Lymphatic vessels in solid organ transplantation and immunobiology
Abstract
With the recent advances in our understanding of the function and biology of the lymphatic vascular system, it is clear that the lymphatic system plays an integral role in physiology, and in pathological settings, may contribute to either enhance or repress inflammation and disease progression. Inflammation is central to both acute and chronic rejection in the context of solid organ transplantation, and emerging evidence suggests the lymphatic system plays a key role in shaping outcomes. The goals of this review are to highlight and contextualize the roles of lymphatic vessels and lymphangiogenesis in immunobiology, the impact immunosuppressive therapies have on the lymphatic system and emerging evidence of organ-specific heterogeneity of lymphatic vessels in the context of solid organ transplantation.
Abbreviations
-
- Ad.VEGFR3-Ig
-
- adenoviral-expressed anti-VEGFR3 immunoglobulin
-
- APC
-
- antigen-presenting cell
-
- BALT
-
- bronchus-associated lymphoid tissue
-
- CAV
-
- cardiac allograft vasculopathy
-
- CLEC-2
-
- C-type lectin domain family member 1B (also known as CLEC1B)
-
- DAMP
-
- damage-associated molecular pattern
-
- DC
-
- dendritic cell
-
- FGF
-
- fibroblast growth factor
-
- FGFR
-
- fibroblast growth factor receptor
-
- FKBP12
-
- FK506 binding protein 12
-
- FOXC2
-
- forkhead box protein C2
-
- FOXP3
-
- forkhead box P3 transcription factor
-
- GALT
-
- gut-associated lymphoid tissue
-
- HA
-
- hyaluronic acid
-
- HEV
-
- high endothelial venule
-
- IFNγ
-
- interferon gamma
-
- IL13
-
- interleukin 13
-
- IL1β
-
- interleukin 1 beta
-
- IL2
-
- interleukin 2
-
- IL4
-
- interleukin 4
-
- IRI
-
- ischemia-reperfusion injury
-
- ISHLT
-
- International Society for Heart and Lung Transplantation
-
- LEC
-
- lymphatic endothelial cell
-
- LN
-
- lymph node
-
- LTβR
-
- lymphotoxin-beta receptor
-
- LV
-
- lymphatic vessel
-
- LYVE1
-
- lymphatic vessel endothelial hyaluronan receptor 1
-
- MALT
-
- mucosa-associated lymphoid tissue
-
- mTOR
-
- mechanistic target of rapamycin (also known as mammalian target of rapamycin)
-
- mTORC1
-
- mechanistic target of rapamycin complex 1
-
- NFAT
-
- nuclear factor of activated T cells
-
- NFκB
-
- nuclear factor kappa B
-
- NRP2
-
- neuropilin 2
-
- PDPN
-
- podoplanin
-
- PIK3CA
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
-
- PROX1
-
- prospero homeobox transcription factor 1
-
- SLO
-
- secondary lymphoid organ
-
- TGFβ1
-
- transforming growth factor beta 1
-
- TLO
-
- tertiary lymphoid organ
-
- TNFα
-
- tumor necrosis factor alpha
-
- VEGF
-
- vascular endothelial growth factor
-
- VEGF-A
-
- vascular endothelial growth factor A
-
- VEGF-C
-
- vascular endothelial growth factor C
-
- VEGF-D
-
- vascular endothelial growth factor D
-
- VEGFR3
-
- vascular endothelial growth factor receptor 3 (known as Flt4 in the mouse)
1 INTRODUCTION
The lymphatic system is composed of lymphatic vessels (LVs), vascular structures similar to blood vessels, which are filled with lymph rather than blood. These LV networks are present throughout the body and are central for the maintenance of tissue fluid balance, the dietary uptake of lipids and the regulation of inflammation.1 The lymph is rich in lipids and lymphocytes (which transit to lymph nodes [LNs]). The lymphatic system is comprised of a series of LVs (capillaries, pre-collecting vessels, collecting vessels) which function in the transport of interstitial fluid, proteins (including antigen), and inflammatory cells to lymphoid organs (thymus, spleen, LN)2 (Figure 1).
