Sirolimus vs mycophenolate mofetil (MMF) in primary combined pancreas and kidney transplantation. Results of a long-term prospective randomized study
Abstract
The study was intended to compare pancreas graft survival rates in two groups of pancreas and kidney transplant recipients prospectively randomized to treatment either with sirolimus or MMF. From 2002 to 2013, 238 type 1 diabetic recipients with end-stage kidney disease were randomized 1:1 to sirolimus or MMF treatment. Noncensored pancreas survival at 5 years was 76.4 and 71.6% for sirolimus and MMF groups, respectively (P > .05). Death-censored pancreas survival was better in the sirolimus group (P = .037). After removal of early graft losses pancreas survival did not differ between groups (MMF 83.1% vs sirolimus 91.6%, P = .11). Nonsignificantly more grafts were lost due to rejection in the MMF group (10 vs 5; P = .19). Cumulative patient 5-year survival was 96% in the MMF group and 91% in the sirolimus group (P > .05). Five-year cumulative noncensored kidney graft survival rates did not statistically differ (85.6% in the sirolimus group and 88.8% in MMF group). Recipients treated with MMF had significantly more episodes of gastrointestinal bleeding (7 vs 0, P = .007). More recipients in the sirolimus group required corrective surgery due to incisional hernias (21 vs 12, P = .019). ClinicalTrials No.: NCT 03582878.
Abbreviations
-
- CNI
-
- calcineurin inhibitor
-
- MMF
-
- mycophenolate mofetil
-
- mTOR
-
- mammalian target of rapamycin
-
- PDRI
-
- Pancreas Donor Risk Index
-
- PRA
-
- panel reactive antibodies
-
- Siro
-
- sirolimus
1 INTRODUCTION
Simultaneous pancreas and kidney transplantation is an accepted therapeutic option for most type 1 diabetic recipients with end-stage kidney disease expected to tolerate both a major surgical procedure and long-term immunosuppressive therapy. According to the data from International Pancreas Transplant Registry, current immunosuppressive protocols consisting of tacrolimus and mycophenolate mofetil (MMF) with or without low-dose steroids are associated with excellent short-term patient and graft survival rates.1, 2 There are still challenges present, especially, however, with high rates of early postoperative complications and recurring cases of pancreas graft failure due to chronic rejection, recurrence of autoimmunity, and beta-cell attrition. In addition, certain metabolic side effects of immunosuppressive drugs have been shown to affect the long-term mortality of recipients with functioning grafts.3 Therefore, widening the range of safe and well-tolerated long-term immunosuppression therapies available to patients with calcineurin inhibitor (CNI)-related nephrotoxicity or MMF intolerance is recommended.4
Mammalian target of rapamycin (mTOR) inhibitors were first introduced as part of organ transplant immunosuppressive protocols at the beginning of this century5 under the assumption that their distinct mechanism of action would offer an alternative or complement to CNIs. Though the efforts of CNI avoidance, especially in the era of steroid tapering and withdrawal, have proven less than effective,6 combination therapy involving mTOR inhibitors in low doses is generally advocated. mTOR inhibitors may also offer an alternative to MMF, which is associated with hematopoietic and gastrointestinal toxicity, particularly in diabetic recipients suffering from gastrointestinal neuropathy.
mTOR inhibitors have yet to be extensively tested in pancreas transplant recipients, with most of the data coming from clinical trials involving kidney transplant recipients.7 According to the results of these studies, mTOR inhibitors seem to be safe and effective when administered in low doses. To date, there have been only two prospective randomized trials to compare the efficacy of sirolimus and MMF (both in combination with tacrolimus) in simultaneous pancreas and kidney recipients.8, 9 And although patient and graft survival rates did not significantly differ between tacrolimus- and sirolimus-treated groups in these studies, discrepant incidences of pancreas rejection were reported. Ciancio et al8 found lower incidence in their sirolimus-treated group and a lower sirolimus intolerance rate in comparison with preliminary results from the EUROSPK 02 international study.10
The aim of this study was to compare 5-year patient and graft survival rates in type 1 diabetic kidney and pancreas recipients prospectively randomized for treatment either with sirolimus or MMF in combination with tacrolimus and early steroid withdrawal. We hypothesized that pancreas graft survival in the cohort on sirolimus/tacrolimus combination will not differ from those on MMF/tacrolimus treatment.
