Treatment with 3-aminobenzamide during ex vivo lung perfusion of damaged rat lungs reduces graft injury and dysfunction after transplantation
DATA AVAILABILITY STATEMENT:
The data that support the findings of this study are available from the corresponding author upon request.
Abstract
Ex vivo lung perfusion (EVLP) with pharmacological reconditioning may increase donor lung utilization for transplantation (LTx). 3-Aminobenzamide (3-AB), an inhibitor of poly(ADP-ribose) polymerase (PARP), reduces ex vivo lung injury in rat lungs damaged by warm ischemia (WI). Here we determined the effects of 3-AB reconditioning on graft outcome after LTx. Three groups of donor lungs were studied: Control (Ctrl): 1 hour WI + 3 hours cold ischemia (CI) + LTx; EVLP: 1 hour WI + 3 hours EVLP + LTx; EVLP + 3-AB: 1 hour WI + 3 hours EVLP + 3-AB (1 mg.mL−1) + LTx. Two hours after LTx, we determined lung graft compliance, edema, histology, neutrophil counts in bronchoalveolar lavage (BAL), mRNA levels of adhesion molecules within the graft, as well as concentrations of interleukin-6 and 10 (IL-6, IL-10) in BAL and plasma. 3-AB reconditioning during EVLP improved compliance and reduced lung edema, neutrophil infiltration, and the expression of adhesion molecules within the transplanted lungs. 3-AB also attenuated the IL-6/IL-10 ratio in BAL and plasma, supporting an improved balance between pro- and anti-inflammatory mediators. Thus, 3-AB reconditioning during EVLP of rat lung grafts damaged by WI markedly reduces inflammation, edema, and physiological deterioration after LTx, supporting the use of PARP inhibitors for the rehabilitation of damaged lungs during EVLP.
Abbreviations
-
- 3-AB
-
- 3-aminobenzamide
-
- 3-NT
-
- 3-nitrotyrosine
-
- BAL
-
- bronchoalveolar lavage
-
- CI
-
- cold ischemia
-
- EVLP
-
- ex vivo lung perfusion
-
- ICAM-1
-
- intercellular adhesion molecule
-
- IL-10
-
- interleukin-10
-
- IL-6
-
- interleukin-6
-
- IR
-
- ischemia-reperfusion
-
- LDH
-
- lactate dehydrogenase
-
- LTx
-
- lung transplantation
-
- MDA
-
- malondialdehyde
-
- MNs
-
- mononuclear cells
-
- NAD+
-
- nicotinamide dinucleotide
-
- NF-κB
-
- nuclear factor kappaB
-
- PA
-
- pulmonary artery
-
- PARP
-
- poly(ADP-ribose) polymerase
-
- PBS
-
- phosphate-buffered saline
-
- PEEP
-
- positive end-expiratory pressure
-
- PGD
-
- primary graft dysfunction
-
- PMNs
-
- polymorphonuclear neutrophils
-
- PV
-
- pulmonary vein
-
- RR
-
- respiratory rate
-
- SelE
-
- E-selectin
-
- SelP
-
- P-selectin
-
- SPC
-
- static pulmonary compliance
-
- VCAM-1
-
- vascular cell adhesion molecule 1
-
- VT
-
- tidal volume
-
- WI
-
- warm ischemia
1 INTRODUCTION
A major limitation to lung transplantation (LTx) is the lack of available organs, due to the limited number of donors and the high proportion of organs deemed unsuitable for transplantation.1 In recent years, ex vivo lung perfusion (EVLP) has been developed to increase donor lung utilization, representing a breakthrough in clinical LTx.2 EVLP allows the assessment of a donor graft in optimal conditions,3, 4 and may serve as a platform to deliver therapies for the repair of damaged organs (concept of reconditioning, or rehabilitation).1, 2
In our own studies, we recently reported reduced ex vivo injury and dysfunction of donor rat lungs damaged by warm ischemia (WI) and treated with 3-aminobenzamide (3-AB), a prototypical inhibitor of poly(ADP-ribose) polymerase 1 (PARP-1), via an EVLP platform.5 PARP-1 is a nuclear enzyme activated by damaged DNA, notably under conditions of oxidative stress. Activated PARP consumes cellular nicotinamide dinucleotide (NAD+) and promotes polyADP-ribosylation (PARylation) in multiple target proteins, impairing cellular metabolism and signaling, to trigger cell death and dysfunction.6, 7 In our aforementioned study,5 we did not explore whether the beneficial effects of ex vivo PARP inhibition improved the outcome of the grafts after LTx. Such an observation would be of great significance, owing to the recent development of the first PARP inhibitors for clinical use (for oncologic indications).6, 8 We therefore conducted the present study to address the hypothesis that PARP inhibition with 3-AB during EVLP would recondition damaged rat lung grafts to improve transplant outcomes.
