The impact of direct-acting antiviral agents on liver and kidney transplant costs and outcomes
See also: Brown
Abstract
Direct-acting antiviral medications (DAAs) have revolutionized care for hepatitis C positive (HCV+) liver (LT) and kidney (KT) transplant recipients. Scientific Registry of Transplant Recipients registry data were integrated with national pharmaceutical claims (2007-2016) to identify HCV treatments before January 2014 (pre-DAA) and after (post-DAA), stratified by donor (D) and recipient (R) serostatus and payer. Pre-DAA, 18% of HCV+ LT recipients were treated within 3 years and without differences by donor serostatus or payer. Post-DAA, only 6% of D-/R+ recipients, 19.8% of D+/R+ recipients with public insurance, and 11.3% with private insurance were treated within 3 years (P < .0001). LT recipients treated for HCV pre-DAA experienced higher rates of graft loss (adjusted hazard ratio [aHR] 1.341.852.10, P < .0001) and death (aHR 1.471.681.91, P < .0001). Post-DAA, HCV treatment was not associated with death (aHR 0.340.671.32, P = .25) or graft failure (aHR 0.320.641.26, P = .20) in D+R+ LT recipients. Treatment increased in D+R+ KT recipients (5.5% pre-DAA vs 12.9% post-DAA), but did not differ by payer status. DAAs reduced the risk of death after D+/R+ KT by 57% (0.190.430.95, P = .04) and graft loss by 46% (0.270.541.07, P = .08). HCV treatment with DAAs appears to improve HCV+ LT and KT outcomes; however, access to these medications appears limited in both LT and KT recipients.
Abbreviations
-
- aHR
-
- adjusted hazard ratio
-
- DAA
-
- direct-acting antiviral
-
- eGFR
-
- estimated glomerular filtration rate
-
- ESLD
-
- end-stage liver disease
-
- ESRD
-
- end-stage renal disease
-
- HCV
-
- hepatitis C virus
-
- HRSA
-
- Health Resources and Services Administration
-
- KT
-
- kidney transplant
-
- LT
-
- liver transplant
-
- MELD
-
- model for end-stage liver disease
-
- NAT
-
- nucleic acid testing
-
- OPTN
-
- Organ Procurement and Transplantation Network
-
- PCD
-
- pharmaceutical claims data warehouse
-
- SRTR
-
- Scientific Registry of Transplant Recipients
-
- SVR
-
- sustained viral response
-
- TRR
-
- transplant recipient registration
-
- USD
-
- United States dollars
-
- US
-
- United States
1 INTRODUCTION
Direct-acting antivirals (DAAs) have dramatically altered the care of patients with chronic hepatitis C virus (HCV) infection.1-6 For the last 2 decades, chronic HCV infection was the leading indication for liver transplant (LT) in the United States. Treatment of HCV infection prior to 2014 consisted primarily of interferon- and ribavirin-based treatment regimens, which had limited efficacy, were associated with debilitating side effects, and, generally, were contraindicated in patients with decompensated cirrhosis or, in the case of ribavirin, advanced chronic kidney disease (CKD).7, 8 DAAs reduce morbidity and result in sustained virological response 12 weeks after completing treatment (SVR12) rates >94% for most genotypes in both compensated and decompensated patients.4, 9, 10 DAAs have also been safely used in the post LT setting to prevent recurrent inflammation and fibrosis, which was universal in the absence of effective pretransplant treatment. Historically, recurrence of HCV in the liver graft resulted in cirrhosis in 20%-30% of LT recipients and fibrosing cholestatic hepatitis C in 2%-9% within the first 12 months.11, 12 DAAs have been shown to achieve SVR12 after LT in multiple clinical trials, although the impact of DAAs on longer-term LT and KT outcomes has not been reported.12
Among patients with end-stage renal disease (ESRD), HCV prevalence is 5 times greater than in the general population.13, 14 Historically, HCV-infected ESRD patients on dialysis were 60% more likely to die than their noninfected counterparts. Prior to DAAs, interferon-based regimens had low levels of efficacy and high rates of intolerability in this population and were generally contraindicated in HCV-infected KT recipients because of unacceptable rates of rejection and allograft dysfunction. Because most HCV-infected KT recipients in the pre-DAA era were therefore either untreated or intolerant to pretransplant interferon/ribavirin, these patients experienced higher rates of graft loss and death than noninfected patients.15 In contrast, DAAs are both safe and well tolerated in patients with advanced CKD, with SVR12 rates from 95% to 98%.16, 17 DAA treatment in KT recipients successfully eradicates the virus without negatively affecting graft function in clinical series.18, 19
