The anti-inflammatory function of the apoptotic secretome
Abstract
Apoptotic cells release select metabolites that act on neighboring live cells to reduce inflammation and promote homeostasis.
Summary and Analysis
, , , et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020; 580: 130–135.
Apoptosis is a caspase-regulated programmed mechanism of cell death known to be important in embryogenesis, homeostasis and tissue regeneration. The authors used various cell lines and mouse primary cells subjected to different pro-apoptotic stimuli to characterize the nature and function of the secretome from apoptotic cells. They found that the secretion of select metabolites synthesized by the transcriptional activity of metabolic enzymes was still active during apoptosis. Sensed by neighboring cells, these metabolites promoted anti-inflammatory responses in vitro and reduced disease in vivo in mouse models of rheumatoid arthritis and lung transplantation.
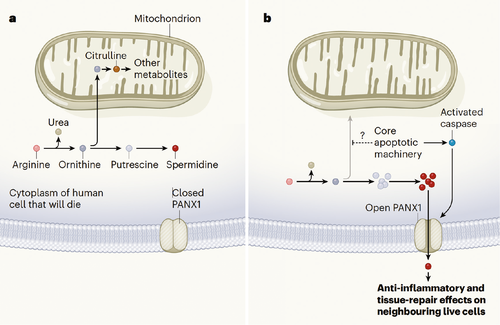
The authors subjected Jurkat cells to apoptosis via UV irradiation or anti-Fas treatment and used untargeted metabolomics profiling on the supernatants at a time when plasma membrane integrity was not compromised. They then identified metabolites that intersected with those secreted by bone marrow–derived macrophages subjected to anthrax toxin and mouse thymocytes treated with anti-Fas. These included the five conserved metabolites adenosine monophosphate (AMP), guanosine-5′-monophosphate (GMP), creatine, spermidine and glycerol-3-phosphate. These were also produced in other cell lines subjected to the same and other apoptosis-inducing stimuli and were reduced upon treatment with the pan-caspase inhibitor zVAD, which prevents apoptosis. Importantly, only select metabolites were released into the supernatants out of the many identified in the cellular pellets. The authors found that some of this selectivity was due to the opening of specific plasma membrane channels, such as pannexin 1 (PANX1) activated during apoptosis. Genetic or pharmacological inhibition of PANX1 prevented the release of 20% of the metabolites. In addition, apoptotic cells maintained transcription of spermidine synthase and increased the production of spermidine in the arginine-to-spermidine metabolic pathway compared with live cells. Thus, the retention of activity of some metabolic pathways and the activation of select channels were two mechanisms by which apoptotic cells could release specific metabolites.
To investigate whether neighboring cells sensed the released metabolites, the authors performed RNA-seq on Chinese ovary cells (CHO) incubated with the supernatants of control and apoptotic Jurkat cells treated, or not, with PANX1 inhibitors. They found PANX1-dependent induction of genes involved in anti-inflammatory and actin rearrangement processes, demonstrating that healthy cells can sense and alter selective intracellular programs in response to apoptosis in their neighbors. This was verified in vivo in Panx1fl/flxCD4-cre mice (in which PANX1 is deleted from thymocytes but not thymic myeloid cells) treated with dexamethasone to induce thymocyte apoptosis. Indeed, wild-type thymic myeloid cells upregulated the expression of genes involved in the glycosylation and transcription of Il10, a phenomenon reduced in cells from Panx1fl/flxCD4-cre mice. Administration of a mixture of three to six of the PANXR1-dependent metabolites after initiation of disease was sufficient to reduce joint inflammation and progression to bone destruction in C57BL/6 mice triggered by injection of serum from arthritic K/BxN mice, a model in which apoptosis occurs and myeloid cells participate in the inflammatory process. Similarly, intraperitoneal injection of the three-metabolite cocktail on days 1 and 3 post-transplantation in C57BL/6 recipients of a C57BL/10 lung allograft resulted in reduced graft inflammation on day 7. Thus, they showed that apoptotic cells are not inert until cleared by phagocytes. Instead, they release select metabolites that can alter the transcriptome and metabolic pathways of neighboring cells, limiting inflammation.
The lung transplant model utilized by these authors directly highlights the potential therapeutic application of the apoptotic secretome in the setting of transplantation. The mechanism by which graft inflammation is reduced upon administration of the apoptotic metabolites, and also the duration and extent of the effect, need to be more thoroughly investigated, but hold promise for harnessing this natural pathway to limit immunopathology. As an extra thought, in these times of a high incidence of post-COVID-19 acute respiratory distress syndrome anticipated to be due to an antiviral or damage-associated cytokine storm, novel therapies that restrict inflammation may be of interest to pursue for both transplanted and nontransplanted patients.
Dr. Alegre is a professor in the Department of Medicine at the University of Chicago. She is also section editor of “Literature Watch.”