Applications of CRISPR technologies in transplantation
Abstract
In transplantation, the ever-increasing number of an organ's demand and long-term graft dysfunction constitute some of the major problems. Therefore, alternative solutions to increase the quantity and quality of the organ supply for transplantation are desired. On this subject, revolutionary Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology holds enormous potential for the scientific community with its expanding toolbox. In this minireview, we summarize the history and mechanism of CRISPR/Cas9 systems and explore its potential applications in cellular- and organ-level transplantation. The last part of this review includes future opportunities as well as the challenges in the transplantation field.
1 INTRODUCTION
Transplantation represents the most successful curative treatment for acute and chronic organ failure. Unfortunately, the gap between the availability of organs for transplantation and the increasing number of patients on the national waiting lists continues. Nowadays, more than 110 000 patients in the United States are waiting for a transplant, and unfortunately around half of them would receive an organ, with around 20 listed patients losing their lives every day (optn.transplant.hrsa.gov).1 This issue has motivated surgeons and scientists to explore innovative ways to find a solution for high organ demand. In this regard, while some researchers are focusing on organoid generation from stem cells or induced pluripotent cells (iPSCs), others are trying to modify genomes of cells or the entire organ of the donor to make the graft more resistant to injury or tolerant for allo- and xenotransplantation.
While moving to a new decade, we witnessed many awe-inspiring scientific discoveries in the last decade. Some of the major mind-boggling breakthroughs in the last 10 years include improvements in next-generation sequencing (NGS) technology, which allow us to sequence an entire human genome for a few hundred dollars rather than billions of dollars, evaluation of multi-omics data at single-cell resolution, producing organoids as a miniaturized organ and genome editing by revolutionary CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology. State-of-the-art technologies have been exploited to understand the molecular and cellular mechanisms of major conditions during transplantation, such as ischemia-reperfusion injury, transplant tolerance, and rejection, among others. In this minireview, we summarize the successes of CRISPR technologies and its combinatorial enrollment with the aforementioned innovations since 2013, and we further address potential future CRISPR applications in solid organ transplantation (SOT).
2 GENOME EDITING
H.W. Boyer and S.N. Cohen's studies of the recombinant DNA technology in the early 1970s paved the foundation of modern genome editing technologies.2 Scientific efforts over the following 2 decades has demonstrated the importance of double-stranded DNA breaks near the target site to achieve precise and efficient genome editing.3, 4 To take advantage of this observation, artificial restriction enzymes have been proposed to eliminate the unwanted consequences of random DNA integration as well as inefficiency. To do this, first, ZFNs (zinc finger nucleases) were engineered to alter genetic material precisely by designing a special peptide for each codon of 18-24 nucleotide long target site.5, 6 Second, TALEN (transcription activator-like effector nuclease) was developed for the same purpose by generating a DNA binding domain that can recognize single specific nucleotides.7, 8 Despite their great advantages in genome engineering, both systems need expensive and time-consuming de novo protein synthesis for each target site. Finally, the CRISPR system adds versatility to genome engineering by recognizing the target DNA on an RNA-dependent “molecular scissors” rather than protein-DNA–dependent mechanism. Due to low feasibility and higher cost of the first 2 methods, recent clinical trials have been performed by the recent CRISPR technologies (Table 1).
| Method | Recognition mechanism | Complexity | Specificity | Cost | Scalability | Overall feasibility | Clinical trial (numbers; field) |
|---|---|---|---|---|---|---|---|
| ZFN (since 1996) | Protein-DNA | High | Low | High | No | Low | 15; mainly for HIV |
| TALEN (since 2009) | Protein-DNA | Moderate | High | High | No | Medium | 6; for blood cancer |
| CRISPR (since 2013) | RNA-DNA | Low | High | Low | Yes | High | 33; sickle cell, blood cancer, CAR-T, diagnostic |
2.1 The rise of CRISPR
CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. These palindromic repeats and spacer sequences in-between were first discovered in Escherichia coli in 1987, long before the establishment of ZFN and TALEN systems.9 Subsequent studies further showed that half of the bacteria and >90% of archaea carry those DNA fragments in their genome10, 11; however, researchers had to wait for almost 20 years to understand their natural function as an adaptive immune system of bacteria and archaea against bacteriophages.12-14 In 2012, E. Charpentier and J.A. Doudna repurposed the type II CRISPR/Cas9 system as a new genome-editing tool, much like ZFN and TALEN, in their in vitro study.15 Three groups further applied this RNA-programmed genome editing to human cells.16-18 Initial findings demonstrate CRISPR/Cas9 as a facile, robust, and multiplexable system compared to other gene-editing tools.