2 MATERIAL AND METHODS
2.1 Animals
Adult Sprague-Dawley rats (10-12 weeks, 350-380 g, Charles River) were used in all the experiments. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 86-23, revised 1996). The experiments were approved by our ethical committee (Authorization Nr 2637).
2.2 Experimental design
- Control (Ctrl, N = 14): The lungs were kept in situ for 1 hour (WI time) and then were flushed with 15 mL of 4°C Perfadex (Xvivo Perfusion). The heart-lung block was then removed and the lungs were preserved in an inflated status (FiO2 = 0.5) for 3 hours in 4°C Perfadex (cold ischemia [CI] time) before LTx.
- EVLP (N = 15): The lungs were kept in situ for 1 hour (WI time), then flushed with 15 mL of 4°C Perfadex. The heart-lung block was then removed. The lungs were preserved in an inflated status (FiO2 = 0.5) for 1 hour in 4°C Perfadex (CI), and were then exposed to 3 hours EVLP (EVLP time), followed by 2 additional hours of CI (in 4°C Perfadex) before LTx.
- EVLP+3-aminobenzamide (EVLP + 3-AB, N = 16): Same as EVLP, except that 3-AB (Sigma-Aldrich) was added during EVLP at a concentration of 1 mg mL−1.
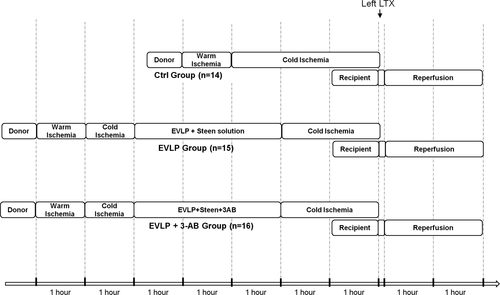
Separate groups of donor lungs were used for the purposes of physiological and biochemical measurements, histology, and mRNA studies, as exposed below.
2.3 EVLP
EVLP was performed as described previously.5 The heart-lung blocks were weighed, mounted in the EVLP system, perfused at progressive flow (2% of theoretical rat cardiac output at 10°C, up to 7.5% of theoretical cardiac output at 37.5°C) with Steen solution (Xvivo Perfusion) equilibrated with 6% O2, 10% CO2, and 84% N2. In EVLP + 3-AB group, 3-AB was added at 1 mg mL−1. At 35°C, ventilation was started (RR 7 min−1, VT 3 mL kg−1, positive end-expiratory pressure (PEEP) 3 cm H2O, FiO2 0.21). At 37°C, VT was set at 6 mL kg−1. Static pulmonary compliance (SPC) was determined by the slope of a quasi-static pressure-volume (P-V) curves generated according to a predefined algorithm.5 At the end of EVLP, the heart-lung blocks were weighed to calculate the weight gain (index of lung water accumulation) and kept inflated (FiO2 = 0.21) in cold Perfadex (4°C) for 2 hours. The lungs were separated and bronchoalveolar lavage (BAL) was done in the right lung while the left lung was prepared for LTx.
