2, 0.3) | 0.2 (0.1, 0.3) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | .022 |
- a The number of participants across categories may not sum to the total number of participants because of missing data.
- b PELD calculated scores were calculated using individual parameters provided to the registry.
Additionally, a significantly higher percentage of patients (P < .001) in group A, 9 out of 26 (34.6%) were dependent on life support against an overall 10.6% life support dependence. Interestingly, when stratified by weight groups, there was no statistical difference in dependence on life support (P = .06). The median and IQR in months of 0.26 (0.11-0.62) for waiting times on the transplant list were significantly shorter for group A (P < .0001). In fact, median waiting times were progressively higher with older age groups and this trend persisted when stratified by patient weight (P < .001). (Table 2 and Table S3B).
In line with their sicker status, serum bilirubin, was highest at a median and IQR value of 16.7 (6.8-29.7) mg/dL in group A vs 8.3 (1.6, 19.9), 10.1 (2.3, 19.8), and 2.2 (0.5, 12.0) for groups B, C, and D respectively. This trend was mirrored when stratified by weight, with the highest bilirubin in patients <5 kg (P < .001) while the median and IQR for international normalized ratio (INR) was 1.5 (1.1-2.2) for all patients undergoing LT, the INR was significantly higher at 3.0 (2.1-3.9) for group A patients (P < .001). INR was also significantly higher for patients <5 kg, with progressively lower INR for the higher weight groups (P < .001). The serum albumin was also significantly lower for group A (P < .0001) and in those who were <5 kg (P < .001). The distribution of creatinine a surrogate for renal function and serum sodium levels differed among age and weight groups, respectively, although their median value was similar across groups.
When evaluating for prior surgery, only 7 out of 29 (24.1%) patients in group A had previous abdominal surgery (Table 3), against an overall 62.6% patients with such surgery (P < .001) and this significance was maintained when stratified by weight (P < .001). In fact, among the total 638 patients with previous abdominal surgery in age groups, 0 to <3 months, 3 to <6 months, 6 months to <1 year, and 1 to <3 years Kasai portoenterostomy was performed in 3, 36, 235, and 182 patients respectively. Among 612 with previous abdominal surgery in the weight groups, 8, 328, and 96 had Kasai portoenterostomy in <5, 5-10, and >10 kg groups.
| Age group | Total (N = 1033)a | P value | ||||
|---|---|---|---|---|---|---|
| 0 to <3 mo (N = 30)a | 3 to <6 mo (N = 98)a | 6 mo to <1 y (N = 417)a | 1 to <3 y (N = 488)a | |||
| Dialysis | ||||||
| No | 15 (53.6%) | 44 (45.8%) | 201 (49.5%) | 256 (53.0%) | 516 (50.9%) | .525 |
| Yes | 13 (46.4%) | 52 (54.2%) | 205 (50.5%) | 227 (47.0%) | 497 (49.1%) | |
| Hepatopulmonary syndrome | ||||||
| No | 28 (100.0%) | 97 (100.0%) | 400 (98.3%) | 468 (97.3%) | 993 (98.0%) | .362 |
| Yes | 0 | 0 | 7 (1.7%) | 13 (2.7%) | 20 (2.0%) | |
| Any previous malignancy | ||||||
| No | 27 (96.4%) | 95 (99.0%) | 403 (98.3%) | 435 (90.6%) | 960 (94.7%) | <.001 |
| Yes | 1 (3.6%) | 1 (1.0%) | 7 (1.7%) | 45 (9.4%) | 54 (5.3%) | |
| Previous abdominal surgery | ||||||
| No | 22 (75.9%) | 52 (53.6%) | 115 (28.0%) | 192 (39.8%) | 381 (37.4%) | <.001 |
| Yes | 7 (24.1%) | 45 (46.4%) | 295 (72.0%) | 291 (60.2%) | 638 (62.6%) | |
| Supplemental feeding | ||||||
| No | 11 (42.3%) | 42 (44.7%) | 148 (36.2%) | 231 (48.5%) | 432 (43.0%) | .003 |
| Yes | 15 (57.7%) | 52 (55.3%) | 261 (63.8%) | 245 (51.5%) | 573 (57.0%) | |
| Route of nutritional intake | ||||||
| Mouth | 11 (44.0%) | 42 (45.2%) | 148 (36.4%) | 231 (48.6%) | 432 (43.2%) | .006 |
| Tube | 8 (32.0%) | 38 (40.9%) | 170 (41.8%) | 182 (38.3%) | 398 (39.8%) | |
| Parenteral (IV) | 4 (16.0%) | 12 (12.9%) | 69 (17.0%) | 52 (10.9%) | 137 (13.7%) | |
| Tube and Parenteral (IV) | 2 (8.0%) | 1 (1.1%) | 20 (4.9%) | 10 (2.1%) | 33 (3.3%) | |
- a The number of participants across categories may not sum to the total number of participants because of missing data.