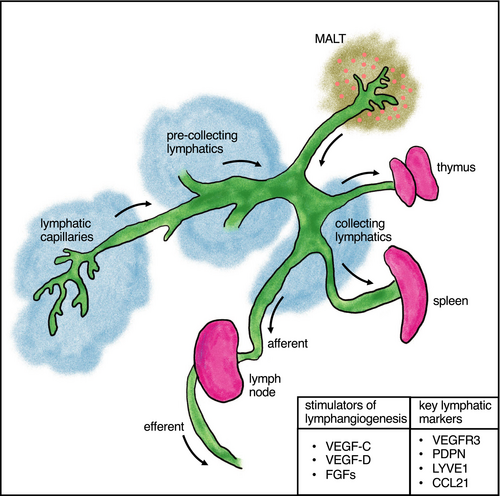
The primary lymphoid organs, the bone marrow and thymus, are major sites of T and B cell production, and secondary lymphoid organs (SLOs) such as the spleen and LNs are key sites for T cell maturation, antigen presentation and the initiation of the adaptive immune response. Tertiary lymphoid structures or organs (TLOs) often develop in sites of chronic inflammation but can also be found natively in some tissues such as the lung (bronchus-associated lymphoid tissue [BALT]) and gastrointestinal tract (gut-associated lymphoid tissue [GALT]), and can function as local immunomodulatory sites.
LVs are lined by lymphatic endothelial cells (LECs), a specialized endothelial cell type that predominantly originate from venous endothelial cells.1, 2 Characteristic lymphatic markers include prospero homeobox transcription factor 1 (PROX1), vascular endothelial growth factor receptor 3 (VEGFR3), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), podoplanin (PDPN), neuropilin 2 (NRP2), the chemokine CCL21, among others.2
The growth of LVs, termed lymphangiogenesis, is primarily mediated by vascular endothelial growth factor (VEGF)-C/VEGFR3 signaling.1 Other mediators of lymphangiogenesis include VEGF-D, fibroblast growth factors (FGFs), ephrin-B2, and hyaluronic acid (HA).1-3 Pro-inflammatory cytokines such as tumor necrosis factor alpha (TNFα) and interleukin 1 beta (IL1β) can induce VEGF-C expression.1 Hemostasis can also stimulate lymphangiogenesis through the release and activation of VEGF-C, which is cleaved (activated) by thrombin and plasmin.4
Wong et al provides a comprehensive overview of regulators of lymphangiogenesis and the development of organ-specific LVs.3 This review summarizes the role of LVs and lymphangiogenesis in solid organ transplantation, with particular focus on its role in heart, kidney, and lung allograft rejection and their intersection with transplant immunobiology.
2 ROLE OF LYMPHANGIOGENESIS IN SOLID ORGAN TRANSPLANTATION
Solid organ transplantation remains one of the few viable options for patients with end-stage disease in a variety of organs. Despite the advent of immunosuppression, allograft rejection remains a primary cause of graft failure beyond 1-year posttransplant. A notable feature common to all solid organ transplants is the lack of surgical reconnection of LVs, in part, due to technical challenges (ie, anatomical exposure, friable nature of tissue), which leads to perturbed lymphatic drainage. Because LVs are essential routes for trafficking of antigen-presenting cells (APCs) and soluble antigens (to SLOs), this may alter local and systemic immunobiology in the allograft, affecting acute and chronic rejection. Regeneration of these severed LVs has been demonstrated as early as 3 days posttransplant in renal transplants in dogs, with evidence of small lymphatics crossing adhesions between the bowel and kidney capsule;5 however, reestablishment of lymphatic flow (drainage to the mesenteric LN) was not seen until 14 days posttransplant. In lung transplants, regeneration of LVs could not be observed until 12 days posttransplant,6 suggesting LV regrowth dynamics may be organ, species, and/or model specific. As our understanding of the unique roles of lymphatics in a variety of organs is rapidly increasing, a recontextualization of the specific role of LVs in solid organ transplantation is warranted to determine whether modulation of LV growth/function can lead to clinical benefit (Figure 2).