2.2 Mechanism of CRISPR/Cas9 System
The most widely used CRISPR/Cas9 system, derived from Streptococcus pyogenes (sp), requires 1 single protein called spCas9 (CRISPR-associated protein), 2 natural noncoding RNA molecules, crRNA (CRISPR RNA) and tracrRNA (trans-activating crRNA) and ribonuclease III.15, 19 By taking advantage of RNA engineering, 2 noncoding RNA molecules are fused to produce a single guide RNA (sgRNA), which has 2 critical features18: 20 nucleotides at the 5′ end determine the DNA target site by Watson-Crick base pairing if the target DNA has a PAM (protospacer adjacent motif) sequence, and the scaffold structure at the 3′ end interacts with Cas9. In other words, the first 20 nucleotides (originally crRNA) of the sgRNA help Cas9 to inspect 3 billion bases in the human genome to find the exact match, like a Google search engine. Once the sgRNA/Cas9 complex finds the right site, Cas9 performs its endonuclease function like a “molecular surgeon” to produce a double-strand cut that is corrected mainly by 2 different DNA repair mechanisms: error-prone “nonhomologous end-joining” (NHEJ) and error-free “homologous repair” (HR). If the donor template is supplied to the target cells, the HR system gets a chance to edit the target site by integrating the given DNA fragment during cell division; however, NHEJ generally repairs the double-strand break by random insertion and deletion of nucleotides in both dividing and nondividing cells.16, 18 These mechanisms comprise the canonical Cas9-mediated cleavage activity. It is also noteworthy that researchers have gone further and developed many other tools by modifying the wild-type Cas9 endonuclease (Figure 1).
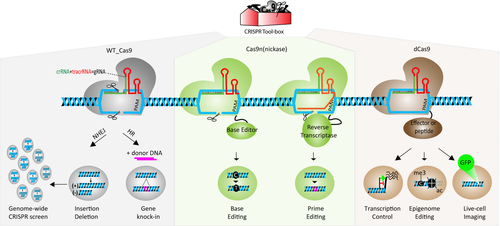
2.3 CRISPR tool box is expanding
Due to ease of use and higher editing efficiency, CRISPR/Cas9 became a popular gene-editing tool to edit the genome of different cell types (cell lines, primary cells, iPSCs) and to generate transgenic animals in a short amount of time.20, 21 These signs of progress inspire researchers to increase their efforts to find novel applications of this system across a variety of fields. The scientific community challenged traditional approaches by utilizing inactive or death Cas9 (dCas9), which lacks nuclease activity on both domains but generates a higher affinity to the target DNA. Therefore, de novo dCas9-fused complex allows us to dock effector protein or peptides on the desired location wherein researchers can change gene expression, alter the epigenome, image the chromatin in live cells, and modify the chromatin architecture.22 Cas9 nickase, which has only one active nuclease domain, initiated the second-generation CRISPR system in which “base editing” and “prime editing” allow us to edit the genome without donor DNA and double-stranded DNA cut23, 24 (Table 2). We further repurposed base editing to silence genes in a safer method called CRISPR-STOP.25 One of the major advantages of the CRISPR system is its scalability or multiplex targeting due to an RNA-based editing mechanism. By designing a genome-wide CRISPR library containing 80 000-100 00 gRNA to target the entire genome, CRISPR screens have become a tremendous tool for functional genomics, where one can test the effect of loss-of-function or gain-of-function at the genomic level in the presence of selectable phenotype or outcome. Studies extrapolating the CRISPR library demonstrate the ease with which researchers can carry out parallel high-throughput screening.26, 27 Collectively, these findings have helped expand the CRISPR toolbox in less than a decade, and each of these tools holds great promise for future translational research (Figure 1).
