The bridge between transplantation and regenerative medicine: Beginning a new Banff classification of tissue engineering pathology
See also: Lefaucheur et al, Nankivell et al, Haas et al.
Abstract
The science of regenerative medicine is arguably older than transplantation—the first major textbook was published in 1901—and a major regenerative medicine meeting took place in 1988, three years before the first Banff transplant pathology meeting. However, the subject of regenerative medicine/tissue engineering pathology has never received focused attention. Defining and classifying tissue engineering pathology is long overdue. In the next decades, the field of transplantation will enlarge at least tenfold, through a hybrid of tissue engineering combined with existing approaches to lessening the organ shortage. Gradually, transplantation pathologists will become tissue-(re-) engineering pathologists with enhanced skill sets to address concerns involving the use of bioengineered organs. We outline ways of categorizing abnormalities in tissue-engineered organs through traditional light microscopy or other modalities including biomarkers. We propose creating a new Banff classification of tissue engineering pathology to standardize and assess de novo bioengineered solid organs transplantable success in vivo. We recommend constructing a framework for a classification of tissue engineering pathology now with interdisciplinary consensus discussions to further develop and finalize the classification at future Banff Transplant Pathology meetings, in collaboration with the human cell atlas project. A possible nosology of pathologic abnormalities in tissue-engineered organs is suggested.
Abbreviations
-
- HCAP
-
- Human cell atlas project
-
- TEP
-
- Tissue engineering pathology
-
- TET
-
- Tissue engineering transplantation
1 INTRODUCTION
Worldwide approximately 1.2 million people need transplantation for end stage organ failure.1 Current transplant protocols reach fewer than 10% of this number. Regenerative medicine/tissue engineering can rehabilitate suboptimal donor organs, thereby considerably expanding the current donor organ pool, and has the potential to save the remaining 90%, by generating or repairing organs. Rapid advances are also being made in other areas to increase the donor pool, including xenotransplantation,2 tolerance induction,3 ex vivo perfusion,4 organ preservation and banking,5 and more traditional approaches.6 If the field of transplantation is to be increased tenfold or more in size to fill the expanding need for organ replacement, regenerative medicine will be the major cause of this improvement, but there will be many other secondary causes in addition. A hybrid of these different modalities working in concert is likely the most appropriate model for the future of transplantation, with tissue engineering/regenerative medicine approaches eventually becoming the dominant approach (Table 1).
| A. Initiatives Optimizing/Improving Allotransplantation as It Is Practiced Now |
|---|
| Tolerance induction |
| Presumed consent |
| Paired exchange |
| Xenotransplantation |
| Cryonic Preservation |
| Improved tissue typing |
| Desensitization therapy |
| Use of HIV and HCV positive donors |
| Buying organs |
| Organ donation after euthanasia |
| B. Initiatives Increasing Organ Supply/Repair Capabilities through Regenerative Medicine/Tissue Engineering—Possible Elimination of Rejection as a Consideration |
| Organ scaffolds |
| 3D printed organs |
| Stem cell repair of organs in vivo |
| Ex vivo perfusion with stem cell repair |
| Practical use of organoids |
| De novo growing of organs simulating embryogenesis |
| Synthetic scaffolds better than natural ones |
| Better understanding of matrix factors |
| Human cell atlas approaches |
| Liquid biopsy approach |
The future safety and efficacy of bioengineered tissues and organs is crucial to long-term transplantation success. It cannot be assumed that every rehabilitated or stem cell-generated organ has normal structure and function and will provide net benefit to the patient. Indeed a first branch point in a taxonomy of engineered organs—be they repopulated scaffolds or cells on chips—is distinguishing deviations from a gold standard healthy functioning native organ arising from engineering decisions or problems of generation, versus problems of deterioration in situ. Pathologic examination of organs produced by regenerative medicine/tissue engineering before transplantation and monitoring after transplantation will be crucial to the success of this new medical discipline. Some of this monitoring will be via soluble biomarkers,7 or possibly employing implantable sensors as well as by more traditional pathology techniques.
In this paper, we define a new pathology discipline, tissue engineering pathology, and propose a basic construct for a new Banff classification of this new discipline, with the aim of having the first full version of the classification finalized through consensus discussions at the 2021, 2023, and 2025 Banff allograft pathology meetings. It will be along organ lines, similar to the current Banff classification scheme8-10 but with a focus entirely separate from the current scheme. Since it is likely that the new Tissue Engineering Pathology classification will overlap with the current Banff classification for at least a decade, it is important that it be constructed in a fashion that will not be confused with the current Transplant Pathology classification, so both classifications can easily be scored simultaneously.