While DAA treatment is revolutionary, access to it has been hampered nationally by its high cost.20-22 Initial regimens resulted in total healthcare expenditures exceeding $100 000 USD (United States dollars) per treatment course. As more DAAs have entered the market and competition has increased, costs have diminished, but remain in excess of $25 000 per course, depending on specific agent. Consequently, major private payers developed preauthorization processes that initially restricted use to patients with defined clinical conditions such as demonstrated hepatic fibrosis, cirrhosis, or advanced kidney disease, despite evidence suggesting clinical benefits even in patients without advanced liver disease.23 While long-term economic analyses suggest that DAAs are cost effective, few are cost saving despite reducing the need for transplant.24-26 Furthermore, the cost savings are accrued far in the future when many patients have changed health insurance, diminishing enthusiasm for broader treatment of patients without qualifying conditions.
While clinical trial experience with DAAs in the posttransplant LT and KT populations have demonstrated high SVR rates, no large-scale, population-based assessment of access to DAA treatment, effects on longer-term transplant outcomes, or increases in the cost of treatment has been conducted. Using a unique data set linking pharmacy claims data and transplant registry outcomes, we developed a national cohort of HCV-positive transplant recipients with sufficient power to assess DAA use in LT and KT recipients before and after introduction of DAAs, characteristics associated with posttransplant HCV treatment, and the independent impact of these medications on patient and graft outcomes.
2 METHODS
2.1 Data sources
We conducted a retrospective cohort study using linked healthcare databases in the United States to ascertain patient characteristics, pharmacy fill records, and outcome events for LT and KT recipients. This study used transplant data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors. Baseline demographic information ascertained for LT and KT recipients from OPTN included age, sex, and race as reported by the transplant centers.
Pharmacy fill data were assembled by linking SRTR records for LT and KT recipients with billing claims from a large US pharmaceutical claims data (PCD) warehouse that collects prescription drug fill records including self-paid fills and those reimbursed by private and public payers. PCD comprises National Council for Prescription Drug Program format prescription claims aggregated from multiple sources including claims warehouses, retail pharmacies, and prescription benefit managers for approximately 60% of US retail pharmacy transactions. Individual claim records include the pharmacy fill date with the national drug code identifying agent and dosage. After Institutional Review Board and HRSA approvals, PCD records were linked with SRTR records for transplant recipients. We applied a deterministic de-identification strategy wherein patient identifiers (last name, first name, date of birth, sex, and ZIP code of residence) were transformed before delivery to the Saint Louis University researchers with Health Information Portability and Accountability Act and Health Information Technology for Economic and Clinical Health (HITECH)–certified encryption technology from PCD. The patient de-identification software uses multiple encryption algorithms in succession to guarantee that the resulting “token” containing encrypted patient identifiers can never be decrypted. However, the algorithm yields the same results for a given set of data elements, such that linkages by unique anonymous tokens are possible.27
2.2 Sample and clinical characteristics
We identified adult LT and KT recipients (age ≥18 years) with SRTR records of transplants between 2007 and 2016 and available pharmaceutical fill records for up to 36 months posttransplant. Recipient clinical and demographic characteristics, characteristics of the donated organ, and other transplant factors including ischemic time and sharing, were defined by the OPTN Transplant Candidate and Recipient Registration forms (Table 1). Patients were identified has being HCV+ based on HCV serostatus at the time of transplant as noted on the Transplant Recipient Registration (TRR) form. As patients who were HCV antibody positive but nucleic acid testing (NAT) negative may be classified as positive on the TRR, we further identified patients who were given an HCV antibody or NAT-positive donor organ as evidence of an active viremic state (as use of HCV+ organs in NAT-negative recipients remains uncommon). Patient insurance coverage was dichotomized as public (Medicaid, Medicare, self-pay) vs private (all others). Patient and graft outcomes were determined from SRTR registry data.