2 PATIENTS AND METHODS
2.1 Study design
The study was carried out as an investigator-initiated, single-center, open-label, randomized trial. Two hundred and thirty-eight type 1 diabetic recipients with end-stage renal disease invited to undergo first-time simultaneous pancreas and kidney transplantation were enrolled in the study, which took place between January 2002 and December 2013. The first 46 subjects had originally participated in the multicenter EUROSPK 02 trial (Clinical Trials No: NCT 00140543). Patient recruitment for the single-center study (Clinical Trials No: NCT 03582878) followed the same inclusion/exclusion criteria and immunosuppressive protocol used in the international study. The study was conducted according to the principles of the Declaration of Helsinki and approved by the ethics committees of the Institute for Clinical and Experimental Medicine and Thomayer Teaching Hospital, Prague (Approval No. 206, January 16, 2001, Prague, Czech Republic) (Figure 1). Data were stored at an institutional server.

2.2 Organ donors
Pancreases from donors (Czech Republic residents) under the age of 45 years with a body mass index lower than 30 kg/m2 and without signs of macroscopic fibrosis, edema, necrosis, or bleeding were accepted. The upper limit for cold ischemia time was set at 15 h for the pancreas and 18 h for the kidney. HTK solution was used in all cases for organ preservation.
2.3 Study participants
Recipient inclusion criteria were as follows: type 1 diabetes mellitus confirmed by history and negative or nearly negative C-peptide level, end-stage renal disease defined as serum creatinine >250 µmol/L and/or creatinine clearance rate <0.5 mL/s or regular dialysis, age >18 years, and signed informed consent. Main exclusion criteria were any other type of diabetes, known hypersensitivity to study drugs, any previous transplant, positive crossmatch, current malignancy or history of malignancy within the last 5 years, pregnancy or lactation, known active liver disease, enzyme serum activity 3 times above the upper reference range, any form of substance abuse or severe psychiatric disorder, cardiac failure as confirmed by a cardiologist, active tuberculosis, any acute infection, noncompliance, or any other condition that in the opinion of the investigator might negatively affect cooperation.
2.4 Randomization and immunosuppressive therapy
Recipients on waiting lists were called to the hospital to undergo combined pancreas and kidney transplantation according to national allocation criteria. After rechecking the inclusion and exclusion criteria and providing their signed informed consent to participate in the study, participants were preoperatively randomized for prophylactic immunosuppressive treatment either with tacrolimus + sirolimus or tacrolimus + MMF. In the first 46 recipients, randomization was performed after opening consecutive sealed envelopes prepared by the EUROSPK 02 coordination team in random order for 50 subjects using a 1:1 ratio. Randomization of all subsequent recipients was by consecutive opening of sealed envelopes provided by the Department of Statistics of the Institute for Clinical and Experimental Medicine. Two sets of 100 subjects were randomly assigned to either therapy with a combination of tacrolimus + sirolimus or tacrolimus_MMF using a ratio of 1:1 for each set of envelopes. Induction therapy consisted of 8 mg/kg polyclonal antibody (Grafalon [previously ATG-Fresenius], Neovii, Lexington, MA) before unclamping the vascular anastomosis followed by 3 mg/kg given daily for 3 consecutive days after transplantation. Tacrolimus was administered at a dose of 0.1 mg/kg before transplantation and then reduced to 0.05 mg/kg twice a day. Adjustments were made to maintain trough levels between 10-15 mg/mL for the first month and 5-10 ng/mL thereafter. MMF was administered at a fixed dose of 2 g per day. Sirolimus therapy started with 5 mg pretransplant, with trough levels then maintained between 5-10 ng/mL. Methylprednisolone (250 mg) was administered before transplantation and following by 3 doses of 125 mg. Oral prednisone was initiated thereafter at 20 mg/day. The dose was gradually tapered before being completely withdrawn 6 weeks after transplantation. Prophylaxis consisted of 4.5 g of piperacillin/tazobactam administered 3 times a day for 4 days, 100 mg of fluconazole daily for 1 week, 450 mg of valganciclovir daily for 6 months, and 960 mg of trimethoprim/sulfamethoxazole biweekly for 9 months. Although the study follow-up was set at 5 years, regular clinical evaluations continued thereafter. Study visits were scheduled every 3 months in the first year and every 6 months thereafter. The study design is shown in Figure 1.