| Date | Cas variants | Purpose | Improvements | Reference | |
|---|---|---|---|---|---|
| 2013 | WT Cas9 | Native Cas9 (spCas9) | Genome editing | Specific genome editing in eukaryotic cell | 16-18 |
| 2016 | e-SpCas9 | Increase the specificity of Cas9 | Mutations in noncatalytic domain and reduced off-target effects | 70 | |
| 2016 | HF1-SpCas9 | 71 | |||
| 2017 | hypa-spCas9 | 72 | |||
| 2018 | xCas9 | Expand the PAM recognition | Broad range of PAM sequence and greater DNA sensitivity | 73 | |
| 2015 | saCas9 | Cas9 alternative in other organisms | Smaller Cas9 with different PAM | 74 | |
| 2015 | Other major Cas molecules | Cas12a (Cpf1) | Finding Cas9 alternative | Smaller Cas9, easy to pack in virus | 75 |
| 2016 | Cas13a (C2c2) | Ability to modify RNA sequence | 76, 77 | ||
| 2018 | Cas14 | Ability to target ss DNA | 78 | ||
| Mutated spCas9 | Fused domains | ||||
| 2013 | Cas9n (nickease) (one mutation) | — | Genome editing | Reduce the chance of random double-strand cut | 79 |
| 2016 | Cytidine deaminase | Base editing (C to T) | Genome editing without ds DNA cut and donor DNA | 23 | |
| 2017 | Deoxyadenosine deaminase | Base editing (A to G) | 80 | ||
| 2019 | Reverse Transcriptase | Prime editing | 24 | ||
| 2013 | dCas9 (death or inactive Cas9) (double mutation) | KRAB | Gene regulation | Site-specific gene silencing | 81 |
| 2013 | VP64,VPR | Site-specific gene activation | 82, 83 | ||
| 2014 | Fokl | Reduce off-target effect | Less off-target activities | 84 | |
| 2015 | p300 | Epigenome editing | Site-specific acetylation of H3K27 | 85 | |
| 2016 | Tet | Epigenome editing | Site-specific removal of methyl group | 86 | |
| 2017 | DNMT3a | Epigenome editing | Site-specific addition of methyl group | 87 | |
| 2017 | GFP | Live-cell imaging | Specific loci labeling in vivo | 88 | |
| 2017 | PYL1, ABI1 | Reorganizing chromatin architecture | Locus-specific chromatin looping | 89 | |
| 2019 | EZH2 | Epigenome editing | Site-specific methylation of H3K27 | 90 | |
3 TRANSPLANTATION STUDIES IN THE AGE OF GENE EDITING
Compared to the current applications of CRISPR technology in medical fields such as oncology, neuroscience, or developmental biology, there is a limited number of applications in transplantation. However, promising improvements are continually developing, particularly those related to xenotransplantation. In this section, the ongoing and potential future applications of this revolutionary system from cell to organ level for transplantation are discussed and summarized (Figure 2).
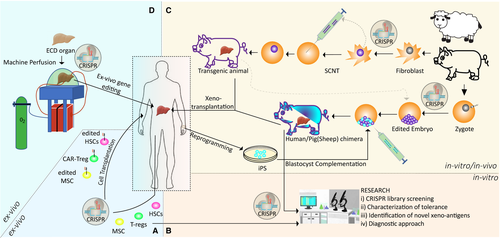
3.1 In vivo and ex vivo gene editing at cellular level
3.1.1 Gene editing of universal human cells for transplantation
Cas9 and gRNA can be efficiently delivered to target cells by using viral (adeno-associated virus, lentiviral) and nonviral (electroporation, liposome, nanoparticles, ribonucleoprotein) methods to exert a therapeutic effect.28, 29 Delivering the CRISPR system into ex vivo isolated cells is relatively more feasible compared to in vivo targeting of solid tissue.30, 31 Therefore, initial CRISPR clinical trials (33 total phase I/II studies) have been performed to cure cancer and blood cell–related diseases by manipulating T cells and hematopoietic stem cells (HSCs) ex vivo.31 Studies regarding pluripotent stem cells (PSCs) and induced stem cells (iPSCs) combined with CRISPR gene editing opened tremendous opportunities to understand regenerative medicine and disease settings. Researchers are not yet able to generate an organ from stem cells on a dish in the laboratory with the current technology. However, researchers have engineered allogeneic stem cells by removing MHC I and II molecules and inserting CD47 protein by CRISPR to produce universal stem cells, which are termed “hypoimmunogenic” and evade immune rejection. This strategy might be used in future projects for organ development in the laboratory.32, 33 On the other hand, in hereditary tyrosinemia type 1 (HT1) disease, allogeneic liver/hepatocyte cell transplantation has potential therapeutic benefits, but the availability of enough donor cells and GvHD (graft-versus-host disease) represent major limitations that are common in cell-based transplantation. In a mouse model of this disease, both in vivo and ex vivo CRISPR-mediated gene editing in hepatocytes improved liver metabolism and demonstrated the potential of the CRISPR system as an alternative therapy for cell/organ transplantation.34, 35 Another potential target for cell-based therapy is a mesenchymal stem/stromal cell (MSC) due to its anti-inflammatory, immunomodulatory, and tissue-repair properties that can play critical roles during solid organ transplantation.36, 37 CRISPR manipulation of those cells will demonstrate potential therapeutic approaches in the near future.38