2.4 Orthotopic left LTx
Rat left LTx was performed according to de Perrot.11 The recipient rat was anesthetized (induction with inhaled 5% isoflurane, and ip ketamine, 80 mg kg−1, xylazine, 8 mg kg−1, and buprenorphone 0.05 mg kg−1; maintenance with inhaled isoflurane 1.5%), tracheotomized, and ventilated (FiO2 0.5, RR 90 min−1, VT 7 mL kg−1, PEEP 3 cm H2O). The right jugular vein was cannulated for intravenous infusion (0.9% NaCl, 10 mL kg−1 h−1 throughout the experiment). The left carotid artery was canulated to monitor blood pressure. Via a left posterolateral thoracotomy, the left hilum was dissected, the native lung removed, and the pulmonary artery (PA) and pulmonary vein (PV) clamped incised and encircled with 7-0 silk sutures (Teleflex). VT was reduced to 5 mL kg−1(single right lung ventilation). The donor left lung was weighed, and its PA and PV were canulated with a 16-gauge cuff (Abbocath Catheters, Hospira Inc) and sutured into the recipient's PA and PV. The lung was connected to a second ventilator (FiO2 0.5, RR 90 min−1, VT 4 mL kg−1, PEEP 5 cm H2O), and its PV and PA were unclamped for reperfusion. After 2 hours, the rat was exsanguinated, and the graft was harvested, weighed, and used for BAL analysis or histology. Blood was centrifuged (10 minutes, 5000 rpm) and plasma was stored for further analysis.
2.5 Measurements
2.5.1 Physiological measurements
The graft peak airway pressure (PAWP) was monitored, and SPC was determined as the slope of the quasi-static P-V curve generated by a low-flow stepwise inflation up to 4 mL kg−1. At the end of reperfusion, a blood gas analysis was done from the graft's pulmonary vein (Abbott i-STAT analyzer and CG4+ cartridge).
2.5.2 Bronchoalveolar lavage and biochemical measurements
A BAL was performed in the right lung at the end of EVLP, with 6 mL phosphate-buffered saline (PBS), and in the left, transplanted lung, at the end of reperfusion, with 4 mL PBS. After centrifugation (5000 rpm, 10 minutes), the levels of lactate dehydrogenase (LDH) and the cytokines interleukin-6 (IL-6) and interleukin-10 (IL-10) were determined in the clear supernatants, using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) as described previously.5, 9 The ratio IL-6/IL-10 was calculated as an index of inflammatory/anti-inflammatory balance.12, 13 In the BAL from the left transplanted lung, the cell pellets were resuspended in PBS, cells were counted in a hemocytometer, cytospinned (800 rpm, 5 minutes), and stained (Diff-Quik) to count polymorphonuclear neutrophils (PMNs) and mononuclear cells (MNs). In addition, IL-6 and IL-10 were also measured in plasma samples obtained at the end of experiments.
2.5.3 Measurements of PARP activity, myeloperoxidase, nitrotyrosine, and malondialdehyde
PARP activation was determined both at the end of EVLP (right lung) and after transplantation (left lung), by quantifying the amount of poly(ADP-ribose) in lung tissue extracts, expressed in pg/mg protein, as described previously.5 To assess the amount of PMNs from the donor lungs at the end of EVLP, we determined the activity of myeloperoxidase (MPO) in tissue extracts from the right lung after EVLP, expressed in ng/mg protein, using an MPO ELISA kit (Hycult Biotech). To evaluate oxidative stress following transplantation, we measured the amount of nitrotyrosine (a marker of peroxynitrite generation) in BAL fluid, expressed in pg/mL, using a commercial ELISA (Rat 3-Nitrotyrosine ELISA kit; BlueGene Biotech), as well as the levels of malondialdehyde (MDA, a marker of lipid peroxidation) in lung tissue extracts, expressed in ng/mg protein (Rat Malondialdehyde ELISA kit, MyBioSource).