2.5 Primary and secondary endpoints
The primary outcome of the study was to compare 5-year pancreas graft survival. Pancreas graft failure was defined as death, graftectomy, retransplantation, or resumption of regular insulin therapy lasting more than 30 days (at any dose).
Secondary outcomes were to assess patient survival, kidney survival, and wound healing time. Kidney graft failure was defined as death, retransplantation, resumption of regular dialysis, or graftectomy. Wound healing time was counted from the date of transplantation to the date of complete wound re-epithelialization without any external drainage or wound secretion. We separately recorded wound complications characterized either by fluid collection requiring drainage, antibiotic therapy, or relaparotomy, or by wound dehiscence requiring relaparotomy or persistent wound toilet (lasting longer than 4 weeks). We also recorded the formation of incisional hernias requiring surgical correction. After hospital discharge, patients were monitored weekly until 3 months, then monthly until 6 months and quarterly thereafter. Clinical and laboratory data were recorded electronically. Laboratory tests were conducted in respect of cell blood count, lipase, amylase, creatinine, urea, proteinuria, sodium, potassium, HbA1c, and tacrolimus and sirolimus trough levels. Lipid profiles were checked as part of annual visits.
Immunosuppressive protocol failure was defined as permanent discontinuation (lasting more than 2 months) of the immunosuppressive drug assigned or failure of steroid withdrawal.
A kidney or pancreas biopsy was performed in case of suspected rejection. Biopsy samples were evaluated by an experienced transplant pathologist (LV) and classified according to Banff 97 and subsequent updated criteria for kidney10, 11 and pancreas12, 13 transplantations.
All recipients in the study were monitored every 3 months at diabetes outpatient clinic and standard visit tests included pancreatic lipase, amylase, creatinine, urea, and immunosuppression levels. Every elevation of lipase activity over the upper reference range or elevation glycemia or HbA1c was an indication for pancreas biopsy. Acute cellular rejections were treated by methylprednisolone, steroid resistant rejections were treated by ATG (Fresenius).
2.6 Patient and graft survival
All study participants with at least one functioning graft were regularly followed at the Institute for Clinical and Experimental Medicine over 3-month intervals. Visits were monitored by study coordinators every 3 months during the first year and yearly thereafter. All data were recorded on the institution's patient tracking system, with the operator name and date given for all subsequent changes recorded.
Patient and graft survival rates were calculated using the Kaplan-Meier method. Survival curves were compared using the logrank test. Data were analyzed using intention-to-treat analysis.
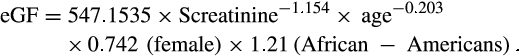
Delayed kidney graft function in recipients with pretransplant dialysis was defined as the need for dialysis in the first week posttransplant. In patients transplanted preemptively, delayed kidney graft function was defined as a decrease in serum creatinine level by less than 10% over the first 3 consecutive days.
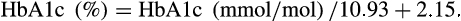
2.7 Transplant surgery and wound management
Two different pancreas transplantation techniques were used. The technique used until 2008 (n = 152) involved a whole pancreas being placed in the right pelvic fossa extraperitoneally. Vessels were anastomosed to the iliac artery and vein, with the duodenal segment anastomosed via peritoneal fenestration of the jejunum.14 The post-2008 (n = 88) technique involved placing the pancreas in retrocolic intraperitoneal position, superior mesenteric vein anastomosis, and enteric drainage of pancreas fluid.15 The kidney was placed in the left pelvic space with standard anastomoses of graft vessels to the recipient iliac artery and vein. Urethral catheter was extracted after 4 days. A suction drain was placed around the kidney graft and, in most cases, extracted within 4 days. A corrugated drain was placed next to the pancreas graft and then removed 7-14 days later.