3.1.2 Gene editing and tolerance
Tolerance, the specific absence of a harmful alloimmune response to a graft tissue in the absence of immunosuppression, is the main step for successful complete immunosuppressive drug (IS) withdrawal following solid organ transplantation. Dendritic and regulatory T cells (Tregs) have been studied for a while to understand their roles in immune tolerance.39, 40 Therefore, these cells became the natural target of CRISPR gene editing with the potential to provide further insights into the mechanisms behind their protective role in immunotolerance and improve their activity. Scientists can engineer both cells to induce tolerance.41, 42 In a recent study, Cas9 and gRNA targeting CD40 were encapsulated into nanoparticles and successfully disrupted CD40 in dendritic cells both in vivo and in vitro.41 Currently, approximately 50 clinical trials are using Tregs, and 15 of them have been conducted for solid organ transplantation (SOT) (clinicaltrials.gov).43 Adoptive cell therapy (ACT) of polyclonal Treg cells has been tested clinically in living donor liver and kidney transplantation and demonstrated the efficacy in IS drug cessation.44, 45 Alloantigen-specific Treg cells are more potent inhibitors than the polyclonal Treg cells; however, producing a high yield of these cells is very challenging.46 Researchers can generate these antigen-specific Treg cells using CRISPR technology by (a) engineered T cell receptors (TCRs), (b) chimeric antigen receptors (CARs) like CAR-T cells in immune-oncology, or (c) overexpression of FoxP3 in antigen-specific effector CD4+ cells. CRISPR-dCas9–mediated epigenome editing has been shown to change FoxP3 expression significantly.47, 48 As of today, there is no clinical trial using CRISPR-engineered Treg cells. Collectively, alloantigen-specific CRISPR gene editing will improve the specificity, stability, and efficiency of Treg cells, and it might allow scientists to produce off-the-shelf Treg cells with advanced engineering. Treg adoptive cell therapy in which RNA-guided nuclease technology and Treg are merged will be one of the most effective treatments for SOTs. Furthermore, genome-wide CRISPR-screening studies shed light on details of molecular mechanisms behind the Treg differentiation, like revealing the activation and differentiation of helper Th2 cells.49
3.2 Gene editing at the organ level
The increasing need for organ donors for end-stage patients forces researchers and clinicians to evaluate alternative options (ie, xenotransplantation, organoids, 3-dimensional [3D] bioprinting). These alternative methods are taking advantage of developments from CRISPR technology to shorten the needed time for transferring applications from bench to bedside.
3.2.1 Xenotransplantation and humanized pig liver
Farm animals and nonhuman primates (NHPs) have been considered alternative sources for organ supply since the 1900s, together with some medical, legal, and ethical issues.50 Chimpanzees and baboons have been used in initial trials, but in the modern era of xenotransplantation, porcine sources are currently preferred due to their similar organ size, ease of breeding, high offspring number, and less disease transmission risk compared to NHPs. However, there are still 3 major barriers preventing successful pig-to-primate (human) organ transplantations: (a) Glycans on the surface of porcine endothelial cells act like xenoantigens (α-Gal, Neu5Gc, SDa) and cause hyperacute rejection (HAR), (b) dysregulated coagulation due to the disagreement between the pig and human coagulation system (THBD, TFPI, CD39), and (c) porcine endogenous retroviruses (PERVs) in porcine genome can transfer vertically into human cells and cause xenosis. Due to the efficiency and simplicity of CRISPR gene editing, researchers have tested ~40 different combinations of pig-gene knock-out and human-gene knock-in into the porcine genome with great efficiency in less than a decade.51, 52 Recently, G. Church and colleagues established the most advanced transgenic pig by using CRISPR technology in which they successfully deleted 3 pig genes (GGTA1, CMAH, and B4GALNT2), and specifically inserted 9 human transgenes (CD46, CD55, CD59, B2M, HLA-E, CD47, THBD, TFB1, and CD39) in a single locus in addition to the inactivation of 25 PERV loci in pig cells.53 Biotech companies like eGenesis, Qihan, and United Therapeutics have accelerated their progress to begin the first human trial. Further improvements can be anticipated in these transgenic animals after evaluating the results of the first trials, leading to a better understanding of xenoimmune response mechanisms.
