DC Metabolism Controls Lung T Cell Polarization
Abstract
Dendritic cells’ metabolism determines their adjustment to specific tissues and the type of adaptive immunity they instruct following inflammatory challenges.
, , , et al. mTOR regulates metabolic adaptation of APCs in the lung and controls the outcome of allergic inflammation. Science 2017; 357(6355): 1014-1021.
Summary and Analysis
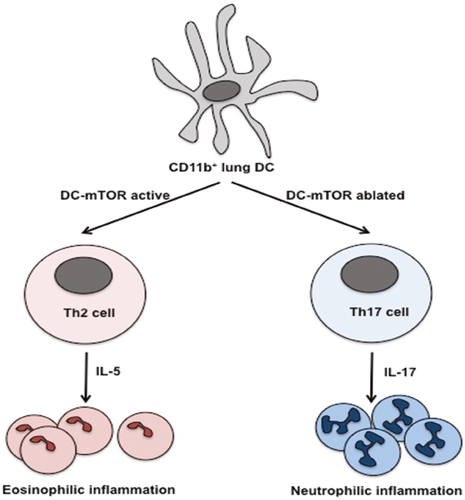
Following intense interest in the transcription factors that program differentiation and effector function of immune cells, the immunology community is now embracing the importance of cell metabolism in these processes. The mechanistic target of rapamycin, also known as the mammalian target of rapamycin (mTOR), composed of mTOR complexes 1 and 2 (mTORC1 and mTORC2), is a serine/threonine kinase that plays a key role in cell survival, proliferation, differentiation and migration, and is inhibited by rapamycin. mTOR integrates input from growth factors, amino acids, oxygen and energy levels with output of protein translation, but also of cell metabolism, as it promotes lipid synthesis and reduces fatty acid oxidation (FAO). Within T cells, mTORC1 promotes T helper (Th)1 and Th17 differentiation, but inhibits induced T regulatory (iTreg) cell differentiation and restrains T cell memory, whereas mTORC2 facilitates Th2 differentiation. The functional consequences of mTOR in dendritic cells (DCs) and antigen-presenting cells (APCs) in vivo are less well understood. To address this question, Sinclair and colleagues generated CD11c-Cre X Mtorfl/fl (MtorΔAPC) mice in which mTOR is genetically ablated in DCs and some macrophage subsets. These mice exhibited abnormal DC subset composition at steady state only in select tissues, and exaggerated Th17 responses in the inflamed lung, in a manner dependent on abnormal DC metabolism.
DCs can be divided into CD8α+/CD103+ DCs and CD11b+ DCs. The growth factors needed for their homeostatic maintenance, Flt3L and granulocyte macrophage colony-stimulating factor (GM-CSF), are known to activate mTOR. DC presentation of antigen to T cells is essential to initiate adaptive immune responses. Several groups have mapped the transcription factors within DCs that endow them with the ability to polarize T cells, such as DC-Irf8 for Th1, and DC-Irf4 for Th2 and Th17 differentiation. Sinclair and colleagues demonstrate that inhibition of mTOR signaling conditionally in DCs, independently of transcription factor expression, has a profound impact on T cell polarization in select tissues.
At steady state, DC composition in the MtorΔAPC mice was minimally altered in most tissues, but was abnormal in skin and lung, with loss of Langerhans cells but not CD103+ cells in the skin, and loss of CD103+ cells and alveolar macrophages in the lung via increased cell death–dependent, but translation-independent, mechanisms. These data infer a role for tissue-specific factors in imprinting an mTOR-sensitive metabolic phenotype in APCs, and indeed, lung but not spleen APCs displayed a distinctive metabolic signature in the absence of mTOR. Intraperitoneal treatment of wild-type mice with rapamycin or with the inhibitor of lipid anabolism fatostatin recapitulated the loss of alveolar macrophages. Intratracheal challenge with the allergen house dust mite (HDM), which drives a Th2 response that attracts an eosinophilic infiltrate in wild-type mice, resulted in a Th17 response and a neutrophilic infiltrate in MtorΔAPC. Conversely, severity of experimental allergic encephalomyelitis, a Th17-driven model of multiple sclerosis, was not exacerbated in MtorΔAPC mice, further underscoring the tissue-specific impact of this genetic alteration. Neutrophilic lung inflammation was due to an altered function of CD11b+ DCs that made more of the Th17-promoting cytokine interleukin (IL)-23. These DCs had elevated FAO. Its inhibition by etomoxir reduced their activation phenotype and the Th17 polarization in the lung, demonstrating the causal role of altered APC metabolism in the abnormal lung inflammatory response to HDM allergen. The data highlight the importance of the microenvironment in driving tissue adaptation of DCs and reveal how the metabolic program can superimpose on the transcriptional program to modulate DC-dependent adaptive immune activation.
This work has implications for transplantation. mTOR can be activated by damage- and microbial-associated molecular patterns, both of which are present following transplantation of organs bearing commensal flora and undergoing various degrees of ischemia/reperfusion injury. With the increased incidence of obesity and metabolic syndrome, abnormal access of APCs to glucose and fatty acids may modulate mTOR-dependent metabolic programming of donor and host APCs. Additionally, some transplant patients receive rapamycin as an immunosuppressive drug with the intent to reduce T cell proliferation; this treatment may also affect DCs and promote their capacity to drive differentiation of alloreactive Th17 cells, as well as ablate alveolar macrophages. The fact that inhibition of mTOR appears to preferentially impact lung APCs may explain why it is in lung transplantation that Th17 cells have been observed more often in the clinic. Targeting metabolic pathways selectively in DCs may be a therapeutic avenue to reset the balance between homeostasis and inflammation and to tailor immune responses following transplantation.
Dr. Alegre is a professor in the Department of Medicine at the University of Chicago. She is also section editor of “Literature Watch.”