Malignancy including tumors in the pediatric population remain relatively rare.21-24 While a history of malignancy was lowest in group B (1.0%), followed by group C (1.7%), group D had the largest percent of patients with prior malignancy at 45 out of 480 LTs (9.4%). When compared against all LTs there was a statistically lower percent of patients with malignancy in group A (P < .001) and highest for those >10 kg (P < .001). Interestingly, a significant difference in the route of nutrition (tube vs PO) and supplemental nutrition intake were noted among the groups with tube and parenteral nutrition accounting for the highest percent in Group A and those less than 5 kg (all P < .01), though no differences in hepatopulmonary syndrome or the need for dialysis were noted among the groups. (Table 3 and Table S4B). Donor type differed by age (P = .04). However, no differences in donor gender, ethnicity, race, or blood type were noted among the groups.
The distribution of patients receiving ABO incompatible grafts were similar across age and weight groups. (Table 4 and Table S5B). No statistically significant differences in patient survival (P = .494) or graft survival (P = .078) were noted between ABO compatible vs incompatible transplants. Additionally, ABO incompatible transplants did not have a higher risk of death after adjustment for age (P = .485) or weight (P = .394).
| Age group | Total (N = 1033)a | P value | ||||
|---|---|---|---|---|---|---|
| 0 to <3 mo (N = 30)a | 3 to <6 mo (N = 98)a | 6 mo to <1 y (N = 417)a | 1 to <3 y (N = 488)a | |||
| Donor type | ||||||
| Deceased—brain death | 22 (75.9%) | 70 (72.9%) | 289 (71.9%) | 382 (79.9%) | 763 (75.9%) | .040 |
| Deceased—donation after cardiac death (DCD) | 0 | 1 (1.0%) | 2 (0.5%) | 7 (1.5%) | 10 (1.0%) | |
| Living | 7 (24.1%) | 23 (24.0%) | 110 (27.4%) | 85 (17.8%) | 225 (22.4%) | |
| Unknown | 0 | 2 (2.1%) | 1 (0.2%) | 4 (0.8%) | 7 (0.7%) | |
| Donor gender | ||||||
| Male | 14 (56.0%) | 45 (50.0%) | 228 (58.9%) | 269 (59.0%) | 556 (58.0%) | .436 |
| Female | 11 (44.0%) | 45 (50.0%) | 159 (41.1%) | 187 (41.0%) | 402 (42.0%) | |
| Donor ethnicity | ||||||
| Hispanic or Latino | 0 | 12 (12.6%) | 48 (12.3%) | 72 (15.5%) | 132 (13.5%) | .313 |
| Not Hispanic or Latino | 17 (63.0%) | 56 (58.9%) | 238 (61.2%) | 267 (57.3%) | 578 (59.2%) | |
| Not reported | 10 (37.0%) | 27 (28.4%) | 103 (26.5%) | 127 (27.3%) | 267 (27.3%) | |
| Donor race | ||||||
| White | 17 (63.0%) | 47 (53.4%) | 238 (63.0%) | 247 (55.1%) | 549 (58.3%) | .081 |
| Black | 4 (14.8%) | 21 (23.9%) | 49 (13.0%) | 93 (20.8%) | 167 (17.7%) | |
| Other | 1 (3.7%) | 4 (4.5%) | 22 (5.8%) | 15 (3.3%) | 42 (4.5%) | |
| Not reported/unknown | 5 (18.5%) | 16 (18.2%) | 69 (18.3%) | 93 (20.8%) | 183 (19.4%) | |
| Donor weight, kg | ||||||
| N | 23 | 82 | 347 | 421 | 873 | |
| Median (IQR) | 20.0 (8.0, 40.9) | 16.4 (7.7, 59.0) | 36.0 (10.6, 68.0) | 20.0 (12.0, 64.0) | 22.7 (11.0, 65.0) | .022 |
| Donor blood type | ||||||
