A national mandatory-split liver policy: A report from the Italian experience
DATA AVAILABILITY STATEMENT:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Abstract
To implement split liver transplantation (SLT) a mandatory-split policy has been adopted in Italy since August 2015: donors aged 18-50 years at standard risk are offered for SLT, resulting in a left-lateral segment (LLS) graft for children and an extended-right graft (ERG) for adults. We aim to analyze the impact of the new mandatory-split policy on liver transplantation (LT)-waiting list and SLT outcomes, compared to old allocation policy. Between August 2015 and December 2016 out of 413 potentially “splittable” donors, 252 (61%) were proposed for SLT, of whom 53 (21%) donors were accepted for SLT whereas 101 (40.1%) were excluded because of donor characteristics and 98 (38.9%) for absence of suitable pediatric recipients. The SLT rate augmented from 6% to 8.4%. Children undergoing SLT increased from 49.3% to 65.8% (P = .009) and the pediatric LT-waiting list time dropped (229 [10-2121] vs 80 [12-2503] days [P = .045]). The pediatric (4.5% vs 2.5% [P = .398]) and adult (9.7% to 5.2% [P < .001]) LT-waiting list mortality reduced; SLT outcomes remained stable. Retransplantation (HR = 2.641, P = .035) and recipient weight >20 kg (HR = 5.113, P = .048) in LLS, and ischemic time >8 hours (HR = 2.475, P = .048) in ERG were identified as predictors of graft failure. A national mandatory-split policy maximizes the SLT donor resources, whose selection criteria can be safely expanded, providing favorable impact on the pediatric LT-waiting list and priority for adult sick LT candidates.
Abbreviations
-
- BMI
-
- body mass index
-
- CIT
-
- cold ischemic time
-
- CNT
-
- National Transplantation Centre
-
- ERG
-
- extended right graft
-
- ITU
-
- intensive therapy unit
-
- LDLT
-
- living donor liver transplantation
-
- LFTs
-
- liver function tests
-
- LLS
-
- left lateral segment
-
- LT
-
- liver transplantation
-
- MELD
-
- model of end-stage liver disease
-
- MOF
-
- multiorgan failure
-
- PELD
-
- pediatric end-stage liver disease
-
- PNF
-
- primary nonfunction
-
- SLP
-
- split liver policy
-
- SLT
-
- split liver transplantation
-
- UNOS
-
- United Network of Organ Sharing
1 INTRODUCTION
Over the past few years, the transplant community has made great efforts to increase the pediatric priority in liver organ allocation; however, the mortality of children candidates for liver transplantation (LT), who are disadvantaged because of the lack of size-matched donors, has been steady at approximately 10% every year.1, 2 Split liver transplantation (SLT) was introduced to expand the pool of grafts available for pediatric recipients, providing a left lateral segment (LLS) to a child and an extended-right graft (ERG) to an adult recipient.3 After the initial splitting experience, advances in surgical techniques and a better understanding of recipient/donor matching led to excellent SLT outcomes both in pediatric and adult recipients.4-9
Different organ allocation systems encouraged the implementation of SLT to expand the graft availability from deceased donors for children, without disadvantaging adult LT candidates.10 The SLT rate varies worldwide: in Europe, SLT represents approximately 6% of all LT.11 In the United States, SLT comprises approximately 1% of all LT, despite the fact that it is estimated that 20% of deceased donors meet United Network of Organ Sharing (UNOS) guidelines for split livers.12 In Italy, after the encouraging results of the initial SLT experience of the North Italian Transplant programme,13 since 2015 the National Transplantation Centre (CNT) in collaboration with the Italian College of Liver Transplantation Programmes defined a mandatory-split liver policy (SLP) in order to increase the number of splitting procedures nationwide and to reduce pediatric LT-waiting list mortality.
This study aims to analyze liver allocation in Italy after the introduction of the new SLP and its impact on the pediatric/adult LT-waiting list and on SLT outcomes.
2 MATERIALS AND METHODS
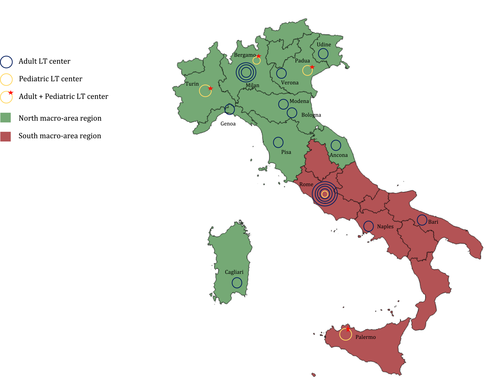
2.1 Liver allocation policy in Italy
In Italy there are 21 LT centers divided into 13 regions governed by the CNT network and grouped into two macro-areas (Figure 1). Since the CNT's establishment, liver allocation policies have seen several modifications. The first national SLT allocation program was approved in 2006; transplant centers could voluntary decide whether or not to participate. At that time, donors aged ≤14 years were preferentially allocated to pediatric recipients (<18 years), whereas donors ≥15 years were allocated to adults. Donors aged 10-50 years with stable hemodynamics, intensive therapy unit (ITU)-stay ≤5 days, transaminases ≤3 times normal, and absence of steatosis on the ultrasound scan were defined as “splittable.” The decision of whether or not to perform the splitting procedure was the choice of the LT center to which the graft was assigned.