2.8 Statistical analysis
All analyses were performed on an intention-to-treat basis, with patients grouped according to the treatment arm in which they were randomized. We assumed that the primary endpoint (noncensored pancreas graft survival rate) of the sirolimus group would not differ by more than 15% from that of the MMF group. On this basis, it was estimated that about 100-120 recipients be included in each arm to achieve a statistical power of at least 80% with a type I error of 0.05. For sample size estimation, nQuery software (Statsols, Cork, Ireland) was used. Survival curves were plotted using the Kaplan-Meier technique and compared using the logrank test. Differences were tested at a significance level of 0.05. Categorical variables were compared using Fisher's exact test. Numerical variables were expressed as means with standard deviations and compared using the parametric test in the case of normal distribution. The nonparametric test was used where variable distribution differed from Gaussian distribution. Bonferroni correction was used to adjust the significance levels in case of multiple testing. Statistica software was used for data analysis, curve plotting and difference testing (Statistica version 12, TIBCO Software Inc, Palo Alto, CA).
3 RESULTS
3.1 Baseline demographic data (Supplementary data)
3.1.1 Patient survival
Cumulative 5-year patient survival was 96% (95% confidence interval, 88-100) in the MMF group and 91% (95% confidence interval, 85-96) in the sirolimus group, a statistically nonsignificant difference. According to the logrank test, no statistical differences were found between whole survival curves (Figure 2). In the sirolimus group, a total of 11 recipients died within the 5-year period due to embolization (1), bleeding (1), sepsis (5), car accident (2), cardiac arrest (1), and sepsis 1 year after failure of both grafts (1). Five deaths were recorded in the MMF group due to bleeding (1), sepsis (1), embolization (1), ischemic stroke (1), and heart failure (1).
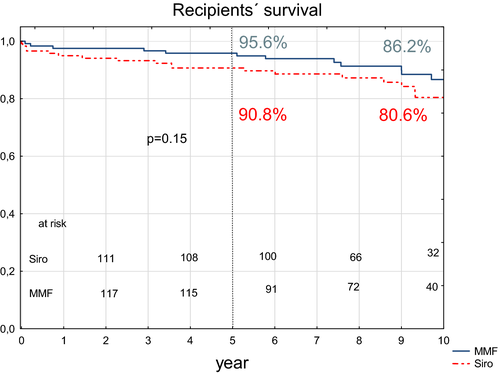
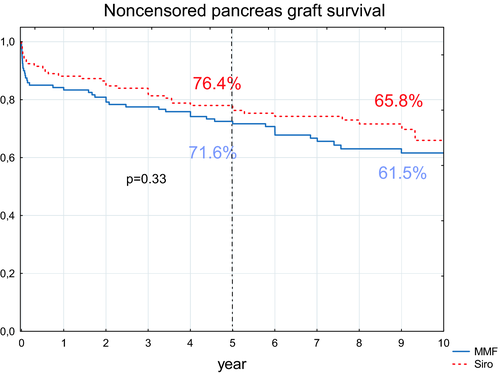
3.1.2 Pancreas and kidney graft survival
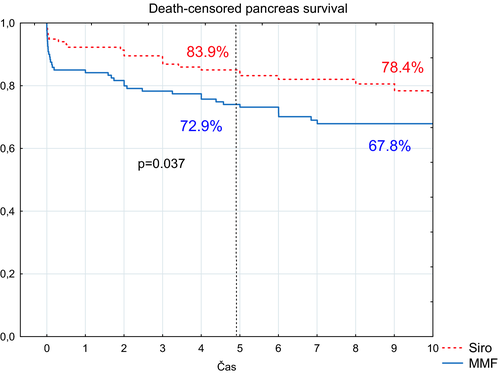
Noncensored pancreas survival at 5-year follow-up was 76.4% (95% confidence interval, 69-84) and 71.6% (95% confidence interval, 64-80) for the sirolimus and MMF groups, respectively (P > .05) (Figure 3). Death-censored pancreas survival was significantly better in the sirolimus group (P = .037) (Figure 4). In the sirolimus group, nine pancreas grafts failed due to technical reasons (with proven rejection in one case) (Table 1). In the MMF group, 17 pancreas grafts were lost due to technical reasons (with proven rejection in seven cases). More grafts, albeit nonsignificantly, were lost due to rejection in the MMF group (10 vs 5; P = .19). However, when merging the number of rejection cases with thrombosis cases caused by rejection, the difference significantly favored the sirolimus group (6 vs 17 cases; P = .014). Two and three grafts in the MMF and sirolimus groups, respectively, were lost due to pancreatitis. Three and nine patients with functioning pancreata died in the MMF and sirolimus groups, respectively. Notably, two subjects in the sirolimus group died as the result of a car accident.