| A | 5 (18.5%) | 26 (28.3%) | 126 (32.6%) | 132 (28.1%) | 289 (29.6%) | .656 |
| B | 4 (14.8%) | 14 (15.2%) | 38 (9.8%) | 51 (10.9%) | 107 (11.0%) | |
| AB | 0 | 2 (2.2%) | 9 (2.3%) | 9 (1.9%) | 20 (2.1%) | |
| O | 18 (66.7%) | 50 (54.3%) | 214 (55.3%) | 277 (59.1%) | 559 (57.3%) | |
| Donor-recipient ABO incompatible | ||||||
| No | 22 (81.5%) | 75 (81.5%) | 330 (85.7%) | 398 (84.9%) | 825 (84.8%) | .7415 |
| Yes | 5 (18.5%) | 17 (18.5%) | 55 (14.3%) | 71 (15.1%) | 148 (15.2%) | |
- a The number of participants across categories may not sum to the total number of participants because of missing data.
3.4 Transplant procedure, immune suppression, and posttransplantation
Transplant procedure type differed by age group (P < .001) where Group A had the highest percentage of patients with a partial liver transplant (44.4%), however the type of procedure was not statistically significant when stratified by the weight groups (P = .12). Duct to duct anastomosis was significantly less as compared to Roux-en-Y choledochojejunostomy among all age groups (P < .001). No differences in biliary stent placement, warm or cold ischemia times or portal vein thrombosis, were noted among the age or the weight groups (Table 5 and Table S6B).
| Age group | Total (N = 1033) | P value | ||||
|---|---|---|---|---|---|---|
| 0 to <3 mo (N = 30)a | 3 to <6 mo (N = 98)a | 6 mo to <1 y (N = 417)a | 1 to <3 y (N = 488)a | |||
| Procedure type | ||||||
| Whole liver | 10 (37.0%) | 47 (48.5%) | 161 (39.0%) | 246 (50.8%) | 464 (45.4%) | <.001 |
| Partial liver | 12 (44.4%) | 31 (32.0%) | 134 (32.4%) | 105 (21.7%) | 282 (27.6%) | |
| Split liver | 5 (18.5%) | 19 (19.6%) | 118 (28.6%) | 131 (27.1%) | 273 (26.7%) | |
| Unknown | 0 | 0 | 0 | 2 (0.4%) | 2 (0.2%) | |
| Biliary anastomosis | ||||||
| Duct-to-duct | 4 (14.8%) | 10 (10.9%) | 32 (7.9%) | 96 (20.0%) | 142 (14.1%) | <.001 |
| Roux-en-Y choledochojejunostomy | 20 (74.1%) | 71 (77.2%) | 335 (82.5%) | 358 (74.7%) | 784 (78.1%) | |
| Other | 3 (11.1%) | 11 (12.0%) | 39 (9.6%) | 25 (5.2%) | 78 (7.8%) | |
| Biliary stent | ||||||
| None | 18 (78.3%) | 66 (72.5%) | 254 (63.2%) | 309 (64.9%) | 647 (65.2%) | .381 |
| External | 0 | 2 (2.2%) | 18 (4.5%) | 14 (2.9%) | 34 (3.4%) | |
| Internal | 5 (21.7%) | 23 (25.3%) | 130 (32.3%) | 153 (32.1%) | 311 (31.4%) | |
| Warm ischemia time, min | ||||||
| N | 23 | 73 | 322 | 390 | 808 | |
| Median (IQR) | 39.0 (33.0, 53.0) | 38.0 (30.0, 47.0) | 39.0 (30.0, 52.0) | 39.0 (30.0, 50.0) | 39.0 (30.0, 51.0) | .616 |
| Total cold ischemia time, h | ||||||
| N | 24 | 82 | 353 | 431 | 890 | |
| Median (IQR) | 7.3 (5.1, 9.0) | 6.0 (4.5, 8.6) | 6.1 (3.8, 8.0) | 6.0 (4.2, 8.0) | 6.0 (4.0, 8.0) | .392 |
| Portal vein thrombosis in native liver | ||||||
| No | 24 (92.3%) | 91 (95.8%) | 381 (94.8%) | 455 (95.0%) | 951 (94.9%) | .857 |
| Yes | 2 (7.7%) | 4 (4.2%) | 21 (5.2%) | 24 (5.0%) | 51 (5.1%) | |
- a The number of participants across categories may not sum to the total number of participants because of missing data.