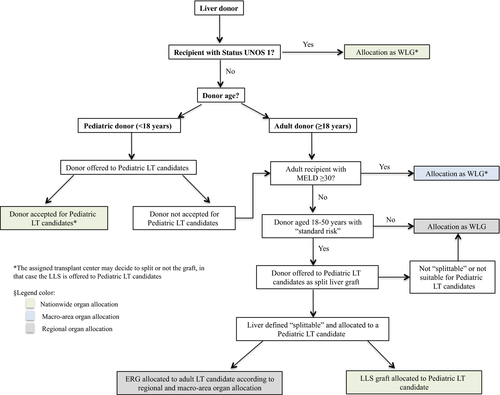
In 2015, a consensus redefined the Italian criteria for LT candidate stratification not only based on urgency but also on the principles of utility and transplant benefit. The current liver allocation system is based on the Model for End-Stage Liver Disease (MELD)/Italian Score for Organ Allocation (ISO), which is defined by biochemical MELD and exceptions.14 At present, liver grafts are shared according to the following principles: (1) nationwide, for (a) UNOS status 1 patients; (b) pediatric candidates according to the pediatric LT allocation system15; (2) macro-areas for adult LT candidates with MELD ≥30; and (3) regionally for adult patients with MELD <30.14 Additionally, the age of the donors preferentially assigned to pediatric recipients increased from 14 to 17 years and a national mandatory SLP was adopted. In the new SLP, all deceased donors aged 18-50 years with standard risk (defined as the absence of potential transmissible infections or neoplastic diseases) are mandatorily offered to pediatric transplant centers according to the pediatric national LT-waiting list15 unless a UNOS 1 status or MELD ≥30 adult candidate is on the waiting list. If the deceased donor is “splittable,” the LLS graft is allocated to a pediatric recipient. According to the adult rules, the ERG is then allocated to a recipient not only on the basis of the MELD/ISO score14 but also taking into account clinical parameters and donor-to-recipient size matching (Figure 2).
2.2 Study design
This study analyzed all deceased donors used in Italy for LT after the introduction of the new SLP and all recipients transplanted with LLSs and ERGs derived from split procedures. For the outcome analysis, the same number of SLTs performed consecutively before the introduction of the new SLP was used as a control group. To evaluate the impact of the new SLP, data from adult and pediatric LT-waiting lists as well as data on living donor liver transplantation (LDLT) activity were considered. Organ allocation, donor, recipient, and surgical data were recovered from the CNT prospective databases and retrospectively analyzed. To define the impact of the split liver procuring centers, the pediatric centers, which performed all split procedures, were stratified according to the number of split procurements performed/year (high-volume, ≥15 procedures; low-volume, <15 procedures).
2.3 Splitting technique
Since the beginning of the Italian SLT experience dating back to the mid-1990s, split liver has been performed in situ by a surgical team composed of a pediatric and an adult transplant surgeon. Ex situ split is carried out only if the in situ procedure might compromise the recovery of other organs (ie, donor hemodynamic instability) or for intraoperative technical issues, which did not allow the in situ technique.
Before 2015, parenchymal transection was preferentially performed using the transumbilical technique16 and the celiac tripod was kept in continuity with the left hepatic artery. According to the new SLP, parenchymal transection (transumbilical or transhilar17) and vessels’ division are decided in agreement between pediatric and adult surgeons intraoperatively. The celiac tripod can be assigned either to the LLS or the ERG according to (1) donor-to-recipient size matching, (2) donors’ vascular anatomy (vessels’ sizes, number of branches, segment IV branches’ origin), and (3) recipients’ vascular anatomy and clinical status (urgency, retransplantation, hepatic artery thrombosis). The main portal vein is assigned to the ERG and the left portal vein to the LLS. Only in case of disagreement regarding vessels’ division, the final decision is taken by the adult center for donor ≥18 years, whereas by the pediatric centers for donor <18 years. Intraoperative cholangiography is not routinely performed.
2.4 Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics, version 22.0 (IBM, Chicago, IL). Donor/recipient characteristics and clinical data are shown (wherever applicable) as either median with range or mean ± standard deviation. Univariate data were analyzed using the Mann-Whitney test and Fisher's exact test. A P value of <.05 was considered significant. Normal distribution continuous data were analyzed by parametric test (Student's t test). Survival rates were calculated using the Kaplan-Meier method for univariate analysis and Cox-regression for multivariate analysis.
3 RESULTS
3.1 Liver allocation and split liver procurement
Between August 2015 and December 2016, 1537 cadaveric donors were used for LT, including 58 (3.8%) pediatric donors (<18 years), 1066 (69.4%) adults >50 years and/or nonstandard risk, and 413 (26.8%) adults aged 18-50 years with standard risk. In the latter group, 161 (39%) donors were allocated to UNOS status 1 or MELD ≥30 patients; the remaining 252 (61%) were proposed for SLT, of whom 53 (21%) were accepted. One hundred and one (40.1%) were excluded from split because of the clinical characteristics of the donor at the time of offer (n = 85) or at laparotomy (n = 16). In 88 cases (34.9%), the split procedure was not performed for absence of suitable pediatric recipient, and in 10 cases (4%) due to logistic issues (Table 1). All donors refused for SLT were allocated to adult recipients as a whole graft. The donors accepted for SLT were significantly younger, had lower body weights, and received less vasopressor compared to those not split (Table 2).
| Number (%) | |
|---|---|
| At the time of donor offer | 171 (85.9) |
| Donor | |
| Abnormal LFTs | 30 (15.1) |
| Steatosis on US scan | 20 (10.1) |
| Hemodynamic instability | 20 (10.1) |
| Hepatic lesions on US scan | 7 (3.5) |
| Comorbidities | 4 (2) |
| Prolonged ITU stay | 4 (2) |
| Recipient | |
| Inadequate donor/recipient size matching | 65 (32.7) |
| No suitable recipients on the waiting list | 13 (6.5) |
| Logistic issue | 8 (4) |
| At donor laparotomy | 28 (14.1) |
| Donor | |
| Steatosis at liver biopsy | 6 (3) |
| Intraoperative vascular anomalies | 6 (3) |
| Intraoperative hemodynamic instability | 4 (2) |
| Recipient | |
| Inadequate donor/recipient size matching | 10 (5) |
| Intraoperative logistic issue | 2 (1) |
- ITU, intensive therapy unit; LFTs, liver function tests; US scan, ultrasound scan.