2.6 Determination of adhesion molecules mRNA in transplanted lungs
The mRNA expression levels were measured in lungs exposed or not exposed to BAL at the end of experiments (n = 9-11 rats/group) to exclude any possible confounding effect of the process of lung lavage on the determination of mRNA. Frozen tissue samples were pulverized in liquid nitrogen, and RNA was extracted from the tissue powder using peqGOLD TriFast reagent. One microgram of mRNA was reverse transcribed into complementary DNA (cDNA) using High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific), followed by real-time PCR using PowerUp SYBR Green Master Mix Ex (Applied Biosystems) on a QuantStudio 12K Flex System (Applied Biosystems). The mRNA expression of the following adhesion molecules was evaluated: P-selectin (SelP), E-selectin (SelE), vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), using the following oligonucleotides: SelP: Fwd 5′-GGCTTCTACTTCTGGCCTGG-3′, Rev 5′-GGCTTCTACTTCTGGCCTGG-3′; SelE: Fwd 5′-TCCCTTGGTAGTGGCACTCT-3′, Rev 5′-GGCAGCTACTAGCAGGAACG-3′; VCAM-1: Fwd 5′-GACACCGGGAAAGCTCTTGT-3′, Rev 5′-TCGGAGCAACGTTGACATAAAGA-3′; ICAM-1: Fwd 5′-GCCTGGGGTTGGAGACTAAC-3′, Rev 5′-TCGCTCTGGGAACGAATACAC-3′. Gene specific expression was normalized to the ribosomal subunit gene Rps18: Fwd 5′-ACTTTTGGGGCCTTCGTGTC-3′, Rev 5′-GCCCAGAGACTCATTTCTTCTTG-3′, and was expressed relative to the expression in lungs obtained from the Ctrl group. All the samples were tested in triplicate.
2.7 Histological analysis
At the end of reperfusion, grafts from n = 4/group (not subjected to BAL) were fixed with optimal cutting temperature compound and 4% paraformaldehyde, embedded in paraffin, longitudinally sectioned at 5 µm, and stained with hematoxylin & eosin. Sections were digitalized (Hamatsu Photonics) and uploaded to an image software (Slidepath, Leica Biosystems). The degree of lung injury was quantified according to the score proposed by Ruiz-Remolina et al,14 based on the degree of alveolar septa congestion, alveolar hemorrhages, alveolar edema, and cellular infiltration, with each item being scored as absent (0), mild (1), moderate (2), or severe (3). Ten histological fields per section were blindly evaluated for the calculation of the score.
2.8 Analysis of data
Data analysis was performed by Graphpad prism 6 (GraphPad Software Inc). All the results are presented as individual dots and means ± standard error of the mean (SEM). In EVLP experiments, comparisons between the EVLP and EVLP + 3-AB groups were done using an unpaired t test. In all other conditions, we applied one-way or two-way analysis of variance (ANOVA) followed by post hoc Tukey or Bonferroni adjustments when appropriate. The analyses were performed on log-transformed data in cases of not normal distribution, as determined by the Shapiro-Wilk normality test. Statistical significance was assigned to a P < .05.
3 RESULTS
3.1 Physiological and biochemical variables during EVLP
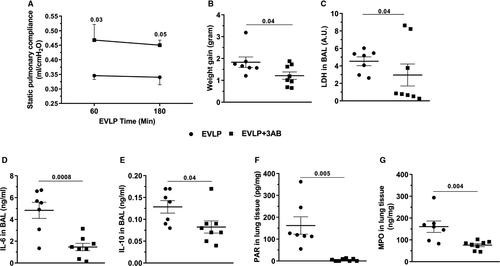
EVLP data are shown in Figure 2. SPC was significantly higher (Figure 2A) in lungs from EVLP + 3-AB compared to EVLP, at 60 and 180 minutes. At the end of EVLP, weight gain of the heart-lung blocks was significantly attenuated in EVLP + 3-AB (Figure 2B), whereas BAL LDH (Figure 2C), a marker of cellular damage, BAL IL-6 (Figure 2D), and IL-10 (Figure 2E) were significantly lower in EVLP + 3-AB. The accumulation of poly (ADP-ribose) (PAR) in lung tissue (indicative of PARP activity) was fully suppressed by 3-AB (Figure 2F), and lung MPO activity was significantly reduced in EVLP + 3-AB (Figure 2G).
