Renal infarct in a COVID-19–positive kidney–pancreas transplant recipient
Abstract
The novel coronavirus disease 2019 (COVID-19) is associated with increased risk of thromboembolic events, but the extent and duration of this hypercoagulable state remain unknown. We describe the first case report of renal allograft infarction in a 46-year-old kidney–pancreas transplant recipient with no prior history of thromboembolism, who presented 26 days after diagnosis of COVID-19. At the time of renal infarct, he was COVID-19 symptom free and repeat test for SARS-CoV-2 was negative. This case report suggests that a hypercoagulable state may persist even after resolution of COVID-19. Further studies are required to determine thromboprophylaxis indications and duration in solid organ transplant recipients with COVID-19.
Abbreviations
-
- COVID-19
-
- coronavirus disease 2019
-
- DVT
-
- deep vein thrombosis
-
- MMF
-
- mycophenolate mofetil
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
1 BACKGROUND
In December 2019, the novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in Wuhan, China. COVID-19 has now spread worldwide and caused significant morbidity and mortality with 3.8 million cases and upward of 267 000 deaths at the time of writing.1 COVID-19 primarily affects the respiratory system but is increasingly recognized to have multisystemic manifestations.2-4 There is a high prevalence of arterial and venous thromboembolic events in patients with severe COVID-19.5, 6 At this time there are no guidelines delineating which patients should be anticoagulated and for how long. Immunosuppressed patients, such as transplant recipients, may be more susceptible to COVID-19 infection and its subsequent complications. Here, we describe a kidney–pancreas transplant recipient who developed a clinically significant coagulopathy resulting in segmental infarction of his kidney allograft in the context of COVID-19 infection.
2 CASE
A 46-year-old man presented to a screening center with dyspnea and cough. He had a history of simultaneous kidney–pancreas transplant 13 years ago for type 1 diabetes mellitus complicated by diabetic nephropathy and neurogenic bladder. Maintenance immunosuppression consisted of tacrolimus, mycophenolate mofetil (MMF), and prednisone. He had a history of hypertension and dyslipidemia but no history of coagulopathy or conditions predisposing him to arterial or venous thromboembolism. His body mass index was 26.6 kg/m2. He was tested for SARS-CoV-2 using a qualitative polymerase chain reaction test (Seegene Allplex 2019-nCoV Assay), and the diagnosis of COVID-19 was confirmed on April 2, 2020.
He was admitted to our hospital 2 weeks later (April 15) for nausea, diarrhea, weakness, worsening cough, and hypoxemia requiring supplemental oxygen. He was treated for COVID-19 with lopinavir/ritonavir, hydroxychloroquine, and azithromycin for 5 days as well as 7 days of cefuroxime. Tacrolimus was held due to lopinavir/ritonavir administration, but MMF and prednisone were continued at the same doses. Throughout his hospitalization, he received high-dose thromboembolism prophylaxis with enoxaparin 40 mg subcutaneously every 12 hours, as per institutional protocol, which was modified to reflect the higher rate of thromboembolic events observed in COVID-19 patients. During this admission, he had 3 electrocardiograms to monitor QTc, all of which demonstrated sinus rhythm. His creatinine was elevated from a baseline of 120 to 147 μmol/L and remained elevated at time of discharge. Other laboratory values are summarized in Table 1. He was discharged home on April 20 without anticoagulation following improvement in clinical status. At the time of discharge, he still tested positive for SARS-CoV-2.
| First hospitalization | Second hospitalization | |
|---|---|---|
| WBC (ref 3.5-10.5 × 109/L) | 7.1 | 27.4 |
| Lymphocyte count (ref 0.8 to 3.5 × 109/L) | 0.5 | 0.8 |
| Hb, g/L (ref 125 to 170) | 159 | 141 |
| Platelet (ref 130 to 380 × 109/L) | 330 | 404 |
| Creatinine, μmol/L (ref 62 to 100) | 128 | 153 |
| Random glucose, mmol/L (ref 4 to 11) | 5.0 | 7.6 |
| Lipase (14 to 85 U/L) | — | 20 |
| D-dimer, μg/L (ref ≤500) | 1133 | 744 |
| C-reactive protein, mg/L (ref ≤10) | 58.4 | 39.5 |
| Ferritin, μg/L (ref 30-400) | 915 | 699 |
| Fibrinogen, g/L (ref 190-450) | — | 7.9 |
| INR (ref 0.9-1.2) | — | 1.2 |
| PTT, seconds (ref 22-30) | — | 24 |
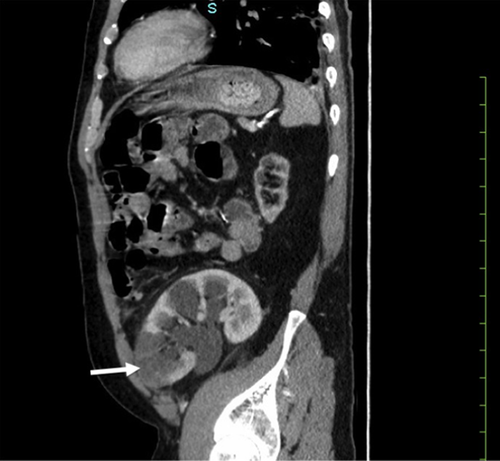
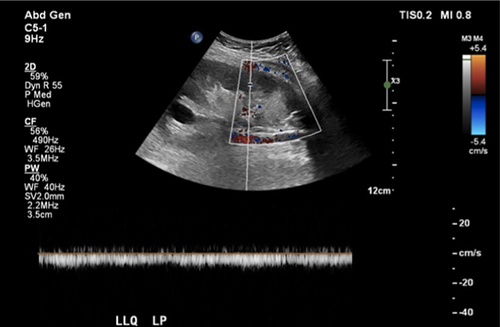