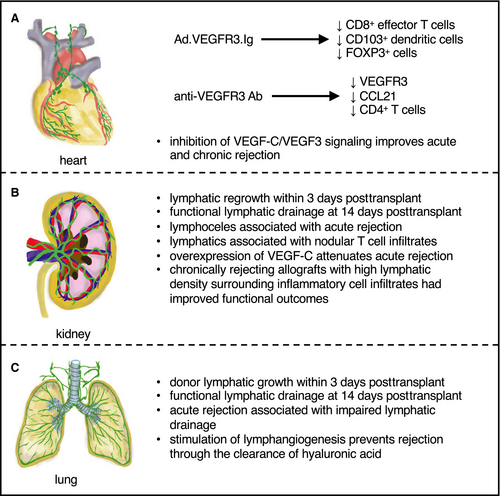
2.1 Heart transplantation
The heart contains a robust LV network extending from the subepicardium to the myocardium and subendocardium. In humans, the most extensive lymphatic network extends from the base of the heart proximal to the pulmonary artery and drains into a cardiac LN in the right mediastinum.7 The primary function of cardiac lymphatics is to prevent myocardial edema through the drainage of interstitial fluid.
Previous reports have shown that the density of LYVE1+ and PROX1+ LVs significantly decreased one month posttransplant, and patients with at least one rejection episode of International Society for Heart and Lung Transplantation (ISHLT) grade IIIa or higher had significantly lower density of VEGF3 posttransplant.8 Efforts to target LVs have yielded new insight into our understanding of their role in heart transplantation. A role for VEGFR3 in immune cell trafficking from peripheral tissues to SLOs via lymphatic CCL21 production has been demonstrated.9 LVs in the allograft expressed VEGFR3, contained APCs, and produced the dendritic cell (DC) chemoattractant CCL21.9 Treatment of transplant recipients with adenoviral-expressed anti-VEGFR3 immunoglobulin (Ad.VEGFR3-Ig) significantly prolonged rat cardiac allograft survival. Ad.VEGFR3-Ig therapy did not affect allograft lymphangiogenesis, but rather, reduced CCL21 production and CD8+ effector T cell entry into allografts, concomitant with reduced DC recruitment and increased forkhead box P3 transcription factor (FOXP3) expression (in the spleen), suggesting that targeting VEGFR3 signaling in lymphatics perturbed their immunomodulatory function, rather than their growth. In a complementary approach, a neutralizing anti-VEGFR3 antibody reduced arteriosclerosis, the number of activated lymphatics expressing VEGFR3 and CCL21, and graft-infiltrating CD4+ T cells in chronically rejecting mouse cardiac allografts.10
Cardiac allograft ischemia-reperfusion injury (IRI) associated with transplantation also results in increased VEGF-C/VEGFR3 signaling, leading to increased cardiac lymphangiogenesis.10 This led to LV activation, increasing inflammation within cardiac allografts, whereas pharmacological inhibition of VEGF-C/VEGFR3 reduced both acute and chronic cardiac allograft rejection10 (Figure 2A). In line with the immunomodulatory role of LVs and lymphangiogenesis in heart transplantation, a recent report demonstrated that lymphatic flow is significantly increased in murine heart allografts.11 Of note, this increased lymphatic flow was correlated with an increase in the number of donor-derived cells in the mediastinal draining LNs and an increase in cardiac allograft vasculopathy (CAV), with increased graft infiltration by CD4+ T cells, CD8+ T cells, and CD68+ macrophages.11 Along these lines, targeted delivery of immunotherapeutics to the LNs prolonged cardiac allograft survival in a mouse model, suggesting the transport of immune cells (and antigens) via the lymphatics to the draining LNs exacerbates cardiac allograft rejection.12
Together, cardiac lymphatics remain an untapped avenue for therapeutic intervention in cardiovascular health and disease.7 Efforts primarily targeting VEGFR3 signaling pathways have demonstrated improvements in reverse cholesterol transport, coronary artery disease, myocardial infarction, and cardiac allograft survival (reviewed in Brakenhielm et al7). Overall, current evidence supports the notion that the role of LVs is deleterious in the context of acute and chronic rejection in cardiac transplantation.
2.2 Kidney transplantation
Renal lymphatics originate from the renal lobule and follow the main arteries or veins toward the hilum.13 The intrarenal lymphatic system originates from the superficial network, which lines the fibrous capsule. These LVs lack valves, which allows for bidirectional lymph flow, and drain either directly to the hilum, or connect to the deeper cortical lymphatic capillaries that run along the capsular arterial branches.13 Renal lymph is formed from the interstitial fluid in the cortex, contains capillary filtrate and fluid reabsorbed from the renal tubules, and is regulated by interstitial fluid volume and intrarenal venous pressure.13 The renal lymphatics play an important role in controlling the pressure, volume, and protein content of the interstitial fluid and also participate in regulating inflammation.