| HCV D+/R+ | HCV D-/R+ | HCV D-/R- | HCV D+/R- | |
|---|---|---|---|---|
| Liver pre-DAA | ||||
| Total subjects | 1132 | 14 640 | 20 864 | 0 |
| PCD eligibility at Tx | 926 | 11 348 | 15 902 | 0 |
| 1 y PCD eligibility post-Tx | 985 | 12 488 | 18 433 | 0 |
| HCV Rx: 3 mo post-Tx | 3 | 98 | 3 | 0 |
| HCV Rx: 1 y post-Tx | 59 | 820 | 29 | 0 |
| HCV Rx: post-Tx | 335 | 3907 | 153 | 0 |
| Listing to Tx in d (mean) | 303 | 282 | 225 | NA |
| Liver post-DAA | ||||
| Total subjects | 1166 | 6254 | 14 320 | 133 |
| PCD eligibility at transplant | 890 | 4908 | 10 716 | 85 |
| 1 y PCD eligibility post-Tx | 483 | 3183 | 6071 | 47 |
| HCV Rx: 3 mo post-Tx | 47 | 176 | 6 | 1 |
| HCV Rx: 1 y post-Tx | 155 | 614 | 26 | 5 |
| HCV Rx: post-Tx | 181 | 793 | 31 | 11 |
| Listing to Tx in d (mean) | 328 | 336 | 241 | 315 |
| Kidney pre-DAA | ||||
| Total subjects | 1377 | 3173 | 95 715 | 232 |
| PCD eligibility at transplant | 1066 | 2480 | 75 378 | 184 |
| 1 y PCD eligibility post-Tx | 1256 | 2925 | 89 704 | 212 |
| HCV Rx: 3 mo post-Tx | 0 | 2 | 4 | 0 |
| HCV Rx: 1 y post-Tx | 11 | 9 | 6 | 1 |
| HCV Rx: post-Tx | 215 | 298 | 77 | 12 |
| Listing to Tx in d (mean) | 395 | 826 | 708 | 411 |
| Kidney post-DAA | ||||
| Total subjects | 1083 | 1608 | 54 412 | 273 |
| PCD eligibility at transplant | 852 | 1247 | 42 892 | 157 |
| 1 y PCD eligibility post-Tx | 482 | 756 | 26 383 | 82 |
| HCV Rx: 3 mo post-Tx | 19 | 19 | 2 | 1 |
| HCV Rx: 1 y post-Tx | 144 | 82 | 13 | 11 |
| HCV Rx: post-Tx | 181 | 119 | 25 | 11 |
| Listing to Tx in d (mean) | 396 | 871 | 749 | 384 |
- D/R, donor/recipient hepatitis status; DAA, direct-acting antiviral; HCV, hepatitis C virus; Rx, treatment for HCV; PCD, pharmaceutical claims data; Tx, transplant.
2.3 HCV treatments
Using pharmacy fill records, we identified claims for approved HCV medications and combinations. In the pre-DAA era, defined as before January 2014, HCV treatment was defined as pegylated interferon, interferon, and ribavirin. DAA-era HCV treatments, with or without ribavirin, are shown in Table S1.
2.4 Analyses
2.4.1 Demographic characteristics
Donor and recipient characteristics were drawn from the SRTR data. Pre- and post-DAA-era differences were assessed using Student t test and χ2 analyses as appropriate.
2.4.2 Propensity to receive HCV treatment
Kaplan-Meier survival analysis was performed to identify the proportion of patients receiving HCV treatment by era and primary payer. Multivariate regression analyses were separately performed for LT and KT recipients to assess factors correlated with HCV treatment before and after introduction of DAAs. Donor and recipient characteristics, including primary payer, were included as independent variables.
2.4.3 Cost of treatment analysis
The direct cost of HCV treatment was calculated using pharmacy claims for LT and KT recipients before and after introduction of DAAs.