| Variables | Total donor offered for SLT | Donor refused for donor characteristicsa | Donor refused for absence of suitable recipientsa | Donors accepted for SLT | P value |
|---|---|---|---|---|---|
| Number (%) | 252 | 101 (40.1%) | 98 (38.9%) | 53 (21%) | - |
| Age (years) | 41 (18-50) | 43.5 (18-50) | 41 (18-50) | 38 (18-50) | .044 |
| Gender (female) | 88 (34.9%) | 38 (37.6%) | 29 (29.6%) | 21 (39.6%) | .366 |
| BMI | 25 (16-46) | 25 (18-46) | 26 (18-46) | 24 (18-32) | <.0001 |
| Weight (kg) | 75 (30-150) | 75 (42-120) | 80 (30-150) | 70 (50-90) | <.0001 |
| Height (cm) | 170 (130-192) | 170 (146-190) | 175 (130-192) | 170 (150-190) | .158 |
| Blood group | |||||
| 0 | 94 (37.3%) | 45 (44.6%) | 22 (22.4%) | 27 (50.9%) | |
| A | 103 (40.9%) | 37 (36.7%) | 50 (51.0%) | 16 (30.2%) | |
| AB | 14 (5.6%) | 5 (4.9%) | 9 (9.2%) | 0 (%) | .002 |
| B | 41 (16.3%) | 14 (13.9%) | 17 (17.3%) | 10 (18.9%) | |
| Use of vasopressors (yes) | 198 (78.6%) | 76 (75.2%) | 69 (70.4%) | 42 (86.8%) | <.0001 |
| Use >1 vasopressors | 49 (19.4%) | 25 (24.8%) | 13 (13.3%) | 11 (20.8%) | .088 |
| ITU stay (days) | 3 (0-37) | 2 (0-37) | 3 (0-16) | 3 (0-19) | .648 |
| AST (U/L) | 45 (7-15285) | 55 (9-15285) | 40 (7-497) | 42 (9-628) | .023 |
| ALT (U/L) | 41 (5-5575) | 56 (6-5575) | 32 (5-971) | 34 (9-530) | .035 |
| Total bilirubin (mg/dL) | 0.4 (0.1-7.8) | 0.4 (0.1-7.8) | 0.4 (0.1-3.04) | 0.3 (0.1-2.6) | .038 |
| GGT (U/L) | 39 (5-988) | 42 (5-988) | 33 (8-537) | 33 (5-624) | .378 |
| Serum sodium (mmL/L) | 150 (130-187) | 150 (131-183) | 151 (130-187) | 148 (131-173) | .438 |
| Cause of death | |||||
| Cerebrovascular accident | 112 (44.4%) | 40 (39.6%) | 46 (46.9%) | 26 (49.1%) | |
| Trauma | 93 (36.9%) | 38 (37.6%) | 37 (37.8%) | 18 (34%) | .248 |
| Anoxia | 38 (15.1%) | 20 (19.8%) | 12 (12.2%) | 6 (11.3%) | |
| Others | 9 (3.6%) | 3 (3%) | 3 (3.1%) | 3 (5.7%) | |
- ALT, alaninoaminotransferase; AST, aspartatoaminotransferase; GGT, gamma-glutamyltransferase; BMI, body mass index; ITU, intensive therapy unit; LFTs, liver function tests; US scan, ultrasound scan.
- a Liver graft from donor refused for SLT due to donor or recipient characteristics was used as whole liver graft.
In the pediatric donor group (n = 58), 45 (78%) livers were transplanted as whole grafts into 26 (58%) pediatric and 19 (42%) adult recipients. In the other 11 (19%) cases, livers were split generating 10 LLSs, 10 ERGs, one left and one right lobes. Two (3%) pediatric grafts were reduced on the back table. In 4 cases, split liver procurements were performed in donors >50 years. The clinical characteristics of split liver donors <18 and >50 years are summarized in Table S1.
In summary, 68 split liver procedures were performed after the introduction of the new SLP, generating one left lobe, one right lobe, 66 LLSs (one LLS was discarded because of vascular injury), and 67 ERGs. For outcomes evaluation, the left and right lobes and two ERGs that were part of multiorgan transplantations were excluded, resulting in 66 LLSs and 65 ERGs enrolled in the study. All LLSs were transplanted into pediatric recipients. In 57 (87.7%) cases, the ERG was transplanted into adults, and in 8 (12.3%) cases into a child (Table 3). Two children, with UNOS 1 status, received ERGs from donors >18 years.