3.2 Physiological variables after LTx
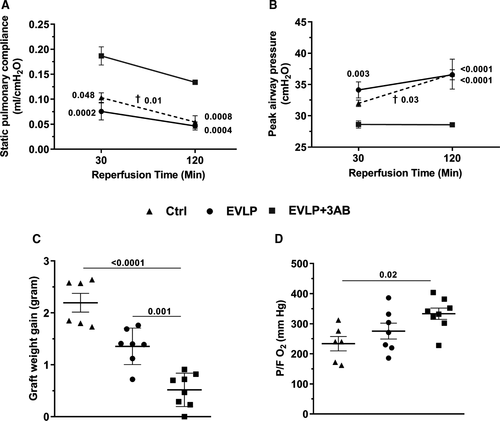
SPC (Figure 3A) and PAWP (Figure 3B) did not show any significant differences between the Ctrl and EVLP groups, both after 30 and 120 minutes of reperfusion, whereas both variables were significantly ameliorated in the EVLP + 3-AB group when compared to the two other groups. The weight gain of the graft (Figure 3C) was significantly reduced in the EVLP + 3-AB group in comparison to the Ctrl and EVLP groups. Oxygenation capacity (P/FO2 in the pulmonary vein, Figure 3D), was significantly increased in EVLP + 3-AB compared to Ctrl, but did not significantly differ between the EVLP and EVLP + 3-AB groups.
3.3 Markers of PARP activation and oxidative stress after LTx
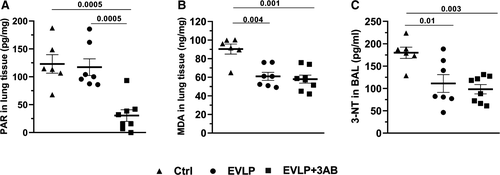
As shown in Figure 4A, comparable amounts of PAR accumulated in lungs from the Ctrl and EVLP groups, which were significantly reduced by EVLP + 3-AB. The levels of MDA (Figure 4B) and 3-NT (Figure 4C) were significantly reduced in the EVLP group in comparison to the Ctrl group, with no further reduction in the EVLP + 3-AB group.
3.4 BAL and plasma cytokines after LTx
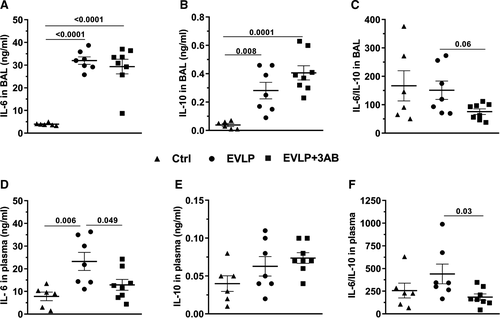
In the BAL, IL-6 (Figure 5A) and IL-10 (Figure 5B) displayed low levels in Ctrl, while both cytokines were significantly higher in EVLP and EVLP + 3-AB groups, without any significant differences between them. The IL-6/IL10 ratio (Figure 5C) was reduced in EVLP + 3-AB in comparison to EVLP (P = .06). With respect to plasma cytokines, we noticed that IL-6 was significantly higher in EVLP as compared to Ctrl and EVLP + 3-AB (Figure 5D), whereas no statistically significant differences were noted for IL-10. The IL-6/IL-10 ratio tended to be higher in EVLP in comparison to Ctrl, and this ratio was significantly lower in lungs from the EVLP + 3-AB group.