2.4.4 Survival analysis
Posttransplant survival was assessed in HCV+ patients who did and did not receive HCV treatment in the pre- and post-DAA eras. Multivariate Cox proportional hazard models were constructed with HCV treatment as a time-varying covariate. Models were separately constructed for patients noted to be HCV+ on the TRR and for HCV+ patients who received HCV+ donor organs. Donor and recipient characteristics were included as covariates in the model.
2.4.5 Statistical significance
For all models, P < .05 was used to determine statistical significance. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
2.4.6 Approval
This project was reviewed and approved by the Institutional Review Board of Saint Louis University.
3 RESULTS
Between 2007 and 2016, 58 509 LTs were performed; pharmacy claims data for at least 1 year after transplant were available for 41 690 (71%) of these (Table 1). Among patients with claims, 15 671 (38%) were HCV donor negative and recipient positive (D-/R+), 1468 (3.5%) were D+/R+, and 47 (0.1%) were recorded as D+/R-. In the same period, 157 873 KTs were performed; pharmacy claims data were available for 121 800 (71%). In this population, 3681 (3.0%) were D-/R+, 1738 (1.4%) were D+/R+, and 294 (0.2%) were D+/R-.
3.1 Use of HCV treatment
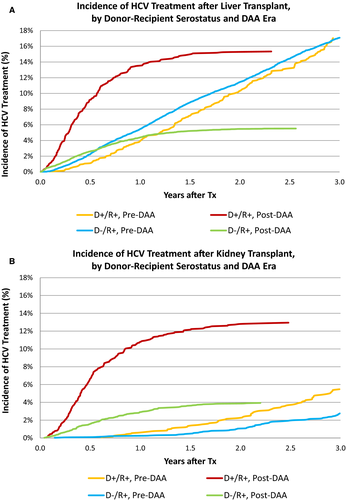
Overall, 12.9% of patients undergoing LT received HCV medications within 3 years after transplant. Among serologic subgroups, treatment prevalence varied from 0.75% of HCV D-/R- patients to 35.2% of D+/R+ patients (Figure 1A). In the pre-DAA era, 4.0% of HCV D+/R+ and 5.5% of D-/R+ received HCV treatment within 1 year. Post-DAA, 13.5% of D+/R+ patients and 4.4% of D-/R+ patients received treatment within 1 year. By 3 years, 17.0% D+/R+ and 17.1% D-/R+ patients received treatment in the pre-DAA era. Post-DAA, 15.3% of D+/R+ and 5.5% of D-/R+ recipients had received treatment at the end of follow-up (2.3 years and 2.6 years, respectively). Clinical factors associated with use of HCV treatments pre-DAA include male sex (adjusted hazard ratio [aHR] 0.830.911.00), diabetes (aHR 0.800.890.98), hypertension (aHR 1.031.131.25), higher model for end-stage liver disease (MELD) score (15-30, aHR 1.011.101.20; >30, aHR 1.001.181.38 vs MELD<15), and black race (aHR 0.720.810.90). Post-DAA, donor HCV+ status (aHR 1.661.952.29), higher MELD score (15-30, aHR 1.231.421.65; >30, aHR 1.431.812.28), and black race (aHR 0.650.790.96) were associated with the likelihood of HCV treatment (Table S2A).
The impact of payer on LT recipient access differed by DAA era. Pre-DAA, HCV+ LT recipients with private insurance were equally likely to be treated with HCV medications (Figure 2A). After adjustment for donor and recipient characteristics, HCV+ recipients with private insurance were somewhat more likely to be treated (aHR 1.011.101.19). However, in the post-DAA era, both D+/R+ and D-/R+ recipients with private insurance were significantly less likely to receive HCV treatment (Figure 2B). Nearly 20% of D+R+ recipients with public insurance, compared with 11% of recipients with private insurance (P < .0001), received treatment. After adjustment for other donor and recipient characteristics, D+/R+ recipients with private insurance were 45% less likely than publicly insured recipients to receive HCV treatment (aHR 0.430.550.71, P < .0001). Among D-/R+ recipients, 6.2% with public insurance received DAA treatment, compared with 5.0% with private insurance (aHR 0.740.840.96, P = .01).