| New split liver policy | Whole liver transplantation | Split liver transplantation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of deceased donors | Total | LLS | ERG | Left lobe | Right lobe | ||||||||
| Total | Pediatric recipient | Adult recipient | Pediatric recipient | Adult recipient | Pediatric recipient | Adult recipient | Pediatric recipient | Adult recipient | Pediatric recipient | Adult recipient | |||
| Pediatric (<18 y) | 58 (3.8%) | 47 | 28a | 19 | 22 | 10 | - | 6 | 4 | 1 | - | - | 1 |
| Adult 18-50 y, standard risk | 413 (26.8%) | 360 | - | 360 | 105 | 52 | - | 2 | 51b | - | - | - | - |
| Adult >50 y, non-standard risk | 1066 (69.4%) | 1062 | 6 | 1056 | 8 | 4 | - | - | 4 | - | - | - | - |
| Total | 1537 | 1469 | 34 | 1435 | 135 | 66 | - | 8 | 59 | 1 | - | - | 1 |
| Enrolled in the outcome analysis | - | - | - | 66 | 65 | - | - | - | |||||
| Old split liver policy | Whole liver transplantation | Split liver transplantation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLS | ERG | Left lobe | Right lobe | ||||||||||
| Type of deceased donors | Total | Pediatric recipient | Adult recipient | Total | Pediatric Recipient | Adult recipient | Pediatric recipient | Adult recipient | Pediatric recipient | Adult recipient | Pediatric recipient | Adult recipient | |
| Pediatric (<18 y) | 96 (4.4%) | 85 | 41 | 44 | 22 | 11 | - | 4 | 7 | - | - | - | - |
| Adult 18-50 y, standard risk | 574 (26.2%) | 525 | 6 | 519 | 97 | 46 | - | 3 | 44c | 1 | 1 | - | 2 |
| Adult >50 y, nonstandard risk | 1518 (69.4%) | 1510 | 4 | 1506 | 16 | 8 | - | 1 | 7 | - | - | - | - |
| Total | 2188 | 2120 | 51 | 2069 | 135 | 65 | - | 8 | 58 | 1 | 1 | - | 2 |
| Enrolled in the outcome analysis | 65 | 65 | |||||||||||
- ERG, extended right graft; LLS, left-lateral segment; y, years.
- a Two liver grafts from pediatric donor were reduced on backtable.
- b 2 ERGs combined with kidney transplantation were excluded from the outcome analysis.
- c 1 ERG combined with pancreas transplantation was excluded from the outcome analysis.
The 68 consecutive split liver procedures chosen as the control group were performed between June 2013 and August 2015. In the control group, 49 (72%) split procedures were performed in donors aged 18-50 years, 11 (16.2%) in donors <18 years, and 8 (11.8%) in donors >50 years (Table 3). These were standard split liver procedures in 66 (97.1%) cases and full-left/full-right split in 2 (2.9%) cases. One LLS graft was not transplanted because of vascular injury. Similar to the study group, left and right lobes and one ERG used for combined liver-pancreas transplantation were excluded, resulting in 65 LLSs and 65 ERGs enrolled in the study.
The clinical characteristics of the donors who underwent split liver procurement in the new and old SLP were similar except for the ITU-stay, which was longer in the study period (3 [0-19] vs 2 [0-11] days, P = .039) (Table 4).
| Donor variables | New split liver policy | Old split liver policy | P value |
|---|---|---|---|
| Number | 66 | 65 | |
| Age (years) | 36 (5-66) | 33 (8-58) | .836 |
| <18 years | 10 (15.4%) | 11 (16.7%) | |
| 18-50 years | 52 (80%) | 46 (70.8%) | |
| >50 years | 4 (6.2%) | 8 (12.3%) | |
| Gender (female) | 25 (51%) | 24 (49%) | 1.000 |
| BMI | 23 (16-32) | 23 (19-33) | .692 |
| Weight (kg) | 68 (25-90) | 70 (42-101) | .421 |
| Height (cm) | 170 (125-190) | 170 (145-194) | .245 |
| Blood group | |||
| 0 | 34 (51.5%) | 30 (46.2%) | |
| A | 22 (33.3%) | 30 (46.2%) | .211 |
| B | 10 (15.2%) | 5 (7.7%) | |
| Use of vasopressors | 45 (66.7%) | 35 (53.8%) | .155 |
| Use of >1 vasopressors | 12 (18.2%) | 11 (16.9%) | .291 |
| ITU stay (days) | 3 (0-19) | 2 (0-11) | .039* |
| AST (U/L) | 40 (9-628) | 42 (8-357) | .639 |
| ALT (U/L) | 29 (9-530) | 34 (8-269) | .978 |
| Total bilirubin (mg/dL) | 0.5 (0.1-3) | 0.4 (0.1-8) | .078 |
| GGT (U/L) | 30 (5-624) | 28 (5-545) | .712 |
| Serum sodium (mmL/L) | 148 (131-173) | 148 (124-193) | .363 |
| Cause of death | |||
| Cerebrovascular | 31 (46.9%) | 33 (50.8%) | |
| Trauma | 24 (36.4%) | 21 (32.3%) | .632 |
| Anoxia | 7 (10.6%) | 10 (15.4%) | |
| Others | 4 (6.1%) | 1 (1.5%) | |
- ALT, alaninoaminotransferase; AST, aspartatoaminotransferase; GGT, gamma-glutamyltransferase; BMI, body mass index; ITU, intensive therapy unit.
3.2 Outcomes of SLT
3.2.1 Left-lateral segment graft transplantation
Table 5 shows the surgical and recipient characteristics of LLS transplantation. During the study period, the LLS recipients were significantly younger compared to those transplanted in the control period, including 77.3% vs 58.5% children <24 months of age (P = .025).