Lymphoceles, the accumulation of lymph without an apparent epithelial lining, has long been associated with renal transplantation. The incidence of lymphocele posttransplant has been reported to be between 0.03% and 33.9%; however, symptomatic ranges are lower (0.03%-26%).14-16 Kidney transplants undergoing acute rejection have a significant risk for the development of lymphoceles.17 During transplant recipient preparation of the recipient for implantation, the iliac artery and vein are exposed, and in this process, LNs surrounding these vessels are often damaged. In addition, surgical damage of LVs in the donor kidney occurs during procurement. Together, these may both contribute to the overall disruption of the renal lymphatic system, although the implications or long-term clinical consequences remain to be determined.
De novo lymphangiogenesis has been reported in 61%-74% of renal protocol biopsies from kidney transplant patients, although there was no significant difference in LV density in acute cellular rejection versus those below the threshold for acute rejection or chronic allograft nephropathy.18 Sequential biopsies in human kidney transplants revealed that grafts with higher lymphatic densities within nodular areas of cellular infiltrates (resembling TLOs) had improved kidney functional outcomes compared to those with lower LV density 1 year posttransplant.18 Another study demonstrated kidney transplants with higher perivascular lymphatic density had a reduced proportion with progression of interstitial fibrosis/tubular atrophy grade from 3-12 months posttransplant and was associated with a reduced rate of estimated glomerular filtration rate decline after 12 months.19 In line with this, VEGF-C overexpression in mouse kidney allografts significantly attenuated acute rejection20 (Figure 2B). Overall, perivascular lymphatic density or lymphatic density in proximity to TLOs may be the best indicators of early outcomes in kidney transplants, in part, related to their ability to provide a route for immune cell emigration.
In chronically rejected human renal grafts, concomitant lymphangiogenesis and ectopic germinal center formation has been reported.18 Another report demonstrated that high LV density with nodular infiltrates is associated with graft loss,21 suggesting that in chronically rejecting kidney transplants, the inability to clear immune cell infiltrates may occur despite the high density of LVs, which may be a reflection of LV dysfunction rather than lack of (re)growth. In a rat kidney transplants, lymphangiogenesis is associated with chronic renal allograft injury, and the mechanistic target of rapamycin (mTOR) inhibitor sirolimus was shown to be a potent inhibitor of lymphangiogenesis.22 In rat kidney transplants immunosuppressed with cyclosporine, there was an 18-fold increase in the number of interstitial LVs with no change in the number of perivascular LVs.23 This increase in interstitial LVs was correlated with the extent of interstitial fibrosis,23 which mirrors previous reports of de novo tubulointerstitial lymphangiogenesis in failing human kidney transplants.24
Whereas some reports demonstrate lymphangiogenesis associated with acute and chronic rejection, recent experimental evidence demonstrates that enhancing lymphangiogenesis may improve acute transplant survival.20 One variable that may be considered in comparing differences between reports is the use of immunosuppressive agents. For example, chronic lymphedema has been reported in renal transplant recipients given sirolimus,25 whereas observations in many animal models did not utilize immunosuppressants.20 Another observation that can be inferred is that in renal allografts, LV density alone reflect outcomes, and further investigation of the proximity and interaction of LVs with inflammatory cell infiltrates may yield more conclusive insights. In a model of renal injury and fibrosis, lymphangiogenesis within the kidney and the corresponding draining renal LNs promoted intrarenal inflammation and fibrosis,26 whereas in another model, enhancing renal lymphatic expansion was shown to prevent hypertension through the reduction of renal immune cell accumulation.27 Clearly the role of lymphangiogenesis in regulating inflammation and immune cell transit is context dependent, and further kidney transplant studies are warranted to determine what conditions may promote a more tolerogenic environment.