He presented to the hospital 8 days later (April 28) with a 1-day history of sharp left lower quadrant pain. On examination, vital signs were normal but his abdomen was exquisitely tender with rebound tenderness and guarding in the left lower quadrant. There were no signs of deep venous or arterial thrombosis. The remainder of his cardiac and respiratory examinations was unremarkable. Laboratory values at the time of second admission are listed in Table 1. A contrast computed tomography demonstrated a new cortical hypodensity in the lower pole of the transplant kidney in keeping with segmental renal infarction (Figure 1A). The transplanted pancreas was unremarkable. Moderate hydronephrosis and distended bladder were also visualized in keeping with neurogenic bladder. The radiologic findings were suggestive of large vessel arterial thromboembolism, although no thrombus was seen on computed tomography (arterial phase images were not done). Renal ultrasound with Doppler demonstrated decreased blood flow to the lower pole consistent with segmental infarct, with no arterial or venous thrombus seen (Figure 2). A bilateral leg Doppler was negative for deep vein thrombosis. A repeat electrocardiogram showed sinus rhythm. Anti–phospholipid antibody panel (anti–β2-glycoprotein 1 and anti-cardiolipin IgG/IgM antibodies, lupus anticoagulant) was negative. Factor V Leiden and prothrombin gene mutations were absent, and antithrombin activity was normal. Repeat swab for SARS-CoV-2 performed at the time of readmission and 48 hours thereafter returned with negative results.
Therapeutic anticoagulation with enoxaparin 80 mg subcutaneously every 12 hours was initiated with a plan to switch to apixaban 5 mg orally twice daily at discharge for at least 3 months. Outpatient echocardiogram, Holter monitor, and age-appropriate malignancy screening were arranged prior to patient's discharge home.
3 Discussion
COVID-19 is thought to affect the kidneys through several postulated mechanisms, including systemic illness, renal hypoperfusion, cytokine storm, and direct effects of SARS-CoV-2 itself on the nephron.7 There is mounting evidence that COVID-19 patients are predisposed to both venous5, 8, 9 and arterial10 thromboembolic events.
The pathophysiology of COVID-19–induced hypercoagulability appears to be multifactorial. First, the hyperinflammatory state caused by the SARS-CoV-2 virus activates coagulation pathways and creates a hypercoagulable state.11, 12 This may precipitate disseminated intravascular coagulation. Endothelial dysfunction provoked by the cytokine storm as well as direct adhesion of the virus to endothelial cells via angiotensin-converting enzyme 2 receptor further exacerbates hypercoagulability and may be responsible for microvascular disease manifestations.11 Another potential mechanism of SARS-CoV-2–induced coagulopathy, albeit less well characterized, is the association with the production of anti-phospholipid antibodies.13 COVID-19 patients frequently demonstrate high erythrocyte sedimentation rate, C-reactive protein, D-dimer, and fibrinogen, along with thrombocytopenia.11, 12, 14 Reports have demonstrated a potential role for D-dimer in both the prediction and prognostication of DVT in COVID-19 infection.13, 15
Early case reports and case series have highlighted that kidney transplant recipients often present with similar initial symptoms to the general population.16-18 Immunosuppressive medications potentially predispose them to more severe or atypical forms of the disease.19 Consistent with this, they are more likely to deteriorate rapidly and have poorer outcomes.19, 20
Reports also suggest that COVID-19 patients may benefit from thromboprophylaxis.9, 21 The American Society of Hematology has stated that it is reasonable to consider thromboprophylaxis beyond hospitalization in certain populations.22 Whether solid organ transplant recipients present an even higher risk of thromboembolism in the setting of COVID-19 remains unclear. Since we are the first to report this complication, further investigation is required before making recommendations for thromboembolic prophylaxis in all solid organ transplant recipients with COVID-19.
In summary, we present the case of a kidney–pancreas transplant recipient with moderate to severe COVID-19 complicated by late kidney allograft segmental infarction. This is the first case of a thromboembolic event in a SARS-CoV-2–positive solid organ recipient. We hypothesize that COVID-19–related hypercoagulable state is responsible for this thromboembolic event. Furthermore, this event occurred after the resolution of symptoms. The extent of thromboembolic risk in transplant patients with COVID-19 requires further investigation, as does the potential role for prolonged thromboprophylaxis in patients with moderate to severe COVID-19 infection. This case report highlights an unexpected consequence of COVID-19 and adds to the recent reports suggesting an association with large vessel arterial thrombosis.
ACKNOWLEDGMENTS
The authors thank the patient for allowing his case to be presented in this article. The authors of this manuscript have no sources of funding to declare.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