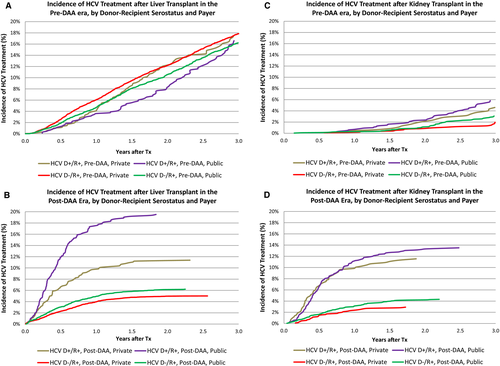
As expected, HCV treatment was substantially less common in KT recipients; overall, 1.4% received HCV treatments within 3 years of transplant. Utilization was highest, at 22.8%, for D+R+ recipients; 11.3% of D-/R+ recipients received treatment (Figure 1B). Pre-DAA, 2.8% of D-/R+ and 5.5% of D+R+ recipients received treatment with HCV medications within 3 years. Post-DAA, 3.9% of D-/R+ and 12.9% of D+/R+ recipients received treatment (P < .0001). Differences by payer in the post-DAA era were modest. Among D+R+ recipients, 13.5% with public insurance received treatment compared with 11.5% with private insurance (P = .35). Among D-/R+ recipients, 4.3% with public insurance received treatment, compared with 2.9% with private insurance (P = .05). After adjustment for donor and recipient characteristics, HCV treatment in R+ D+ KT patients was more likely than D- patients before and after DAA introduction (Pre-DAA: aHR 1.432.02 2.84 vs Post DAA aHR: 2.312.93 3.71). In the post-DAA era, other patients whose race was not black or white and older patients were more likely to be treated (Table S2b).
3.2 Impact of HCV treatment on patient and graft survival
LT outcomes among all HCV+ recipients have improved in the post-DAA era (Figure 3A). Among all HCV+ LT recipients, after adjustment for donor and recipient characteristics, HCV requiring treatment pre-DAA was associated with a significantly higher risk of posttransplant mortality (aHR 1.471.681.91, P < .0001) and all-cause graft failure (aHR 1.641.852.10, P < .0001) (Figure 4A). In contrast, there was not increased risk of death or graft failure post-DAA (aHR for death: 0.740.941.19, P = .61; aHR for graft failure: 0.740.941.19, P = .62) (Figure 4; Table S3A). In D-/R+ recipients, HCV treatment pre-DAA was not associated with an increased risk of death (P = .96) or graft failure (P = .40). Among D+/R+ post-DAA, the reduction in the risk of death (aHR 0.340.671.32, P = .25) and graft failure (aHR 0.320.641.26, P = 0.20) within 3 years of transplant was not statistically significant (Table S3B).
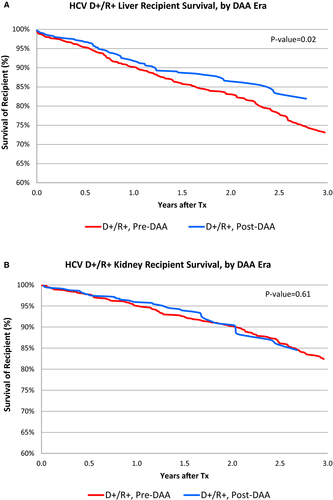
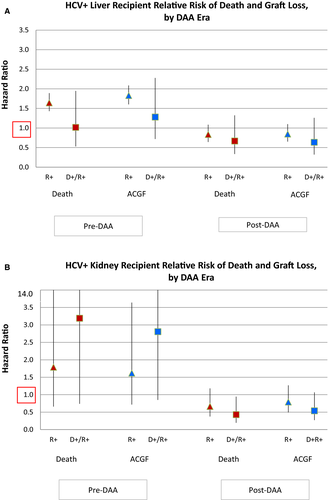
Among HCV+ KT recipients, there was no significant improvement in unadjusted patient survival post-DAA compared with pre-DAA (Figure 3B). In the adjusted analysis, pre-DAA HCV treatment was associated with a non–statistically significant increased risk of death (aHR0.67 1.804.87, P = .25) and graft failure (aHR 0.711.613.62, P = .24) (Figure 4B; Table S4A). There was a nonsignificant protective effect of treatment for KT recipients post-DAA for mortality (aHR 0.370.661.18, P = .16) and graft failure (aHR 0.490.791.27, P = .33) (Table S3; Table S4A). Among D+R+ KT recipients, DAA treatment was associated with nonsignificant reduction in graft failure (aHR 0.270.54 1.07, P = .08) and a statistically significant lower risk of death after KT (0.190.430.95, P = .04). (Table S4B)
3.3 Economic analysis
The cost of HCV treatment increased dramatically for LT and KT recipients in the DAA era. The mean direct cost of HCV treatment for D+R+ recipients after LT increased from $9772 USD pre-DAA to $120 096 USD in 2014-2017 (P < .0001). For KT, cost of treatment increased from $4489 USD to $106 747 USD (P < .0001).