2.3 Lung transplantation
The pulmonary lymphatic network is present throughout the pleura, in the perivascular connective tissue around the venules and arterioles, within the juxta-alveolar region of the interlobular septum, as well as within the connective tissue of the terminal and respiratory bronchioles.28 Within the lungs, forward lymph flow is dependent on changes in pressure associated with respiration, as lung collecting LVs lack supporting smooth muscle cells.29 In a mouse model of lung transplantation, donor LVs have been reported to begin sprouting toward the recipient within 3 days posttransplant, establishing functional connections by 14 days posttransplant.30 Of note, lymph flow in the pulmonary system flows in two opposite directions: in the periphery of the lung, lymph flows toward the pleura, whereas in other regions, lymph flows toward the hilum.28
In one study, 56 of 99 lung transplant recipients (57%) experienced noncardiogenic pulmonary edema (or pulmonary reimplantation response) in the first week posttransplant.31 Although the reimplantation response (referred to as primary graft dysfunction in more recent literature) is generally thought to be a consequence of IRI, it remains unclear whether lymphatic disruption contributes to this condition. Although edema is a hallmark of lymphatic dysfunction, and is to be expected considering the interruption of the lymphatic system during transplantation, the fact that many patients do not develop a reimplantation response suggests that lymphatic disruption alone is not sufficient to promote acute graft injury. Pulmonary edema in the context of a reimplantation generally resolves within a few days with supportive care, and it remains unclear whether effects persist as a result of this lymphatic perturbation.
Experimentally, pulmonary lymphatic flow and lymphatic drainage were assessed after lung transplantation in dogs and demonstrated a lack of visible drainage to the mediastinal LNs 3 days posttransplant in three of the four dogs investigated32 (Figure 2C). In lung allografts treated with immunosuppression, lymphatic drainage was reestablished as early as 2 weeks posttransplant.32 Acute rejection was associated with the cessation of lymphatic drainage from the transplanted lung to the mediastinal LNs.32 Recent experimental work in murine lung allografts showed that stimulating lymphangiogenesis alleviates rejection, in part, through improving the clearance of HA,33 whereas disruption of lymphatic structures and function in the transplanted lung lead to impaired lymphatic flow and perturbed graft function.29
Chronic airway inflammation predisposes to persistent lymphatic hyperplasia, which may lead to bronchial (lymph)edema and airway obstruction.34 Disruptions in the lymphatic system are also seen in a variety of pulmonary disorders, including increased lymphangiogenesis in pneumonia, regression of LVs in asthma, remodeling, and growth of LVs in idiopathic pulmonary fibrosis, among others (reviewed in Yao & McDonald34). It has been demonstrated that rapamycin can reverse VEGF-C-driven lymphatic abnormalities in the respiratory tract,35 suggesting that the use of mTOR-based immunosuppressants in the context of lung transplantation may contribute to modified lymphatic phenotypes. However, due to concerns with regard to bronchial anastomotic healing, rapamycin use early after lung transplantation may not be advisable.36
Overall, in apparent contrast to hearts, LVs in the lung may serve a beneficial role, in part, through facilitating pulmonary drainage and immune cell transport early after transplantation. An important consideration is that lung grafts, unlike hearts, are able to locally provide a suitable environment for the activation of alloimmune responses, and therefore, do not have a requirement for lymphatic flow to draining LNs to trigger rejection.37 As HA can act as a damage-associated molecular pattern (DAMP), promoting inflammation through activation of TLR4, leading to immune cell activation,38 clearance of this may be central to limiting early alloimmune responses. Interestingly, induction of BALT, a TLO that has been characterized by formation of high endothelial venules (HEVs) and LVs, has been observed in lung allografts that undergo chronic rejection, and has also been associated with a tolerant state.39, 40 Therefore, future studies will have to further elucidate how lymphangiogenesis shapes the fate of pulmonary allografts.
3 INTERPLAY BETWEEN LYMPHATIC BIOLOGY AND TRANSPLANT IMMUNOBIOLOGY
The lymphatic system is intimately involved with the immune system, with LVs serving as the primary conduit for lymphocyte emigration to draining LNs.41 LNs are immunological hubs that localize APCs and lymphocytes to propagate inflammatory responses. LECs can directly mediate immune cell interactions (Figure 3A). DCs can induce the release of CCL21 by LECs, further promoting DC transmigration across the lymphatic barrier.42 In addition, CCL21 promotes tissue egress of DCs through afferent LVs.43 T cell migration to draining LNs has also been shown to require intralymphatic crawling44 (Figure 3A). Lymphotoxin-beta receptor (LTβR) signaling in LECs is required for T cell transmigration, in part through a nonclassical nuclear factor kappa B (NFκB) pathway controlling production of CCL21 and CXCL12 in LECs.45 LECs have been shown to directly regulate T cell tolerance and suppress immunity.46 Beyond direct interactions, LECs have been also shown to be able to capture and archive antigen,47 and migratory DCs can actually acquire and present LEC-archived antigens during LN contraction48 (Figure 3A).