| Variables | New split liver policy (n = 66) | Old split liver policy (n = 65) | P value |
|---|---|---|---|
| Surgical variables | |||
| In situ/ex situ split | 64 (97%) / 2 (3%) | 63 (96.9%) / 2 (3.1%) | 1.000 |
| Cold ischemic time (hours) | 6 (3-10) | 5 (1-8) | .108 |
| Warm ischemic time (minutes) | 47 (24-110) | 40 (30-121) | .238 |
| Recipient variables | |||
| Age (years) | 1.1 (0.1-11.2) | 2.1 (0.1-12.1) | .043 |
| Gender (female) | 33 (50%) | 36 (55.4%) | .601 |
| BMI | 16.4 (13 -23.8) | 16.5 (11.3-22.3) | .848 |
| Weight (kg) | 8.7 (4-35) | 12 (6-30) | .020 |
| Height (cm) | 70.1 (53-135) | 84 (58-152) | .014 |
| Blood group | |||
| 0 | 28 (42.4%) | 22 (33.8%) | |
| A | 23 (34.8%) | 33 (50.8%) | .271 |
| B | 12 (18.2%) | 7 (10.8%) | |
| AB | 3 (4.5%) | 3 (4.6%) | |
| PELD score | 22 (10-43) | 22 (12-39) | 1.000 |
| UNOS status | |||
| 1 | 8 (12.1%) | 7 (10.8%) | |
| 1B | 1 (1.5%) | 6 (9.2%) | |
| 2A | 5 (7.6%) | 7 (10.8%) | .344 |
| 2B | 10 (15.2%) | 10 (15.4%) | |
| 3 | 42 (63.6%) | 35 (53.8%) | |
| Indication for transplantation | |||
| Acute liver failure | 6 (9.1%) | 3 (4.6%) | |
| Autoimmune liver disease | 2 (3%) | 1 (1.5%) | |
| Cholestatic liver disease | 43 (65.2%) | 36 (55.4%) | |
| Tumor | 2 (3%) | 9 (13.8%) | .074 |
| Metabolic liver disease | 7 (10.6%) | 6 (9.2%) | |
| Retransplantation (early/late) | 4 (2/2) (6.1%) | 10 (1/9) (15.4%) | |
| Other disease | 2 (3%) | - | |
| Time on waiting list to transplant (days) | 36 (1-530) | 35 (1-571) | .634 |
- ALT, alaninoaminotransferase; AST, aspartatoaminotransferase; GGT, gamma-glutamyltransferase; BMI, body mass index; PELD, pediatric end-stage liver disease; UNOS, United Network of Organ Sharing.
The overall 1-year patient survivals were 84.8% and 87.7% in the study and control period respectively (P = .408); the overall 1-year graft survival was 78.8% for the new SLP and 80% for the old SLP (P = .610) (Figure 3).

In the study group, 10 (15.2%) patients died within 1 year from SLT, of whom 5 (50%) were urgent SLT. Of 7 (10.8%) children who died during the old SLP, only one (14.2%) had urgent transplantation. The retransplantation rate was similar in the two groups (4 [6.1%] vs 5 [7.7%] [P = .712]) (Table 6). The characteristics of the deceased and retransplanted LLS recipients are detailed in Table S2.
| Variables | New split liver policy (n = 66) | Old split liver policy (n = 65) | P value |
|---|---|---|---|
| Total number of death | 10 (15.2%) | 7 (10.8%) | .456 |
| Cause of death | |||
| PNF | 1 (1.5%) | 2 (3.1%) | .619 |
| Sepsis | 2 (3.0%) | 1 (1.5%) | .568 |
| MOF | 5 (7.6%) | 2 (3.1%) | .077 |
| Tumor recurrence | 0 (0%) | 1 (1.5%) | 1.000 |
| Pulmonary embolism | 1 (1.5%) | 0 (1.5%) | .496 |
| Biliary complication | 0 (0%) | 1 (1.5%) | 1.000 |
| Cerebrovascular accident | 1 (1.5%) | 0 (0%) | .496 |
| Retransplantation | 4 (6.1%) | 5 (7.7%) | .712 |
| Cause of retransplantation | |||
| PNF/DNF | 1 (1.5%) | 3 (4.6%) | .302 |
| Hepatic artery thrombosis | 2 (3%) | 0 (0%) | .496 |
| Portal vein thrombosis | 1 (1.5%) | 1 (1.5%) | .991 |
| Chronic rejection | 0 (0%) | 1 (1.5%) | .312 |
- DNF, delayed nonfunction; LLS, left-lateral segment; MOF, multiorgan failure; PNF, primary nonfunction.
Within the first year of SLT, postoperative technical complications were comparable in the two periods (20 [30.3%] vs 24 [36.9%], [P = .529]) (Table S3).
3.2.2 Extended right graft transplantation
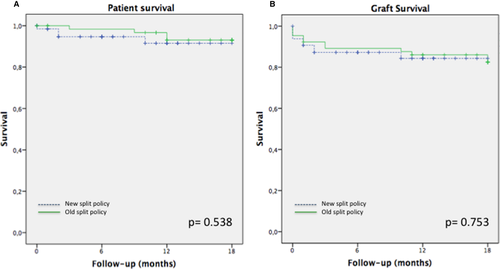
The technical and recipient characteristics of ERG transplantation were comparable in the two periods (Table 7). The 1-year patient survival was 93.8% in both groups (P = .538), whereas 1-year graft survival was 86.2% after the introduction of the new SLP and 83.1% in the control period (P = .753) (Figure 4).