| Graft loss after 5 y | MMF | Sirolimus | P |
|---|---|---|---|
| Thrombosis | 10 | 8 | .829 |
| Thrombosis with rejectiona | 7 | 1 | .076 |
| Pancreatitis | 2 | 3 | .984 |
| Rejection | 10 | 5 | .193 |
| Nondefined | 1 | 0 | .993 |
| All | 30 | 17 | .040 |
- MMF, mycophenolate mofetil.
- a Thrombosis with rejection was defined as clinically diagnosed pancreas thrombosis with histological findings of thrombosis and acute rejection changes. Rejection was classified by the pathologist as the primary reason of thrombosis.
Nonrecipient, death-censored, and death-censored 10-year pancreas graft survival curves are shown in Figures 3 and 4. There was a statistical difference between death-censored survival curves (P = .028) in favor of the sirolimus group.
In recipients with functioning pancreas grafts, mean HbA1c values were numerically lower in the MMF group from 2 to 5 years. However, a statistical difference was recorded only at 4 years (normal values up to 41 mmol/L; Table 2).
| Year | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sirolimus group | 40 ± 8 | 40 ± 8 | 40 ± 8 | 41 ± 12 | 40 ± 6 |
| MMF group | 40 ± 1 | 39 ± 8 | 38 ± 6 | 38 ± 6 | 38 ± 6 |
- No statistical difference was found between groups in mean levels of HbA1c.
- MMF, mycophenolate mofetil; SD, standard deviation.
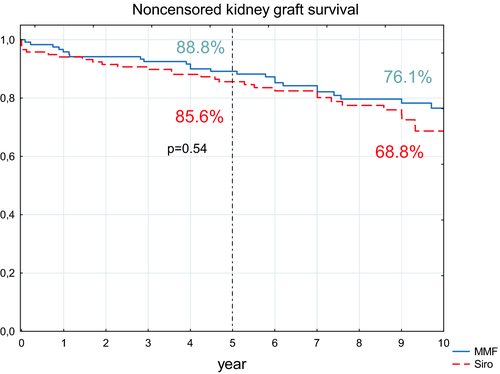
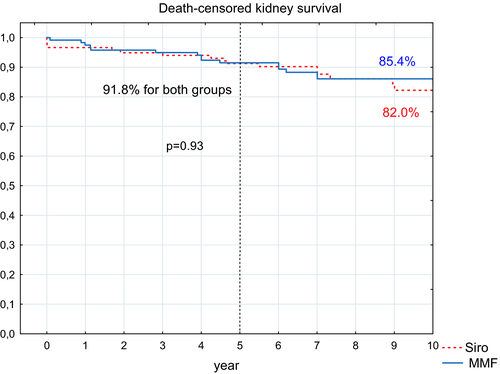
Five-year cumulative noncensored kidney graft survival rates did not statistically differ (85.6% in the sirolimus group and 88.8% in the MMF group, P = .93) (Figure 5). Of those with functioning kidney grafts, four recipients in the MMF group and six recipients in the sirolimus group died. The causes of kidney graft loss are given in Table 3. Death-censored kidney graft survival did not differ between groups (91.8% in both arms) (Figure 6).
| Siro | MMF | P | |
|---|---|---|---|
| Rejection | 1 | 6 | .127 |
| BK nephropathy | 2 | 1 | .988 |
| Pyelonephritis | 1 | 0 | .993 |
- MMF, mycophenolate mofetil.