3.5 Immunosuppression, participant status
No differences in immunosuppression induction, tacrolimus, azathioprine, sirolimus, everolimus, or corticosteroid use, or their combinations were noted among the weight (Table S7B) or age groups (Table 6). However, significant differences in mcophenolate use were noted based on the age groups (P = .02), with the highest mycophonelate use in Group D (33.0%). Interestingly, this pattern was not noted when we stratified by the weight groups (P = .59).
| Age group | Total (N = 1033)a | P value | ||||
|---|---|---|---|---|---|---|
| 0 to <3 mo (N = 30)a | 3 to <6 mo (N = 98)a | 6 mo to <1 y (N = 417)a | 1 to <3 y (N = 488)a | |||
| Antibody therapy as induction | ||||||
| No | 16 (55.2%) | 63 (64.9%) | 261 (64.0%) | 315 (65.6%) | 655 (64.6%) | .700 |
| Yes | 13 (44.8%) | 34 (35.1%) | 147 (36.0%) | 165 (34.4%) | 359 (35.4%) | |
| Tacrolimus | ||||||
| No | 0 | 2 (2.1%) | 2 (0.5%) | 12 (2.5%) | 16 (1.6%) | .077 |
| Yes | 29 (100.0%) | 94 (97.9%) | 408 (99.5%) | 468 (97.5%) | 999 (98.4%) | |
| Mycophenolate mofetil/mycophenolic acid (MMF/MPA) | ||||||
| No | 23 (79.3%) | 78 (81.3%) | 277 (67.6%) | 321 (67.0%) | 699 (68.9%) | .024 |
| Yes | 6 (20.7%) | 18 (18.8%) | 133 (32.4%) | 158 (33.0%) | 315 (31.1%) | |
| Azathioprine | ||||||
| No | 29 (100.0%) | 96 (100.0%) | 409 (100.0%) | 480 (100.0%) | 1014 (100.0%) | b |
| Sirolimus | ||||||
| No | 29 (100.0%) | 96 (100.0%) | 410 (100.0%) | 479 (99.8%) | 1014 (99.9%) | >.999 |
| Yes | 0 | 0 | 0 | 1 (0.2%) | 1 (0.1%) | |
| Corticosteroids | ||||||
| No | 3 (10.7%) | 10 (10.4%) | 54 (13.2%) | 49 (10.2%) | 116 (11.4%) | .563 |
| Yes | 25 (89.3%) | 86 (89.6%) | 356 (86.8%) | 431 (89.8%) | 898 (88.6%) | |
| Everolimus | ||||||
| No | 21 (100.0%) | 75 (98.7%) | 317 (100.0%) | 381 (99.5%) | 794 (99.6%) | .276 |
| Yes | 0 | 1 (1.3%) | 0 | 2 (0.5%) | 3 (0.4%) | |
- a The number of participants across categories may not sum to the total number of participants because of missing data.
- b Statistical comparison was not performed.