3.5 BAL cell counts and histology after LTx
The total number of cells recovered from the BAL (Figure 6A) was comparable between Ctrl and EVLP groups, but was significantly reduced in EVLP+3-AB, as compared to EVLP. Differential cell counts (mononuclear cells -MNs- and PMNs, Figure 6B) indicated nonsignificant differences between groups with respect to MNs, whereas there was a significantly higher number of PMNs in the Ctrl group in comparison to the two other groups. In addition, the EVLP + 3-AB group displayed a striking reduction of PMNs in comparison to the EVLP group. Lung graft histology after LTx is shown in Figure 6D and lung injury severity score in Figure 6C. Lungs from Ctrl (a, 60× magnification; b, 400× magnification) displayed marked leukocyte alveolar infiltration (mainly PMNs, blue circle), massive edema, and early phase diffuse alveolar damage with hyaline membranes. The histological alterations were considerably attenuated in both EVLP (c, d) and EVLP + 3-AB (e, f), as further shown by significantly lower lung injury scores (Figure 6C). The pictures are representative sections from n = 4 animals/group.

3.6 Gene expression of adhesion molecules after LTx
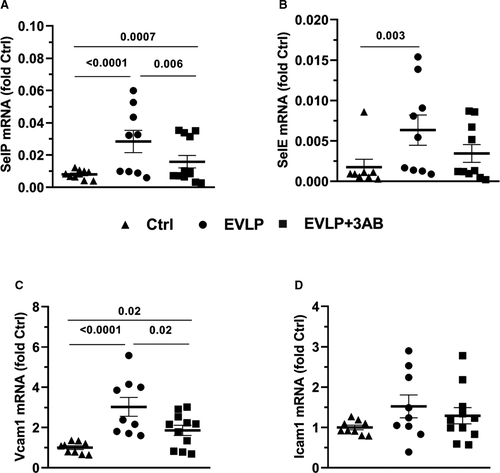
The expression levels of P-selectin (SelP, Figure 7A), E-selectin (SelE, Figure 7B), VCAM-1 (Figure 7C), and ICAM-1 (Figure 7D) are presented as fold increase in comparison to the expression in the Ctrl group. The expressions of SelP and VCAM-1 were significantly increased in EVLP and EVLP + 3-AB, although the increase was significantly less pronounced in the EVLP + 3-AB group. With respect to SelE, its expression only increased in EVLP, whereas it was comparable between Ctrl and EVLP + 3-AB. Finally, we did not notice any significant differences in the expression level of ICAM-1 among the three groups.
4 DISCUSSION
EVLP is gaining wide acceptance for increasing the pool of transplantable lungs.15 EVLP might be particularly valuable to recondition organs damaged by an extended period of WI, as may occur in the setting of donation after circulatory death,1 a context at greater risk of ischemia-reperfusion (IR) injury and primary graft dysfunction (PGD) after LTx.16 Nitro-oxidative stress leading to PARP activation is instrumental in IR injury,17 triggering ATP depletion, widespread PARylation, cell death, and nuclear factor kappaB (NF-κB)–dependent inflammation.18
We recently showed that PARP is activated during EVLP of rat lungs obtained after extended WI (as a model of damaged donor lung),5, 9 and that PARP inhibition with 3-AB is beneficial in this ex vivo setting.5 We confirm these observations, by showing that 3-AB increases SPC, while reducing lung edema and cellular damage during EVLP of damaged lungs. We also confirm that EVLP enhances the expression of cytokines (IL-6, IL-10) in damaged lungs, in accordance with previous findings,19-21 including our own,5, 9, 10 and that 3-AB downregulates cytokines during EVLP, as in our recent study.5 It must be stressed that the degree of cytokine accumulation during EVLP correlates with poor outcomes after LTx.22 Our finding of reduced cytokines with 3-AB implicates therefore a key role of PARP in this process, in line with the reported pro-inflammatory actions of PARP, notably in the lungs.23, 24
To be clinically relevant, the effects of any drug on a lung graft ex vivo should translate into an improved outcome after LTx in vivo. To date, such evidence is scarce. In porcine lungs exposed to CI, EVLP with intravascular trimetazidine,25 inhaled N-acetylcysteine,26 and intrabronchial adenoviral IL-10 Gene Therapy,27 improved post-LTx lung function and inflammation. In lungs exposed to WI, EVLP with inhaled procaterol,28 or intravascular nitroglycerine and db-cAMP29 (in canine models), as well as intravascular ATL802 (an adenosine A2B receptor antagonist)16 and intrabronchial surfactant30 (in porcine models), improved lung function and reduced inflammation after LTx. Our current data add here significant novelty, by showing important in vivo benefits following EVLP reconditioning with 3-AB. Although EVLP, with or without 3-AB, improved the histological status of the transplanted lungs, only EVLP with 3-AB reconditioning provided physiological amelioration, as indicated by significant improvements of SPC, PAWP, graft water accumulation and oxygenation capacity. These findings are clinically highly relevant, since these parameters represent the main transplantation acceptance variables in clinical EVLP protocols.31 Therefore, our observations indicate that 3-AB reconditioning during EVLP helps prevent IR injury leading to PGD in damaged lung grafts.