2 HISTORY AND BACKGROUND
The first publication in transplantation pathology, an article about transplantation of embryo tissue by Nobel laureate Peyton Rous, appeared in 1910.11 The first Banff allograft pathology meeting was in 1991, representing the start of the Banff Classification of Transplant Pathology and Banff Consensus Meetings.8-10 Remarkable progress in transplantation pathology has been made over the past 107 years and particularly the past 26 years. The creation of a Banff Classification of Tissue Engineering would be an important new development in the Banff Consensus Process.
The science of regenerative medicine is arguably older than transplantation—a first major textbook was published in 1901 by Nobel Laureate Thomas Hunt Morgan12 and another by Korshelt on regeneration and transplantation in 1927.13 A major tissue engineering/regenerative medicine meeting took place in 1988 with proceedings published in 1989.14, 15
The history of tissue engineering pathology (TEP) goes back to the 1967 article by Hubacek et al. on reaction to scaffold material.16 The use of the term “tissue engineering” began in 1988 with an article by Vacanti et al. co-authored by pathologist Antonio Perez-Atayde.15, 17 Pathology has been part of tissue engineering from its inception but it makes sense to delineate TEP as a distinct area of pathology now that clinical trials are commencing and the number of publications is increasing rapidly.
Clinical trials of encapsulated islet cells are underway18 and trials with a bioartificial kidney with silicon filter and human cells are slated to start by the end of 2017.19 The range of clinically useful bioengineered constructs is very broad and even includes vascular conduits for hemodialysis without cells.20
3 REGENERATIVE MEDICINE—TISSUE ENGINEERING TRANSPLANTATION (TET) DEFINED
Regenerative medicine—tissue engineering transplantation (TET) is the use of various combinations of bioengineered organs, bio-artificial organs, interventional extra-corporeal normothermic machine perfusion, techniques to enhance stem cell repair in tissues and xenotransplantation to solve the problem of organ shortage and tissue repair once and for all. These techniques may also hold the promise of reducing or avoiding rejection, since the organs implanted could be made more or less genetically identical to the recipient, or protected from rejection through bio-modification or encapsulation. Extra-corporeal perfusion allowing ex vivo repair and rehabilitation of organs to be transplanted is another aspect of regenerative medicine, as is the production of organoids: simplified organs in miniature created by stem cells or progenitor cells.21
The number of publications per year in these fields shows that TET is no longer something of the distant future; it is occurring right now with PubMed “regenerative medicine” publication numbers in 2016 (6216) nearly doubling those in 2013 (3334). A PubMed search on words like “Decellularized” shows how the field has evolved over the past two decades. Table 2 shows the related regenerative medicine standards that exist, in the areas of scaffolds, bioengineered bone, tendon, and meniscus.
| Optimization and Critical Evaluation of Decellularization Strategies to Develop Renal Extracellular Matrix Scaffolds as Biological Templates for Organ Engineering and Transplantation, Caralt et al., Am J Transplant 15: 64-75, 2015. |
|---|
| ASTM F-2529-13 Standard Guide for In Vivo Evaluation of Osteoinductive Potential of Materials Containing Demineralized Bone. https://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/UCM434312.pdf |
| Histopathological scores for tissue-engineered, repaired and degenerated tendon: a systematic review of the literature, Loppini et al., Curr Stem Cell Res Ther 2015;10(1):43-55. |
| Histological scoring systems for tissue-engineered, ex vivo and degenerative meniscus, Longo et al., Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1569-1576. |
4 TISSUE ENGINEERING PATHOLOGY (TEP) DEFINED
Similar to traditional transplantation pathology, TEP consists of all possible pathological abnormalities that might be encountered in the organs or tissues, before, during, or after regenerated organs or tissues are implanted in a recipient: examples include tissue and immunological reactions to scaffold material, missing cells or cells in the wrong places, or a cell population that has not had sufficient time to expand and differentiate. Lack of long loops of Henle is common when kidneys are made from re-aggregated fetal cells in animal models22 and might lead to potentially fatal massive polyuria, but it remains to be seen whether the altered physiology this brings about23 cannot be easily managed by other means. A common challenge across all vascularized solid organs “grown” from scaffolding is establishing the microvascular network that will properly support “energy-hungry” parenchymal cells (cardiac myocytes, hepatocytes, kidney tubular cells) and avoid impaired function, as might occur with long diffusion distances in lungs. Abnormalities of the nerves in engineered tissue could lead to cardiac arrthymias or seizures from epileptogenic foci in the brain. Abnormal growth could lead to teratoma formation or other malignancies.