We next assessed the length of hospital stay posttransplantation. Further corroborating the fact that younger patients tend to be sicker, patients <5 kg and those in group A had the highest post transplant initial hospitalization stay in comparison to all patients with LT. The overall median and IQR for hospital stay post LT was 19.0 (12.0, 30.0) days, however for those less than 3 months (group A) this number was almost 50% more at 31.0 (20.0, 49.0) days (P < .001). Group A patients and those <5 kg weight were also intubated for a longer duration (both ps < 0.001) as shown in Table 7 and Table S9A, respectively. Early (0-30 days) post LT rejection was highest in group D at 16.6% (P = .03), however, this did not reach a statistical significance when stratified by weight (P = .13). At the >1-3-year mark rejection was highest in patients >10 kg (24.2%; P = .02; Table S9B) as well as group D (24.8%; P = .01; Table S8B). In contrast to prior studies,7, 25 no long-term differences in posttransplant hepatic vein thrombosis, hepatic artery thrombosis, portal vein stenosis, hepatic artery stenosis or biliary complications were noted among the age or weight groups, which is likely driven by surgical innovation and improved surgical techniques.26-28
| Age group | Total (N = 977)a | P value | ||||
|---|---|---|---|---|---|---|
| 0 to <3 mo (N = 28)a | 3 to <6 mo (N = 94)a | 6 mo to <1 y (N = 389)a | 1 to <3 y (N = 466)a | |||
| Total 0-30 d follow-up | 28 | 94 | 389 | 466 | 977 | |
| Total 31 d to 1 y follow-up | 26 | 91 | 387 | 457 | 961 | |
| Total >1-3 y Follow-up | 17 | 59 | 226 | 278 | 580 | |
| Initial hospitalization, d | ||||||
| N | 25 | 85 | 370 | 457 | 937 | |
| Median (IQR) | 31.0 (20.0, 49.0) | 26.0 (16.0, 37.0) | 20.0 (14.0, 32.0) | 16.0 (11.0, 25.0) | 19.0 (12.0, 30.0) | <.001 |
| Primary Intubation, d | ||||||
| N | 24 | 81 | 369 | 456 | 930 | |
| Median (interquartile range) | 8.5 (3.5, 12.0) | 3.0 (0.0, 9.0) | 2.0 (1.0, 6.0) | 1.0 (0.0, 3.0) | 1.0 (0.0, 5.0) | <.001 |
| Rejection | ||||||
| 0-30 d | ||||||
| No | 23 (88.5%) | 85 (95.5%) | 323 (84.8%) | 388 (83.4%) | 819 (85.2%) | .030 |
| Yes | 3 (11.5%) | 4 (4.5%) | 58 (15.2%) | 77 (16.6%) | 142 (14.8%) | |
| 31 d to 1 y | ||||||
| No | 17 (68.0%) | 69 (76.7%) | 302 (78.9%) | 337 (73.7%) | 725 (75.9%) | .276 |
| Yes | 8 (32.0%) | 21 (23.3%) | 81 (21.1%) | 120 (26.3%) | 230 (24.1%) | |
| >1-3 y | ||||||
| No | 17 (100.0%) | 51 (87.9%) | 185 (82.2%) | 209 (75.2%) | 462 (79.9%) | .011 |
| Yes | 0 | 7 (12.1%) | 40 (17.8%) | 69 (24.8%) | 116 (20.1%) | |
| Hepatic vein thrombosis (HVT) | ||||||
| 0-30 d | ||||||