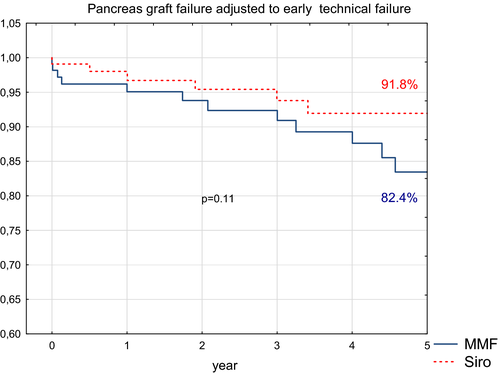
After removal of cases with pancreas technical failure within 90 days after transplantation, 5-year survival did not statistically differ between groups (91% vs 83% in sirolimus and MMF group, respectively n = 0.11) (Figure 7).
3.1.3 Kidney function
In the sirolimus group, mean serum creatinine concentrations were significantly higher compared to the MMF group at 4-year follow-up. Mean MDRD-4 values were consequently lower for the same time point. Data were tested at a significance level of 0.01 after Bonferroni correction to avoid false-positive results (Table 4).
| Mean | MMF | Siro | P | Mean SD MMF | Mean SD Siro | |
|---|---|---|---|---|---|---|
| 1-y | S-creatinine (µmol/L) | 119.8 | 116.7 | .594 | 60.5 | 36.5 |
| MDRD-4 (mL/s) | 0.98 | 0.95 | .385 | 0.30 | 0.26 | |
| 2-y | S-creatinine (µmol/L) | 127.8 | 132.0 | .034 | 108.8 | 75.7 |
| MDRD-4 (mL/s) | 0.63 | 0.58 | .033 | 0.21 | 0.19 | |
| 3-y | S-creatinine (µmol/L) | 131.1 | 138.2 | .069 | 93.9 | 89.5 |
| MDRD-4 (mL/s) | 0.95 | 0.87 | .048 | 0.33 | 0.29 | |
| 4-y | S-creatinine (µmol/L) | 135.3 | 151.7 | .004* | 105.6 | 111.8 |
| MDRD-4 (mL/s) | 0.96 | 0.82 | .007* | 0.37 | 0.31 | |
| 5-y | S-creatinine (µmol/L) | 149.2 | 156.4 | .054 | 131.4 | 124.8 |
| MDRD-4 (mL/s) | 0.94 | 0.82 | .040 | 0.39 | 0.34 |
- Creatinine data were compared using the nonparametric Mann-Whitney test, with MDRD-4 data compared using the two-sided t test.
- MDRD, Modification of Diet in Renal Disease; MMF, mycophenolate mofetil; SD, standard deviation.
- * Significance was set at P ≤ .01 after Bonferroni correction.
3.1.4 Rejection rate
Cumulative 5-year incidence for biopsy-proven rejection episode in the case of all grafts was higher in the group assigned to treatment with MMF (34% vs 21%; P = .03) (Table 5).
| Sirolimus, n = 118 | MMF, n = 120 | P | |
|---|---|---|---|
| Total acute pancreas rejections | 9 (7.6%) | 15 (12.5%) | .282 |
| Grade I | 2 | 8 | .102 |
| Grade II | 2 | 2 | .626 |
| Grade III | 4 | 6 | .748 |
| Total acute kidney rejections | 16 (13.5%) | 26 (21.6%) | .125 |
| Border line | 6 | 7 | .799 |
| Grade I | 6 | 6 | .976 |
| Grade II | 3 | 10 | .083 |
| Grade III | 0 | 0 | ns |
| Acute humoral rejection | 1 | 3 | .634 |
| All episodes for both organs | 25 (21.1%) | 41 (34.1%) | .029 |
- MMF, mycophenolate mofetil.
Within 6 weeks after transplantation acute pancreas rejection was diagnosed in 5 and 3 cases in the MMF and sirolimus groups, respectively (P = .755). Acute kidney rejection was diagnosed in 10 and 5 cases in MMF and sirolimus groups, respectively (P = .19).