It must be stressed that 3-AB may have additional antioxidant effects beyond its actions as a PARP inhibitor,32 which could have contributed to the benefits noted in our study. We addressed this issue by evaluating the effects of 3-AB not only on the activation of PARP (PAR accumulation), but also on markers of oxidative (MDA) and nitroxidative (3-NT) stress in the transplanted lung. 3-AB significantly reduced PARP activation, but it did not change the levels of MDA and 3-NT in comparison to EVLP without 3-AB, implying that PARP inhibition, but not nonselective antioxidant effects, was the primary pharmacological mode of action of 3-AB in our study.
Immune activation plays a major role in lung IR and the development of PGD.33 It depends primarily on the activation of NF-κB,34 which can already occur during EVLP.35 Indeed, we recently reported that inhibiting NF-κB during EVLP suppressed the expression of inflammatory mediators in damaged lung grafts ex vivo.9 We therefore assume that such mechanisms triggered the release of cytokines noted at the end of EVLP, as well as after LTx in EVLP-treated lungs. PARP is known to act as a co-activator of NF-κB,18 a mechanism that most likely explains the decrease of BAL cytokines by 3-AB at the end of EVLP.5 Of interest, this ex vivo attenuation of lung cytokine levels was no longer present after LTx, as shown by comparable increases of BAL IL-6, a key inflammatory cytokine in the context of LTx,36 in both EVLP groups, which suggests that 3-AB delayed, but not fully suppressed, immune activation in our model. Notwithstanding the comparable increase of BAL IL-6, it is noticeable that 3-AB also favored the expression of IL-10 (a protective anti-inflammatory cytokine in the context of LTx)37 in such a way that the IL-6/IL-10 ratio was reduced by 3-AB. This reduced ratio was noted not only in the BAL from the donor lungs, but also in the plasma from the recipient animals. This differential action of 3-AB on IL-6 and IL-10 is consistent with previous findings showing that PARP inhibitors have smaller effects on anti-inflammatory than pro-inflammatory mediators.38 It is worth mentioning that the ratio IL-6/IL-10 represents an important indicator of the pro- to anti-inflammatory balance in the lung, with major prognostic significance in the setting of lung inflammation12 and LTx.13 Then, 3-AB therapy during EVLP appears to switch the immune response from a predominantly pro-inflammatory to a predominantly anti-inflammatory profile after LTx, which is in line with findings reported in unrelated pathophysiological conditions.39 We speculate that such immune modulation could reflect some specific effects of 3-AB on various lymphocyte subpopulations, owing to the multiple actions of PARP in both B and T lymphocytes,40 T regulatory cells (Tregs), and dendritic cells.41
After LTx, damaged lungs displayed a marked infiltration of alveolar spaces by PMNs. It is well established that PMNs recruited within the lung allograft represent crucial cellular effectors of PGD.42 PMNs within the transplanted lungs may arise from two distinct sources, the first one being the donor lung itself, which possesses an important reservoir of marginated neutrophils,43 forming highly toxic NETs (neutrophil extracellular traps)44 and being immediately recruitable upon transplantation,45 and the second one being the circulating PMNs from the recipient.43 The latter pool of PMNs infiltrate the graft upon interaction between leukocyte-derived integrins with selectins and adhesion molecules expressed by the endothelium in the donor lung.43 Reducing the pool of PMNs from either the donor lung, for example, using a leukocyte filter during EVLP,45 or from the recipient blood, for example, using antibodies raised against adhesion molecules,46 significantly limits reperfusion injury in the transplanted lung.