| No | 26 (100.0%) | 88 (97.8%) | 382 (99.7%) | 461 (99.6%) | 957 (99.5%) | .186 |
| Yes | 0 | 2 (2.2%) | 1 (0.3%) | 2 (0.4%) | 5 (0.5%) | |
| 31 d to 1 y | ||||||
| No | 25 (100.0%) | 88 (100.0%) | 370 (99.7%) | 435 (99.8%) | 918 (99.8%) | >.999 |
| Yes | 0 | 0 | 1 (0.3%) | 1 (0.2%) | 2 (0.2%) | |
| >1-3 y | ||||||
| No | 17 (100.0%) | 57 (100.0%) | 225 (100.0%) | 278 (100.0%) | 577 (100.0%) | b |
| Hepatic artery thrombosis (HAT) | ||||||
| 0-30 d | ||||||
| No | 24 (92.3%) | 79 (87.8%) | 354 (92.4%) | 433 (93.7%) | 890 (92.6%) | .269 |
| Yes | 2 (7.7%) | 11 (12.2%) | 29 (7.6%) | 29 (6.3%) | 71 (7.4%) | |
| 31 d to 1 y | ||||||
| No | 25 (100.0%) | 88 (100.0%) | 366 (98.7%) | 434 (99.5%) | 913 (99.2%) | .467 |
| Yes | 0 | 0 | 5 (1.3%) | 2 (0.5%) | 7 (0.8%) | |
| >1-3 y | ||||||
| No | 17 (100.0%) | 57 (100.0%) | 224 (99.6%) | 277 (99.6%) | 575 (99.7%) | >.999 |
| Yes | 0 | 0 | 1 (0.4%) | 1 (0.4%) | 2 (0.3%) | |
| Portal vein stenos (PVS) | ||||||
| 0-30 d | ||||||
| No | 26 (100.0%) | 90 (100.0%) | 377 (98.4%) | 459 (99.4%) | 952 (99.1%) | .491 |
| Yes | 0 | 0 | 6 (1.6%) | 3 (0.6%) | 9 (0.9%) | |
| 31 d to 1 y | ||||||
| No | 25 (100.0%) | 85 (96.6%) | 354 (95.4%) | 424 (97.2%) | 888 (96.5%) | .474 |
| Yes | 0 | 3 (3.4%) | 17 (4.6%) | 12 (2.8%) | 32 (3.5%) | |
| >1-3 y | ||||||
| No | 16 (94.1%) | 56 (98.2%) | 217 (96.4%) | 273 (98.2%) | 562 (97.4%) | .308 |
| Yes | 1 (5.9%) | 1 (1.8%) | 8 (3.6%) | 5 (1.8%) | 15 (2.6%) | |
| Hepatic artery stenosis | ||||||
| 0-30 d | ||||||
| No | 26 (100.0%) | 89 (98.9%) | 377 (98.7%) | 451 (97.4%) | 943 (98.1%) | .537 |
| Yes | 0 | 1 (1.1%) | 5 (1.3%) | 12 (2.6%) | 18 (1.9%) | |
| 31 d to 1 y | ||||||
| No | 25 (100.0%) | 88 (100.0%) | 368 (99.2%) | 429 (98.4%) | 910 (98.9%) | .625 |
| Yes | 0 | 0 | 3 (0.8%) | 7 (1.6%) | 10 (1.1%) | |
| >1-3 y | ||||||
| No | 17 (100.0%) | 57 (100.0%) | 223 (99.1%) | 278 (100.0%) | 575 (99.7%) | .392 |
| Yes | 0 | 0 | 2 (0.9%) | 0 | 2 (0.3%) | |
| Biliary complications | ||||||
| 0-30 d | ||||||
| No | 14 (82.4%) | 48 (84.2%) | 195 (81.9%) | 261 (85.6%) | 518 (84.0%) | .717 |
| Yes | 3 (17.6%) | 9 (15.8%) | 43 (18.1%) | 44 (14.4%) | 99 (16.0%) | |
| 31 d to 1 y | ||||||
| No | 23 (92.0%) | 78 (87.6%) | 356 (93.2%) | 419 (91.7%) | 876 (91.9%) | .382 |
| Yes | 2 (8.0%) | 11 (12.4%) | 26 (6.8%) | 38 (8.3%) | 77 (8.1%) | |
| >1-3 y | ||||||
| No | 16 (94.1%) | 54 (96.4%) | 215 (96.0%) | 262 (94.2%) | 547 (95.1%) | .734 |
| Yes | 1 (5.9%) | 2 (3.6%) | 9 (4.0%) | 16 (5.8%) | 28 (4.9%) | |
- a The number of participants across categories may not sum to the total number of participants because of missing data.
- b Statistical comparison was not performed.