Sixty (50%) recipients in the MMF group had persistently reduced doses of MMF (daily MMF dose <2 g). Acute rejection of either pancreas or kidney graft was diagnosed in 18 of them (30%).
From those without MMF dose reduction, 22 recipients (36%) were diagnosed with acute rejection of any graft (P = .43).
In the MMF Group 6 recipients had acute rejections of both grafts and in 1 patient there were 2 episodes of kidney rejection. In the sirolimus group 1 recipient had 2 episodes of kidney acute rejection and another one had 2 episodes of pancreas rejection.
3.2 Immunosuppression (Supplementary data)
3.2.1 Wound healing and surgical complications (Supplementary data)
Recipients treated with MMF had significantly more episodes of gastrointestinal bleeding related to duodeno-ileal anastomosis of the pancreas graft. More recipients in the sirolimus group required corrective surgery due to incisional hernias in the later posttransplant period (n = 21 in the sirolimus group vs n = 12 in the MMF group, P = .019).
4 DISCUSSION
Over the past two decades, significant progress has been made in understanding the action of mTOR inhibitors, which, apart from regulating cell growth and proliferation (including adaptive T cell activation), affect many different aspects of intermediary metabolism.16 However, initial enthusiasm for their use as an efficient immunosuppressive agent for pancreatic beta cells has gradually waned. Side effects have been reported, with experimental data highlighting impaired peripheral insulin sensitivity and beta-cell regeneration4 as well as associated diabetogenic effects.17 According to the Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients summary report of US data, use of mTOR inhibitors as part of initial immunosuppressive combination therapy in pancreas transplant recipients decreased from approximately 15% in 2005 to <5% in 2016. Furthermore, convincing data regarding their efficacy and safety in combined pancreas and kidney transplantation from controlled prospective clinical trials are limited.8, 9
In this prospective study, we randomized 238 type 1 diabetic subjects to treatment with either sirolimus MMF in combination with polyclonal antibody induction, short-term steroids, and tacrolimus. We were unable to prove statistical differences in the 5-year insulin-independence rate after transplantation, which was used as the primary study endpoint. Five-year pancreas survival in the sirolimus- and MMF-treated groups was 77 and 72%, respectively. Similarly, we found no statistical differences between 10-year pancreas survival curves according to the logrank test. Notably, the proportion of subjects with both functioning grafts alive at 5 years and still taking the study drug was quite high in both arms (78 and 66% in the MMF and sirolimus groups, respectively), enabling a sufficient power of comparison to be reached. To our knowledge, this is the largest randomized study comparing sirolimus with MMF in pancreas and kidney transplant recipients. Moreover, we report favorable results of the use of both immunosuppressive regimens under early steroid withdrawal. In our study, 5-year recipient death-censored pancreas survival rate was better in the sirolimus group (84% vs 73%; P = .037) with lower rate of acute rejection episodes. With regard to pancreas graft efficacy, sirolimus therapy in conjunction with tacrolimus is undoubtedly comparable to MMF and may even be of more benefit.
Patient survival was similar in both groups and in accordance with data presented by the International Pancreas Transplant Registry and various studies.1, 2, 18 Pancreas and kidney graft survival did not differ between groups, as similarly reported by Ciancio et al.8 In contrast with this work, we randomized a larger number of recipients, thus improving statistical test power. However, it must be acknowledged that in our sirolimus group, numerically more recipients died during the study period, although the difference was statistically nonsignificant (P = .16; logrank test). Specifically, sepsis was most frequently recorded as the main cause of death within 5 years after transplantation. There were six deaths in the sirolimus group (including one death a year after failure of both grafts) vs a single case in the MMF group. Two recipients with functioning grafts from the sirolimus group died in a car accident. In the MMF group, a statistically nonsignificant trend of a more frequent delayed kidney graft function was observed. Interestingly, delayed graft function has been more frequently reported in sirolimus-treated subjects in other studies of isolated kidney transplantation.19
Kidney function assessed by serum creatinine levels and MDRD tended to be slightly lower in the sirolimus group throughout the 5-year follow-up but the difference was significant only at 4 years. Although this may suggest a slightly worse renal outcome in this group, it did not translate into worse long-term kidney graft survival.