We found that EVLP reduced the amount of infiltrating PMNs after LTx in comparison to lung grafts not exposed to EVLP, despite an increase in the expression of various adhesion molecules (including P and E-selectin, as well as VCAM-1). This suggests that EVLP primarily reduced the pool of PMNs from the donor lung, in line with data from previous investigators,47 which could reflect the mechanical removal of marginated PMNs during EVLP.48 It seems intriguing at first glance that, despite an increased expression of inflammatory mediators, EVLP-treated lungs displayed such reduction of inflammatory cell infiltration and improved histological status after LTx. It has been indeed frequently reported that EVLP upregulates the expression of many inflammatory genes, due to the activation of pro-inflammatory signaling pathways, including NF-κB, STAT-3, and MAP kinases,9, 35 following ex vivo reperfusion of the graft at normothermia, that is, at full metabolic activity. This apparent paradox has been recently worked out by Yeung et al, who showed that EVLP increased endothelial markers of inflammation, but at the same time reduced the expression of leukocyte-specific inflammatory genes, due to the washout of circulating leukocytes during EVLP.48 It is therefore plausible that the improved histological status and reduced PMN infiltration after EVLP noted in our study resulted from such interruption of leukocyte-dependent inflammatory response.
Treatment with 3-AB during EVLP further decreased PMN accumulation after LTx, which was associated with a reduced MPO activity in lung tissue at the end of EVLP and a decreased lung expression of adhesion molecules (SelP and VCAM-1) after LTx. These observations support that 3-AB reduced the amount of marginated PMNs within the donor lung during EVLP, which is consistent with the known role of PARP in promoting leukocyte transmigration across the lung endothelium.49 Our findings also indicate that 3-AB interfered with the mechanisms of recipient's PMN recruitment after LTx, which agrees with previous reports showing that PARP inhibitors suppressed adhesion molecules and PMN infiltration in various experimental models of IR.50-53
Our study has several limitations. First, we used 3-AB as a PARP inhibitor, which is not available for clinical use, thereby limiting the translational applicability of our study. Recently, olaparib has been introduced as the first clinical PARP inhibitor,6 and this drug should be rapidly evaluated in experimental EVLP for possible clinical implementation. However, owing to the potential of PARP inhibitors to interfere with DNA repair mechanisms,6 additional safety studies in large animals are warranted before such implementation. Second, we only evaluated 3-AB reconditioning at an early (2-hour) time point after LTx. Future studies should address the potential of PARP inhibitors to recondition damaged lungs at later time points, and longer survival experiments should also be conducted. Third, our study may be criticized for some lack of mechanistic insights regarding the modulation of inflammation by EVLP and 3-AB after LTx. Our primary purpose was to determine, from a pharmacological standpoint, whether ex vivo reconditioning with a PARP inhibitor would improve the outcome after LTx. Novel studies will be designed to explore the molecular mechanisms underlying the effects of 3-AB on immune activation and leukocyte trafficking after LTx.
In conclusion, we have demonstrated that ex vivo pharmacological reconditioning of lungs damaged by warm ischemic preservation using the PARP inhibitor 3-AB produces significant benefits after in vivo LTx, as indicated by the reduction of lung edema, the prevention of physiological deterioration, the modulation of the immune response toward a preferentially anti-inflammatory profile, and the suppression of PMN infiltration. Overall, these results support the use of PARP inhibitors for the ex vivo reconditioning of damaged donor lungs.
ACKNOWLEDGMENTS
This work was supported by grants from the Swiss National Fund for Scientific Research (Nr 310030_172975 to T.K. and L.L., and Nr 310030_162629 to L.L.) and by Departmental Funds from the Services of Thoracic Surgery and Adult Intensive Care Medicine of the Lausanne University Hospital (provided by H.B.R. and P.E.).
DISCLOSURE
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.