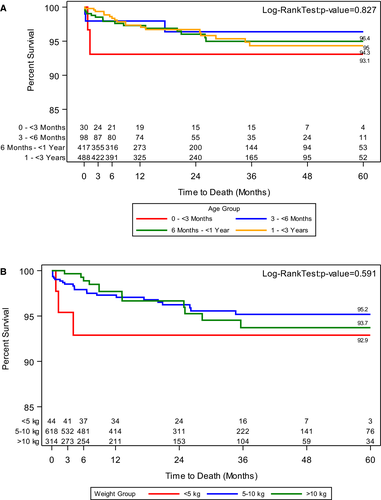
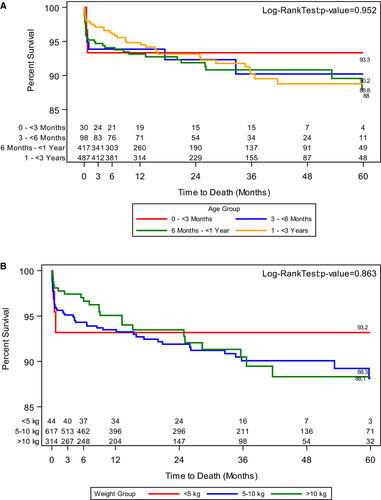
While overall patient survival probability at 3- and 6-months post LT was above 98%, patients in group A had a 93.1% survival at 3 and 6 months (Figure 1A). This trend was mirrored in patients with a weight less than 5 kg (Figure 1B). Patient survival at 12, 24, 48, and 60 months was 97.3%, 96.3%, 94.8%, and 94.8% respectively. From the entire cohort of patients, graft failure was noted in 82 or 7.2% patients. Group A patients also had lower survival at 60 months post LT (Figure 1A). Graft survival at 3- and 6-months post LT was also lower in group A (Figure 2A).
4 DISCUSSION
Liver transplantation is being performed with a greater frequency in younger infants.7, 29 In order to understand and evaluate the variations in the postoperative course, patient and graft survival and the potential pretransplant conditions driving outcomes, we used the SPLIT data base from 2011 onwards to query pre, intra and posttransplant data controlling for age, weight and key transplant associated variables.
Over the past 2 decades survival rates have dramatically improved, especially in the sickest patients. Indeed, both the overall 3-month patient and graft survival noted in our study was much higher than many recent publications7, 30, 31 and this trend was mirrored in all patients weighing over 5 kg and older than 3 months of age.
Previously published outcomes from the SPLIT registry from 2002 reported on 1092 transplants noted survival rates of 83% and 75% for patient and graft survival, respectively.32, 33 Outcomes of liver transplant recipients <90 days old were reported as 87% and 76% for patient and graft survival, respectively, from years 1997-2004.7 In contrast, current outcomes as reported by our study stand at above 93% attesting to the significant improvement of this complicated procedure in the pediatric liver transplant community. While several studies have noted variation in outcomes in younger patients,7, 34, 35 our novel study evaluates one of the largest cohorts of well characterized pediatric liver transplant patients, outcomes stratified by weight and age. This strategy is critical as etiology of liver disease driving liver transplantation differs significantly within the first 3 years of life.36, 37 Thus, comparisons that group all infants or potentially a set weight limit against transplants across all pediatric patients (0-18 years of age) may not provide the rigor in control as needed for such a study.
We noted significant variability in the etiology of diseases leading to transplant within our tight weight and age-controlled cohorts, with fulminant liver failure most common in the youngest patients and those less than 5 kg against the overall preponderance of biliary atresia in older age groups. We also noted that in such patients there was a significantly lower incidence of prior abdominal surgery, likely reflecting this difference due to the higher prevalence of hepatoportoenterostomy (Kasai) surgery in biliary atresia patients.38, 39
Our study also highlights that the initial difference in both patient and graft survival in the younger (93.1% vs 98.7%) and lower weight (92.9% vs 98.7%) patients persists throughout the 60 months follow up period. This information is key as prior studies have noted none to minimal differences in survival outcomes when all infants were compared against all pediatric liver transplantations.3, 7, 40
In line with poorer survival, the youngest patients belonging to group A as well as those under 5 kg weight had significantly higher morbidity, reflected by the highest percent of listing under UNOS status1a/1b. Such patients also had statistically higher serum bilirubin and INR values as well as a higher support on supplemental nutrition. Waiting times on the liver transplant list were also shortest in this cohort, highlighting the urgency of transplantation against possibly more elective surgeries7, 41 in the older and higher weight patients.