| Variables | New split liver policy (n = 65) | Old split liver policy (n = 65) | P value |
|---|---|---|---|
| Surgical variables | |||
| In situ/ex situ split | 58 (89.2%)/7 (10.8%) | 59 (90.8%)/6 (9.2%) | .778 |
| Cold ischemic time (hours) | 7 (4-11) | 6 (3-15) | .125 |
| Warm ischemic time (minutes) | 42 (22-165) | 35 (22-80) | .207 |
| Recipient variables | |||
| Age (years) | 53 (2-71) | 53 (8-69) | .714 |
| Gender (female) | 29 (44.6%) | 38 (58.5%) | .160 |
| BMI | 23.2 (13.4-32) | 23.5 (14.3-43.3) | .257 |
| Weight (kg) | 64 (11-103) | 65 (22-109) | .782 |
| Height (cm) | 168 (88-183) | 165 (86-180) | .279 |
| Blood group | |||
| 0 | 30 (46.2%) | 21 (32.3%) | |
| A | 22 (33.8%) | 37 (56.1%) | |
| B | 12 (18.5%) | 6 (9.2%) | .040 |
| AB | 1 (1.5%) | 1 (1.5%) | |
| Biochemical MELD/PELD | 18 (10-35) | 20 (9-42) | 1.000 |
| UNOS status | |||
| 1 | 2 (3.1%) | 3 (4.6%) | |
| 2A | 5 (7.7%) | 8 (12.3%) | .824 |
| 2B | 38 (58.5%) | 35 (53.8%) | |
| 3 | 20 (30.8%) | 19 (29.2%) | |
| Indication for SLT | |||
| Alcoholic liver disease | 10 (15.4%) | 8 (12.3%) | |
| Autoimmune liver disease | 12 (18.5%) | 9 (13.8%) | |
| Cholestatic liver disease | 4 (6.2%) | 2 (3.1%) | |
| Tumor | 16 (24.6%) | 24 (36.9%) | .109 |
| Viral-related cirrhosis | 16 (24.6%) | 7 (10.8%) | |
| Cryptogenic cirrhosis | 1 (1.5%) | 5 (7.7%) | |
| Metabolic liver disease | 5 (7.7%) | 4 (6.2%) | |
| Retransplantation | 0 (0%) | 1 (1.5%) | |
| Others | 1 (1.5%) | 5 (7.7%) | |
| Time on waiting list to transplant (days) | 91 (1-1682) | 74 (1-1479) | .764 |
- ALT, alaninoaminotransferase; AST, aspartatoaminotransferase; GGT, gamma-glutamyltransferase; BMI, body mass index; MELD, model for end-stage liver disease; LFTs, liver function tests; PELD, pediatric end-stage liver disease; UNOS, United Network of Organ Sharing.
In the study group, 4 (6.2%) patients died within 1 year of SLT, of whom 1 (25%) was transplanted with UNOS 1 status; of 4 (6.2%) deceased patients in the control group, none received urgent transplantation. The retransplantation rate was similar in the two groups (5 [7.7%] vs 7 [10.8%] [P = .638]) (Table 8). The characteristics of the deceased and retransplanted ERG recipients are reported in Table S4.
| Variables | New split liver policy (n = 65) | Old split liver policy (n = 65) | P value |
|---|---|---|---|
| Total number of death | 4 (6.2%) | 4 (6.2%) | 1.000 |
| Cause of death | |||
| Sepsis | 2 (3.1%) | 1 (1.5%) | .559 |
| Myocardial infarction | 1 (1.5%) | 0 (0%) | .315 |
| Tumor recurrence | 1 (1.5%) | 1 (1.5%) | 1.000 |
| MOF | 0 (0%) | 2 (3%) | .496 |
| Retransplantation | 5 (7.7%) | 7 (10.8%) | .638 |
| Cause of retransplantation | |||
| PNF/DNF | 3 (4.6%) | 3 (4.6%) | 1.000 |
| Hepatic artery thrombosis | 2 (3.1%) | 2 (3.1%) | 1.000 |
| Biliary complications | 0 (0%) | 2 (3.1%) | .154 |
- DNF, delayed nonfunction; ERG, extended right graft; MOF, multiorgan failure; PNF, primary nonfunction.
Postoperative complications were observed in 15 (23%) ERG recipients transplanted in the new SLP and in 24 (36.9%) cases in the control group (P = .085) (Table S5).
3.2.3 Univariate and multivariate analysis
By log-rank test, no significant differences in 1-year patient or graft survivals for LLS and ERG transplantations were observed, according to the centers’ split liver procurement volume (Table S6).
Among LLS transplantation, re-transplantation vs first transplantation (HR = 3.349, P = .006), recipient body weight >20 kg vs ≤20 kg (HR = 4.088, P = .001), and donor-to-recipient weight ratio (DRWR) ≤4 vs >4 (HR = 1.380, P = .018) were risk factors for graft failure by univariate analysis (Table S7). In the multivariate analysis, recipient body weight >20 kg vs ≤20 kg (HR = 5.113, P = .048) and retransplantation as an indication of SLT (HR = 2.641, P = .035) were predictors of graft failure (Figures S1, S2).
In ERG transplantation, cold ischemic time (CIT) >8 hours vs ≤8 hours (HR = 2.574, P = .039) and donor ITU-stay >5 days vs ≤5 days (HR = 1.946, P = .046) were identified as risk factors for graft loss according to the univariate analysis (Table S8), and only CIT >8 hours vs ≤8 hours (HR = 2.475, P = .048) in the multivariate analysis (Figure S3).
3.3 Impact of the new split liver policy on the LT-waiting list
Since the new SLP was introduced, the SLT rate increased from 6% to 8.4% and a median of 2 SLTs/week were performed. Compared to the old SLP, the same number of SLTs was achieved in a shorter period (16.5 months in the new SLP vs 26.4 months in the old SLP).
During the study period, 114 pediatric LTs were performed, including 75 (65.8%) SLTs, 34 (29.8%) whole LTs, and 5 (4.4%) LDLTs. During the control period, 150 pediatric LTs were performed, including 74 (49.3%) SLTs, 51 (34%) whole LTs, and 25 (16.7%) LDLTs.
After the introduction of the new SLP, the total number of children receiving a split graft increased from 49.3% to 65.8% (P = .009) and the LDLT rate significantly reduced compared to the control period (4.4% vs 16.7%, P = .0016).