4 DISCUSSION
Introduction of DAAs has markedly improved care for LT and KT recipients with chronic HCV infection. Among LT recipients, our data support findings from smaller clinical studies that suggest that DAAs in the posttransplant setting improve posttransplant outcomes. In the pre-DAA era, HCV reinfection after LT was universal and treatment was generally reserved only for recipients with early aggressive recurrence resulting in fibrosing cholestatic hepatitis C or other complications. Treatment was often ineffective, resulting in accelerated graft loss and death, as confirmed in this analysis. DAAs, by contrast, are well tolerated and recommended for all actively infected patients.7, 9, 28 Based on national data and early follow-up, there is a consistent pattern of improved outcomes in LT recipients with HCV treated with DAAs. In KT recipients, treatment of HCV is less common than in LT recipients. However, a similar protective effect is noted in D+/R+ KT recipients, who have reduced mortality and, likely, graft loss if they receive posttransplant DAAs. Access to these expensive medications, however, appears limited, as <20% of D+/R+ LT and KT recipients receive them, and DAA treatment rates appear to be even lower among privately insured patients.
The availability of effective posttransplant HCV treatment allows LT and KT recipients to time treatment to achieve the greatest benefit.24, 29 Pretransplant patients who are precirrhotic or who have well-compensated disease may benefit from early HCV treatment with stabilization or regression of chronic liver disease, potentially avoiding LT entirely. Ahmed et al recently reported a Markov analysis comparing delayed or immediate HCV treatment among patients waiting for LT.30 The benefit of HCV treatment varied according to clinical condition. Among patients with decompensated liver disease, pre-LT treatment was associated with improved survival (9.3 vs 8.7 quality-adjusted life-years) but higher costs ($304 800 USD vs $283 789 USD). Results were sensitive to MELD score at evaluation, such that pre-LT treatment was of less benefit to patients with greater decompensation. Decompensated patients may be further disadvantaged by pre-LT treatment, because the availability of HCV-infected donor organs has historically been significantly greater, allowing earlier transplant. Among patients with stable liver disease who undergo transplant due to hepatocellular carcinoma, immediate HCV treatment was associated with a gain of 11.5 quality-adjusted life-years vs 10.4 for delayed treatment; however, healthcare expenditures were increased by $82 000 USD per patient. Our data suggest that HCV treatment after transplant is no longer associated with a decrement in graft or patient survival, and may in fact be protective. In light of the organ shortage, reserving treatment of patients with decompensated cirrhosis until after LT may be clinically and economically beneficial, provided they have access to DAAs after transplant.
Dialysis patients infected with HCV are frequently encouraged to seek DAA treatment before transplant despite demonstrated efficacy of DAA in the posttransplant setting.13, 31 In recipients with available, compatible live donors, this strategy can be justified as it allows viral clearance prior to transplant, thereby avoiding potential posttransplant drug-drug interactions and mitigating risk of any early HCV-related complications (for example, new-onset diabetes). However, patients who are waiting for deceased donor organs may delay HCV treatment until after transplant to allow greater access to HCV+ donor organs, which is associated with a marked reduction in expected waiting times.32 Importantly, these data are the first to suggest improved patient and allograft survival among HCV+ KT patients who are treated with DAAs early posttransplant, yet <15% of patients who receive a HCV D+ organ received timely DAA treatment.