However, while we noted a significantly higher percentage of patients less than 3 months of age on life support (P < .001), this did not reach statistical significance when categorized by the weight cohorts. While we remain uncertain as to the rationale for this observation, it is possible that these differences may be secondary to age related maturity differences of key canicular transporters and hepato-biliary receptors like Bile Salt Export Pump (BSEP), sodium-taurocholate co-transporting polypeptide (NTCP), and multidrug resistance 3 (MDR3) regulating hepatic health.42, 43 Additionally, although there we no sex differences based on age, there were significantly higher number of males in group A. Recent data does note higher morbidity and mortality in male neonates and infants in intensive care units for pathologies unrelated to LT in comparison to females,44-46 which may have at least partly contributed to the observed outcomes in group A. While outside the scope of this manuscript evaluation of such sex differences, driving outcomes unrelated to LT may need focused prospective studies.
Another interesting difference was the much higher use of Mycophenolate in the older age group of patients, for which no clear explanation is available but is possible due to variation in immunosuppression practices among the different centers.
Prior studies have reported higher rates of vascular thrombosis in young infants from a combination of technical challenges, including the smaller diameter and integrity of the native and graft vessels, coupled with physiological differences in the young critically ill infants such as low perfusion pressure.7, 47-49
We noted that the most common cause of death post first transplant was respiratory failure when categorized for age or weight, which was followed by hepatic artery thrombosis. However, no differences in ischemia times, portal vein thrombosis, postoperative portal stenosis or hepatic artery stenosis were noted among the age or weight groups, which is important as it highlights significant advancement in surgical techniques over the past few decades.15, 49-51 In order to make specific recommendations regarding preventative measures for each cause of death would require much more detail on risk factors in each case that are not captured by the SPLIT database.
Although not unusual for large registries another potential limitation to this study is patient follow up attrition, which as expected was highest 1-3 years post transplantation. Another important consideration to address is the potential for differential enrollment into the SPLIT registry by centers based on patient outcomes resulting in reporting bias52 as noted in prior studies.7 In order to mitigate such eventualities, SPLIT rigorously reviews underreporting at transplant centers, however since SPLIT registry is not systematically compared with the UNOS data, such remains a potential limitation of this study.
Importantly, the clinical impact here is to recognize that good and improving survival is possible for even the youngest and smallest infants. Additionally, while, it has long been acknowledged that infants under 1 year have the highest liver waitlist mortalities and there is prioritization by UNOS for this cohort availed through more frequent status 1A listing, they deteriorate quickly.53, 54 This system is not without its pitfalls with continued dependence on nonstandardized exception points and concerns for the availability of size matched organs, as well as greater access to centers who can offer technical innovations such as split, live donor, and mono-segmental grafts.55, 56
Lastly, waitlist mortality cannot be assessed from the SPLIT database, but prior data dictates that even those <3-month infants who get onto a waitlist are a highly selected cohort.54, 57 The benefit of transplantation thus is not ambiguous given that mortality in most cases is inevitable, however, calculating the net-benefit of transplantation might need to be done in future studies.
In conclusion, this well categorized, multi-institutional, pediatric liver transplant study highlights that patients less than 3 months of age as well as those lower than 5kg weight have lower patient and graft survival. Such patients also have a greater predominance of fulminant liver failure, higher biliary complication, are sicker with higher INR and bilirubin levels at presentation and require longer hospital stays and life support. This cohort is also likely to be in UNOS 1a/1b status. The significantly higher use of Mycophenolate in the older age groups may need further systematic evaluation. Most importantly, we conclude that strategies targeting aggressive patient selection, postoperative management, and potential LT before reaching UNOS Status 1a/1b, including considerations for living donor transplantation may influence outcomes in this very fragile population.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. AKJ reports honorarium from Alexion Pharmaceuticals outside/unrelated to the submitted work; the other authors have indicated they have no potential conflicts of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings will be available with the Society of Pediatric Liver Transplantation (SPLIT) following SPLIT standardized embargo and policies.