The median time on the LT-waiting list reduced from 229 (10-2121) days to 80 (12-2503) days (P = .045). Child dropout from the LT-waiting list decreased from 2.5% (5/200) to 1.8% (3/163) (P = .735), thus the pediatric waiting list mortality was 4.5% (9/200) in the old SLP and 2.5% (4/163) in the new SLP (P = .398). Of 4 children awaiting LT who died in the study period, 2 (50%) patients were candidates for urgent re-LT. After the introduction of the new SLP, a median of 2 pediatric LTs/week were performed compared to 1 pediatric LT/week during the control period. Moreover, during the study period, one donor every 2 days was offered for SLT to the pediatric LT-waiting list and one split graft/weekly was accepted for a child awaiting LT.
During the new SLP, 1503 adult LTs were performed, including 1435 (95.5%) whole LTs, 60 (4%) SLTs, and 8 (0.5%) LDLTs. In the control period, 2148 LTs were carried out, including 2069 (96.3%) whole LTs, 61 (2.8%) SLTs, and 18 (0.8%) LDLTs. After the new SLP was adopted, the SLT rate in adult recipients increased from 2.8% to 4% (P = .058) and the median time from listing to LT remained stable (282 [0-5951] vs 299 [0-5095] days during the study and control period respectively [P = .142]). Since the introduction of the new SLP, the adult LT-waiting list mortality significantly reduced from 9.7% (369/3814) to 5.2% (149/2891) (P < .001) and the adult LT-waiting list dropout rate remained stable (7.1% [204/2891] vs 6.1% [232/3814] in the new and old allocation programs, respectively [P = .109]). During the study period, a median of 24 adult LTs/week was performed, with 21 adult LTs/week in the control group.
4 DISCUSSION
Improving SLT programs has attracted great interest in recent years as a method of reducing pediatric LT-waiting list mortality.10 Although the SLT rate constantly increased in Europe (>400 SLTs/year since 2002),18 a change in donor demographics resulted in a reduction of deceased liver donors suitable for split procedures. The main issues limiting the expansion of SLT programs worldwide include (1) the need for a national/international organ allocation system with an “intention-to-split” policy, and the cooperation between pediatric and adult LT centers; (2) the lack of international donor selection criteria for SLT; (3) split grafts have been generally perceived by centers as carrying greater morbidity and mortality compared to whole grafts.10, 19, 20
In Italy, the steady increase in average age of deceased donors and the reduction in the number of SLTs causing a lengthening of waiting times for pediatric LT drove the analysis of the Italian SLT data21-27 and the definition in 2015 of a mandatory SLP, which includes three major steps: first, the CNT offers all donors aged 18-50 years at standard risks to the pediatric transplant centers for SLT; second, the pediatric transplant centers decide if the LLS is suitable for pediatric LT candidates; and third, the ERG, which is considered ab initio fit for transplantation as the pediatric donor selection criteria are more narrowing than those adopted for adults, is allocated to the best-matching adult recipient. Subsequently, the graft “splittability” remains only a technical aspect (ie, vascular anomalies).
In the study period, donors within SLT-criteria accounted for 26% of all liver donors, of which 60% were offered for SLT and of these, 21% were effectively used for SLT. Consequently, the introduction of the new SLP significantly increased the number of donors offered for split procedures and SLT rate represented 8.4% of all LT activity, which is higher compared to the recent split data from the Eurotransplant Area (5%).28 Despite the expansion of SLT donor criteria, most of the split procedures were performed in young donors, proving that pediatric centers had more opportunities to choose the best donor for children candidates to LT, with high rate of acceptance.29
Among donors refused for SLT, 40% were not accepted due to the donor quality and 39% due to the absence of suitable recipients, mainly related to donor/recipient size matching. Interestingly, the donors refused due to the lack of size matching recipients were similar to those accepted for SLT for all clinical variables, except for higher body weight. Therefore, almost 40% of potentially “splittable” donors are currently not used for standard splitting procedure, but might represent a consistent donor pool for full-right/full-left SLT or for international networks of organ sharing.20, 30
A mandatory SLP has been adopted also in other countries; thus their split liver donor selection criteria are much more strict when compared to these currently used in Italy.10 In United Kingdom all brain-death donors <40 years, with weight >50 kg, and <5 days of ITU stay are mandatory offered for splitting in absence of superurgent or multivisceral candidates31; in France, donors aged <30 years are first proposed to pediatric LT candidates.32 Contrarily, in the UNOS network splitting is not mandatory and only donors between 18-40 years of age with no more than a single vasopressor, transaminases ≤3 times normal, and body mass index ≤28 are considered for SLT, but are not primarily offered to pediatric centers2; the result is that only 6.3% of liver donors meet the SLT criteria and only 3.8% of these are utilized for SLT.33
Of the criteria used to select donors suitable for split procedures, donor age >50 years and <10 years has been recognized as a crucial risk factor for SLT graft failure.18 In our series, donors aged >50 years were used for SLT in only four UNOS 1 status children because of the impossibility of waiting longer for a graft; thus the “adapted” allocation of ERGs guaranteed good outcomes in all adults.
Notwithstanding the promising results on the use of partial grafts from pediatric donors,24 presently in Italy split liver procedures in donors <18 years are not mandatory. However, our data confirmed that SLTs from young donors have favorable outcomes,34 suggesting that split procedures should be mandatory also in pediatric donors (at least for body weight >40 kg), limiting the use of reduced grafts to selected cases.