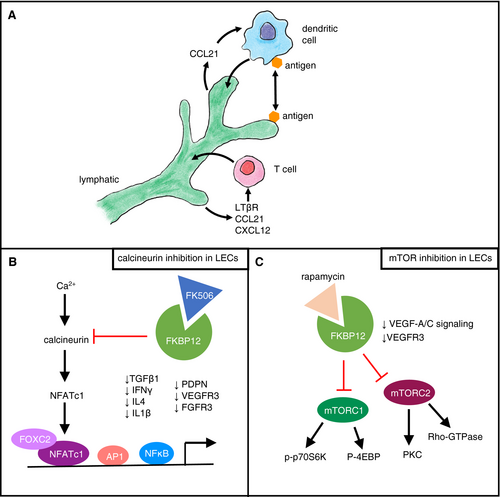
Transplant immunobiology involves a coordinated cascade of allorecognition, T cell activation and differentiation, and effector-mediated functions. As such, a dysfunctional lymphatic system would likely alter immune cell dynamics in the context of a solid organ transplant, where LVs (and their normal draining routes) are severed. Further, the clinically utilized immunosuppressive regimens may have direct effects on lymphatic function and lymphangiogenesis (see below).
Recently, it has been demonstrated that FOXP3+ T cells within BALT contributed to the maintenance of lung allograft tolerance through the suppression of donor-specific antibody production, preventing the formation of antibody-mediated rejection.40 Interestingly, impairment of lymphatic flow in C-type lectin domain family member 1B (CLEC1B, also known as CLEC-2)-deficient mice or inducible lung-specific ablation of LECs resulted in a pro-inflammatory state, characterized by the presence of TLOs (BALT).29 As LVs, along with HEVs, are key to the function and regulation of TLOs, it would be of interest to link FOXP3+ T cell function to LEC status within BALT. It would also be of interest to determine whether a similar phenomenon occurs within kidney allografts, where TLOs have also been reported.
4 EFFECT OF IMMUNOSUPPRESSIVE DRUGS ON LYMPHATIC ENDOTHELIAL CELLS
Transplant recipients are maintained on an immunosuppressive regimen that may include calcineurin inhibitors, mTOR inhibitors, corticosteroids, antimetabolic compounds, or others. Although the advent of the current generation of immunosuppressants have yielded significant improvements in posttransplant outcome, chronic allograft dysfunction and rejection remain pressing clinical challenges, and their secondary effects on stromal cell populations such as LVs and LECs remain to be fully understood.
4.1 Calcineurin inhibitors
Calcineurin inhibitors are the primary mode of immunosuppression currently used. The calcineurin inhibitor tacrolimus (FK506) inhibits T cell activation through complexing with FKBP12 (FK506 binding protein 12), inhibiting calcineurin signaling, ultimately preventing the dephosphorylation of nuclear factor of activated T cells (NFAT) and the transcription of interleukin-2 (IL2).49 NFATc1 signaling has been shown to regulate LEC development, with inhibition resulting in irregular patterning of lymphatic sprouts, in part, through the reduction of PDPN, VEGFR3, and fibroblast growth factor receptor 3 (FGFR3) expression50 (Figure 3B). In particular, decreased calcineurin activity diminished VEGF-A-induced pulmonary lymphangiogenesis.50 NFATc1 signaling also directly interacts with forkhead box protein C2 (FOXC2) on enhancer elements to promote FOXC2-target gene expression, which is essential in multiple aspects of developmental and pathological lymphangiogenesis, including the regulation of lymphatic valve formation.51 In T cells, calcineurin/NFAT signaling has been shown to be required for the switch from catabolic to anabolic metabolism after T-cell receptor and co-stimulation.52 As LEC metabolism has recently been shown to be important in lymphangiogenesis and lymphatic function,53, 54 it would be of interest to investigate the metabolic consequences of tacrolimus on LECs.