One possible way to improve the xenotransplantation is to identify novel non-gal xenoantigens, and powerful genome-wide CRISPR screening might be a suitable method to assess the role of pig genes involved in immune rejection.54 As of today, there is only one study conducted with the pig CRISPR library.55 Noteworthy, future gadgets of CRISPR technology will likely improve the feasibility of "Genome Project-Write (GP-Write)" aiming to write a virus-free genome with the desired modifications.56
3.2.2 Chimeric organs and blastocyst complementation
With current technologies, it is not possible to generate human organs with in vitro conditions; however, an animal can serve as a biological incubator to produce human organs at least partially. In theory, patient-specific and immune-matched chimeric organs can be generated by injecting human pluripotent/stem cells into an animal blastocyst (blastocyst complementation) or a targeted organ of utero (utero transplantation).57 Rat to mice or mice to rat chimeric animals have been generated with great success. However, the ratio of chimerism is very low for interspecies chimeras such as human/pig or human/sheep.58-60 CRISPR gene editing increases the efficiency of human/animal chimerism by disabling particular organ development in the host zygote that can be filled by human cells.61 In future chimeric organ-generation studies, scientists will utilize the most advanced CRISPR-mediated transgenic pigs or sheep as a source of zygote and surrogate animals as well.
4 POTENTIAL FUTURE APPLICATIONS OF CRISPR TECHNOLOGY IN TRANSPLANTATION
In several clinical trials, normothermic pulsatile machine perfusion has been used for organ procurement up to 24 hours with increasing success and progress.62, 63 In a recent study, Clavien and his group were able to increase the viability of poor-quality livers up to 7 days by modifying the regular pump with multiple core physiological units.64 These improvements in machine perfusion and enhancement in delivery methods of CRISPR can surpass the limitation of in vivo organ editing by employing ex vivo gene editing of donor organs inside the pump. We speculate that future studies might focus on re-evaluating the ex vivo approaches associated with normothermic organ perfusions in (a) discarded organ and make them viable for transplantation, (b) poor-quality organs (eg, fatty livers) and treat them before implantation, and (c) induction of transplant tolerance making grafts less immunogenic. Another critical factor during organ transplantation is the ischemia-reperfusion injury to the organ. In our unpublished study, we exploited the genome-wide CRISPR screen to identify the genes whose depletion induces resistance against the oxygen deprivation in an in vitro model. This powerful functional genomic screening will also be very useful for addressing diverse problems of transplantation, such as identifying novel xenoantigens and novel molecules of immunotolerance. After the transplantation procedure, patient monitoring against rejection is important. Recent studies illustrate use of CRISPR-mediated diagnostic tools for virus detection in a shorter period. SHARLOCK and DETECTR are 2 CRISPR-based identification methods that can be tailored to detect biomarkers of rejection from the patient's plasma or urine.65, 66 CRISPR-Chip, which can sense unamplified DNA samples on graphene within 15 minutes, will also be used for the same purpose.67
5 LIMITATIONS OF CRISPR TECHNOLOGY
The off-target effect, efficient delivery methods of CRISPR molecules for in vitro and particularly for in vivo applications and immunogenicity of Cas9 protein against the human immunity system are major barriers for CRISPR technology. Because Cas9 molecules can tolerate some mismatches, especially at the distal gRNA/DNA dimer, intense work should be considered for the design of gRNA and the concentration of Cas9 molecules. Any undesirable change due to off-targets in the human/animal (xenotransplantation) genome might cause serious unwanted effects, such as activation of oncogenes, or any other de-novo single-nucleotide polymorphisms (SNPs) changing the behavior of the cells. Improved Cas proteins, redesigned gRNA molecules, and new strategies like prime editing might eliminate some off-target problems. Transferring Cas9 and gRNA molecules into cells, especially for in vivo treatment associated with major limitations since there is no chance to choose the edited cells as in the case of in vitro or ex vivo treatment. Depending on the organ and disease, researchers might need different levels of editing, partial or full-edited organs, which is not possible with the current delivery method. In addition, it has been shown that >60% of humans have a preexisting humoral and cell-mediated adaptive response against major molecules of the CRISPR system, that is, Cas9.68 Therefore, if a persistent expression of Cas9 is required during the treatment, the immunologic response of Cas9 protein needs to be taken into consideration. A possible solution to this issue might be to modify the epitope of Cas9 protein without altering its function and specificity.69
6 CONCLUSION
The CRISPR system is a natural defense mechanism of bacteria, and repurposing of CRISPR technology for the development of cell/organ transplantation will be meaningful for future medical treatments. So far, researchers have benefited from broad applications of CRISPR technology in basic science. In the era of genomics and artificial intelligence (AI), the next-generation researchers may discover more from “simple” organisms such as bacteria, archaea, and even viruses. It will be very exciting to follow the future progress of these revolutionary technologies for medical research.
ACKNOWLEDGMENT
This research was funded through generous support from James D. Eason Transplant Institute and R01DK080074 (VM), R01DK109581 (VM), and R01DK117183 (AB). We thank members of the Transplant Research Institute, especially C. Watkins and M. McKay, for their critical reading of the manuscript.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
No data were created in the writing of this manuscript.