Kaufman et al were the first to investigate early steroid withdrawal with tacrolimus/sirolimus combination in a small randomized study. The study identified a trend for better patient and graft survival of the tacrolimus/sirolimus group in comparison to standard triple immunosuppression (tacrolimus, MMF, prednisone) therapy, although the results did not reach statistical significance.20 The aim of our protocol was to withdraw steroids by 6 weeks where possible. This was achieved more successfully in the sirolimus arm. At 6 months, the proportion of subjects still on steroids (relatively low at 29 cases altogether) was significantly lower in the sirolimus group (P = .029). In comparison with registry data, this represents quite a low number.21 However, it did not unnecessarily increase the rejection rate, as reported by other authors,22 and may even have prevented some of the undesirable metabolic effects attributed to sirolimus therapy.17, 23
Throughout the study, tacrolimus and sirolimus dose were quite stable with trough levels, usually not exceeding 10 ng/mL. The mean tacrolimus trough level was slightly higher at 2-year follow-up but otherwise comparable throughout the study in both groups.
Impaired wound healing is one of the frequently reported adverse events related to mTOR inhibitor treatment and has been attributed to antiproliferative effects.24 During the inflammatory phase, mTOR inhibitors decrease interleukin and chemokine secretion and inhibit lymphocyte proliferation. During the proliferative and remodelling phases, they inhibit fibroblast migration and vascularization.25, 26 The early studies of pancreas transplant recipients using mTOR inhibitors showed an increased incidence of lymphocele, prolonged wound healing, and more surgical complications.27, 28 However, these results are limited by the low number of participants and use of rather high mTOR inhibitor doses.24, 29 In this work, we demonstrate that a combination of tacrolimus with sirolimus does not significantly prolong postoperative healing and wound re-epithelialization, at least when compared with MMF, an antiproliferative agent reportedly associated with decreased collagen production.30 In agreement with similar experiences of kidney transplantation, incisional hernias did develop and require corrective surgery more frequently in sirolimus-treated recipients. This complication usually occurred 6 to 12 months after transplantation and was more frequent in both groups after switching to intraperitoneal retrocolic placement of the pancreas graft using a single larger medial incision for both pancreas and kidney transplantation.
As expected, more patients in the sirolimus group were treated with statins. In the MMF group we observed more episodes of gastrointestinal bleeding (7 cases vs 0), a complication also reported by other authors.31, 32
In conclusion, our study demonstrates that in combined pancreas and kidney transplantation sirolimus is a well-tolerated and effective drug when combined with medium-dose tacrolimus and early steroid withdrawal. We recognize considerable disadvantage of sirolimus therapy may be the more frequent formation of incisional hernias in the first postoperative year. We therefore speculate that more favorable results might be achieved if sirolimus therapy was initiated only after wound completion. Confirming the effectiveness of such a strategy of course would require a prospective study employing randomization at 3-4 months aimed at either continuing with MMF or switching to an mTOR inhibitor.
ACKNOWLEDGMENTS
We would like to thank Jean-Paul Squifflet from the Centre Hospitalier Universitaire de Liège, Belgium and Jacques Mallaisse from the Centre Hospitalier de l'Université de Montréal, Canada, for designing and initiating the EUROSPK 02 clinical trial. Thanks to Professor Thierry Berney for advice, help, and valuable comments in the preparation of the manuscript.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to describe as described by the American Journal of Transplantation.
FUNDING INFORMATION
This study was sponsored by the Institute for Clinical and Experimental Medicine, Prague, Czech Republic and supported by the Ministry of Health of the Czech Republic under the Conceptual Development of Research Organisations programme (Institute for Clinical and Experimental Medicine – IKEM, IN 00023001).
Open Research
DATA AVAILABILITY STATEMENT
Data supporting the results will be available for 5 years at a website (https://owncloud.ikem.cz).