Our experience showed that the presence of a national organ-exchange organization and the collaboration between pediatric and adult centers were key factors to maximize the use of donors without compromising outcomes.19, 35-37 The availability of surgeons trained to perform split procedures in most of the Italian transplant centers was also essential to implement the national SLT programme.20 The standardization of surgical techniques, consisting of in situ split procedures38 and rules for vascular structures’ division, resulted in limited CIT (organs were shipped from the donor hospital to the recipient center) and low rates of vascular complications, which represent the most common issues in SLT.8, 28, 39-41 These good outcomes justify the enormous logistical efforts of the in situ split technique related to the prolonged donor surgery42; hence, a previous Italian experience reported that standard in situ split procurement added 156 ± 33 minutes to the donor operation, whereas using full-right/full-left split procedure required 185 ± 50 more minutes.27
The introduction of the new SLP did not have an impact on SLT morbidity and mortality, which were comparable to the control period. Children transplanted with LLS showed 1-year patient survival of 85% and graft survival of 79%, in line with the European Liver Transplant Registry18 (patient survival: 89.1%; graft survival: 83.3%) and UNOS43 data (graft survival: 75.5%). Adults receiving ERG had a 1-year patient survival of 94% and a graft survival of 86%, similar to those reported in literature.8
In children receiving LLS, retransplantation and recipient body weight >20 kg were the only risk factors of graft failure, confirming that the recipient status significantly influences SLT outcomes and adequate donor/recipient size matching is essential to avoid “small-for-size syndrome.”44 In ERG transplantation, only CIT >8 hours increased the risk of graft failure in agreement with the UNOS data.43
After the new SLP, the number of liver offers to LT pediatric candidates significantly improved (proportionally to the Italian donation rate), giving more opportunities to children to receive SLT; in consequence the median pediatric LT-waiting list time significantly decreased (from 7 months to less than 3 months), being considerably shorter compared to other series (ie, in the US >40% of children spend over a year on the waiting list).45 Likewise, the pediatric LT-waiting list mortality rate was 2.5%, remarkably lower compared to other reports (7-12%),1, 2, 10 and in half of the cases death occurred in children awaiting urgent re-LT. Because in Italy the majority of children are transplanted with split grafts from deceased donors, we believe that the low pediatric LT-waiting list mortality is mainly related to the pediatric priority in organ allocation. Additionally, in the observational period the LDLT activity reduced, thus the paucity of cases and the different centers’ policy on the use of living-related resource do not allow to interpret data. Hence, the SLT rate is influenced not only by the adoption of mandatory-SLP and donation rate but also by the pediatric LT-waiting list demand (which is variable over the time) and the use of LDLT resource.
On the adult side, the prioritization of UNOS 1 status and MELD ≥30 LT candidates ensured that the mandatory-SLP did not have a negative impact on the adult LT-waiting list mortality and dropout rate. The decreased adult LT-waiting list mortality rate observed in the study period was not related to the new SLP, but it was caused by the higher number of adult LT performed and the introduction of the new liver allocation model (MELD/ISO score), which includes MELD exception and hepatocellular carcinoma priorization.14 In the current system, transplant centers accepting ERG could choose the adult recipient based not only on the MELD/ISO score but also on the recipient clinical status and the donor/recipient size matching, resulting in an increased rate of partial grafts accepted for adults. Moreover, the presence of urgent/MELD ≥30 adult candidates eliminates the obligation of splitting but does not prohibit the possibility for those to receive a SLT (as occurred in 2 cases in our study). ERGs were allocated to a relatively low biochemical MELD score (18 [10-35]), being in line with other series.46 Limitations of our study consist in matching the new SLP with an historical control group and to not compare ERG with whole graft; thus, similar 10-years outcomes of ERG and whole LT performed in Italy were recently reported.9
In conclusion, the current is the first report of a national mandatory SLP. The first donor offer to pediatric LT candidate increases the number of children receiving SLT, resulting in low LT-waiting list dropout and mortality rate, which is mainly limited to urgent transplantation. The prioritization of organ allocation to UNOS 1 status and MELD ≥30 adult candidates ensure that mandatory SLP does not harm the adult LT-waiting list. Split liver donor criteria can be safely expanded, providing optimal graft and patient SLT outcomes. Thus, a significant proportion of potentially “splittable” donors is currently not used for conventional split procedures but might be employed for adult-to-adult SLT or for international organ networks in order to optimize liver allograft resources.
ACKNOWLEDGMENTS
No funding sources were employed. The authors would like to acknowledge Dr. Andrea Ricci for his assistance with statistics and data analysis.
AUTHOR CONTRIBUTIONS
Angelico R: designed the work, analyzed and interpreted data, draft the work, approved the final version, and reviewed the accuracy and integrity of the work.
Trapani S: designed the work, analyzed and interpreted data, drafted the work, approved the final version, and reviewed the accuracy and integrity of the work.
Spada M: designed the work, analyzed and interpreted data, revisited critically the manuscript for intellectual content, approved the final version, and reviewed the accuracy and integrity of the work.
Colledan M: substantial contribution to the conception of the work, interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, and reviewed the accuracy and integrity of the work.
de Ville de Goyet J: substantial contribution to the conception of the work, acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Salizzoni M: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
De Carlis L: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Andorno E: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Gruttadauria S: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Ettorre GM: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Cescon M: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Rossi G: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Risaliti A: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Tisone G: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Tedeschi U: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Vivarelli M: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Agnes S: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
De Simone P: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Lupo LG: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Di Benedetto F: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Santaniello W: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Zamboni F: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Mazzaferro V: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Rossi M: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Puoti F: analyzed and interpreted data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Camagni S: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Grimaldi C: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Gringeri E: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Rizzato L: acquisition and interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Nanni Costa A: substantial contribution to the conception of the work, interpretation of data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
Cillo U: designed the work, interpreted data, revisited critically the manuscript for intellectual content, approved the final version, reviewed the accuracy and integrity of the work.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