The cost of HCV therapy with DAAs increased markedly compared with interferon and ribavirin. The cost of care is further increased for HCV patients with co-existing organ dysfunction. Patients with CKD or ESRD who require HCV treatment incur fourfold higher per member per month costs than HCV patients without ESRD ($5481 USD vs $1922 USD, P < .001). Despite these high costs, DAA treatment in the majority of patients has been found to be generally cost effective, with some regimens characterized as cost saving in the nontransplant population.33, 34 While early treatment regimens, such as those identified in this data set, were very expensive, competition from newly released agents has dramatically reduced the cost of treatment in an effort to increase market share. Treatment costs have also been reduced after shorter durations of care and have been demonstrated to be equally effective. A recent meta-analysis assessing the cost effectiveness of pretransplant treatment revealed that 71% of analyses found second-generation DAAs to be cost saving and 22% cost effective, while only 7% were not cost effective. Further savings may be expected through reduced graft loss and need for retransplantation (LT) and HCV-related kidney disease. Because use of kidneys from HCV-infected donors can markedly reduce wait times for transplant for HCV-infected kidney candidates, the cost-saving realized by a shorter dialysis burden in these patients must be accounted for as well, especially in regions associated with lengthy waiting times.35 It is therefore unclear why access to treatment with DAAs should be limited for transplant recipients, who are even more likely to benefit from treatment than dialysis patients.24, 36
This analysis has several key limitations. First, we lack information regarding viral load and genotype in both the pre- and posttransplant setting. Therefore, it is impossible to determine definitively which patients were actively infected at the time of transplant. This is particularly important in the current era, as HCV seropositivity in the absence of a positive NAT is consistent with virological cure, either related to prior administration of efficacious therapy or spontaneous clearance. This may account for the lower rate of utilization of HCV treatment in the post-DAA era among D-/R+ recipients. Second, the price of HCV medications has fallen since 2016 as new medications have been developed and marketed. This competition has resulted in somewhat lower costs for treatment regimens than reported in this analysis. Therefore, the reduced treatment access for privately insured patients that we found may have improved, as insurance companies start to develop new policies relating to DAAs. Third, despite our assembling the largest cohort reported of treatment in HCV+ recipients, the number of presumptively viremic patients (D+/R+) is still limited in this national study. This may limit inferences about the impact of treatment and comparisons of treatments. Additional data with longer periods of follow-up may provide important insight into the benefits of these treatments. Finally, among KT patients, the severity of HCV-related liver disease, an important outcome determinant, was unknown. However, in this large-scale analysis, it is unlikely to have differed between eras.
In conclusion, HCV treatment patterns have changed with the introduction of highly effective DAAs. Fewer HCV D-/R+ LT patients are treated in the posttransplant setting, because many may have been treated prior to transplant, while rates have increased for D+/R+ patients. In contrast to the reduced survival observed in LT patients treated in the pre-DAA era, HCV treatment with DAAs was not associated with poor outcomes in D+/R+ HCV+ LT recipients. Future studies capturing larger samples may demonstrate improved survival following DAA treatment. In KT patients, HCV treatment remains rare, but is more common in the DAA era and appears to improve outcomes in HCV+ KT recipients. Finally, DAAs were associated with a 10-fold increase in the cost of HCV treatment after LT and KT compared with medications in the pre-DAA era. These data suggest that patients with private insurance have reduced access to DAAs, which may result in higher rates of graft failure and death in patients who receive HCV+ donor organs or remain viremic at the time of transplant. Further study is needed to determine the optimal time and treatment strategy for transplant candidates and recipients infected with HCV.
ACKNOWLEDGMENTS
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing. This work was supported by grants from the Saint Louis University Liver Center and the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK102981.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