Interestingly, application of topical tacrolimus improved LV function with increased contraction frequency and decreased dermal backflow and increased collateral LV formation in the context of secondary lymphedema.55 In this context, the effects were not due to increased expression of VEGF-C or VEGF-A but rather occurred through the suppression of transforming growth factor beta 1 (TGFβ1), interferon gamma (IFNγ), and IL4 and IL13 production.55
Although it is clear tacrolimus can have both direct and indirect effects on lymphangiogenesis and lymphatic function, what remains unclear are the context dependent effects of tacrolimus in solid organ transplantation and the organ-specific LEC and stromal cell responses. Additional investigation into the effects of calcineurin inhibition on LVs is warranted to determine whether tacrolimus may result in perturbation of lymphangiogenesis or LV function and whether counteracting these effects may improve transplant outcomes.
4.2 mTOR inhibitors
mTOR is a central signaling molecule, regulating signaling from insulin, growth factors, and amino acids but also acts as a sensor of nutrients, oxygen, and energy. Rapamycin inhibits mTOR signaling through interacting with FKBP12, resulting in the inhibition of mechanistic target of rapamycin complex 1 (mTORC1) and mTORC2 (Figure 3C). Inhibition of mTOR impairs lymphangiogenesis through inhibition of VEGF-A and VEGF-C signaling via p70S6 kinase.56 Further, rapamycin has been shown to inhibit LEC tube formation via downregulation of VEGFR3.57 In generalized lymphatic anomalies with phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) variants, mTOR inhibition with rapamycin prevented lymphatic hyperplasia and dysfunction in mice expressing an active form of PIK3CA (His1047Arg).58
In rat renal allografts, sirolimus inhibited lymphangiogenesis, leading to improved graft function and a reduction of chronic kidney allograft injury compared to use of the calcineurin inhibitor cyclosporine.22 Indeed, chronic lymphedema has been reported in renal transplant patients attributable to sirolimus in the same location where the arteriovenous fistula used for dialysis occurred.25 Further study of the effects of mTOR inhibitors on lymphangiogenesis and lymphatic function in the context of transplantation is warranted to determine the effects of mTOR inhibition in nonrenal transplants and whether use of mTOR inhibitors in place of calcineurin inhibitors alters effects on LVs and graft outcome.
5 CONCLUDING REMARKS
Overall, lymphangiogenesis is an important physiological process in wound healing and repair, and plays a central role in the regulation of inflammation. In the context of solid organ transplant biology, lymphangiogenesis has been associated with both positive and negative effects. In some cases LVs are associated with acute and chronic rejection,10 whereas in others, LVs have been shown to be beneficial in attenuating acute allograft rejection.20, 33 To this point, future work to distinguish interactions in acute rejection, chronic rejection, and allograft vasculopathy is needed to better understand the role of LVs in differing transplant settings. Further, in some settings, LV density does not correlate with outcomes, whereas proximity to immune cell infiltrates was associated with improved outcomes,18 suggesting that additional investigation is needed focusing on lymphatic function, in particular its role in immunomodulation.
In other solid organ transplant settings, more work is required to understand the role of lymphangiogenesis in transplant outcomes. For example, in a rat model of liver transplantation, lymphangiogenesis has been associated with acute cellular rejection, with de novo growth focused in portal areas and portal-portal bridging areas accompanied with cellular infiltration.59 Along these lines, in a mouse model of corneal transplantation, LVs contributed to graft rejection, and selective inhibition of lymphangiogenesis with anti-VEGFR3 or anti-integrin α5 antibodies was able to able to improve graft survival.60 Collectively, these experimental observations raise the possibility that tissue- and organ-specific differences may exist with regard to the role of LVs.
As chronic allograft dysfunction and rejection remains a pressing challenge in overall graft and patient outcomes despite robust immunosuppressive regimens targeting T cells, greater focus on other stromal compartments such as the lymphatic system are warranted to better understand transplant (immuno)biology and to develop new synergistic therapeutics.
ACKNOWLEDGMENTS
We apologize to those we could not cite due to reference limits. We acknowledge the thoughtful review of this article by Drs. Ola Ahmed, William Chapman, Daniel Kreisel, and Yiing Lin. This work was supported by a Joel D. Cooper Career Development Award from the International Society for Heart and Lung Transplantation.
DISCLOSURE
The author of this manuscript has no conflicts of interest to disclose as described by the American Journal of Transplantation.