The microscopic appearance of bioengineered organs may continue to evolve after implantation. Pathologic examination would be of interest both before implantation and at multiple times after implantation. Where frequent biopsies were not practical or safe, information about the evolving state of the organ could be obtained through soluble biomarkers, cytology, and/or intravital microscopy. Morphology could change considerably after implantation, either deteriorating from normal after implant or normalizing from abnormal, which could result from intrinsic or host-derived stem cell differentiation. In vivo the matrix used to engineer the organ might undergo complete remodeling and the cells could behave quite differently from the way they behaved before implantation.
5 TISSUE ENGINEERING PATHOLOGY AND THE 21ST CENTURY CURES ACT
Section 3033 of the United States 21st Century Cures Act states that a drug is eligible for regenerative medicine advanced therapy (RMAT) designation if it meets certain criteria for efficacy. One can imagine tissue engineering pathology scores and standards becoming a part of submissions requesting such designations.
Current approved therapies are shown in Table 3.
| A. Licensed Cellular Products |
|---|
| 1. Carticel (Autologous Cultured Chondrocytes): For repair of cartilaginous defects of the femoral condyle |
| 2. Provenge (sipuleucel-T): Autologous T-cell immunotherapy for treatment of prostate cancer |
| 3. Laviv (Azficel-T): Autologous fibroblasts for nasolabial fold wrinkles |
| 4. Gintuit (Allogeneic Cultured Keratinocytes and Fibroblasts in bovine collagen): For treatment of mucogingival conditions |
| 5. Maci (Autologous Cultured Chondrocytes on porcine collagen membrane): For repair of cartilage defects of the knee |
| B. Approved Cellular Products (Class III Devices) |
| 6. Dermagraft-TC Organogenesis (Advance Biohealing) PMA/1997 |
| 7. Apligraf (Graftskin) Organogenesis PMA/1998 Human keratinocytes and fibroblasts as skin substitute |
| 8. OrCel Ortec International PMA & HDE/2001 Allogeneic human skin keratinocytes and fibroblasts as skin substitute |
| 9. Dermagraft Organogenesis (Advance Biohealing) PMA/2001 Cryopreserved human fibroblast-derived dermal substitute |
| 10. Epicel Vericel (Genzyme Biosurgery) HDE/2007 autologous cultured keratinocytes |
| C. Point of Care Device for Cellular Therapy (Class III Device) |
| 11. CliniMACS Miltenyi Biotech, Inc HDE/ 2014 For obtaining CD34 + enriched cells from allogeneic HLA-identical sibling donor for reconstitution in AML patients. |
6 COMPROMISES—“GOOD ENOUGH PATHOLOGY”
Many abnormalities in bioengineered organs in animals are qualitatively distinct from abnormalities seen in transplanted or native human organs of today. These often include a microvasculature inadequate to support functional parenchymal cells, missing cells, cells in the wrong places, misshapen structures, or structures that appear perfect by light microscopy but do not properly function.24, 25 As procedural limitations are overcome, these abnormalities in function and pathology will decrease. Until then, these artifacts must be considered part of the disease classification, with the eventual aim of eliminating them. Remuzzi et al.25 highlight “the major physical barriers that limit in vitro recellularization of acellular kidney scaffolds” (getting enough of the right cells to the right places), “the nonuniform focal cell seeding, and the limited cell proliferation with culture time.” We presume these barriers will be overcome in the next six years and then the nascent Banff classification of tissue engineering pathology will change substantially and outlooks overall will improve. The situation is analogous to the situation that existed in transplantation in the beginning when hyperacute rejection was a common threat and immunosuppression was much less effective in controlling acute rejection.26 In an analogous way, the present barriers to success will be overcome and then the classification project this paper describes will be increasingly needed.
7 EXAMPLE #1 OF TISSUE ENGINEERING PATHOLOGY, THE DECELLULARIZED RECELLULARIZED RODENT KIDNEY
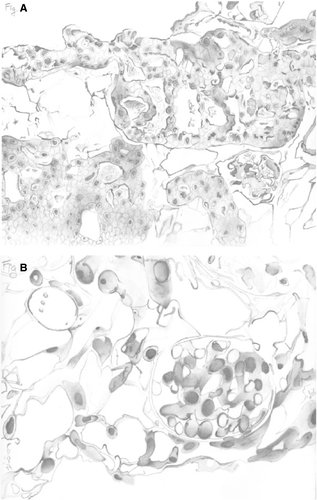
“We had quite a few kidneys blow up in the jar” Harald Ott says at minute 2:33 of the Nature Medicine video.24, 27 It never sounded easy, but those bioengineered rat kidneys that survived the seeding procedure and began functioning had a myriad of morphologic abnormalities that helped shape our thinking about a classification of tissue engineering pathology (Figure 1A,B). An important insight is looking at the recellurized organ “from a device perspective” and thinking about what specific functions it might serve (course video from minute 9).28 The work of Remuzzi et al25 provides considerable additional data on this rat model and suggests that in the first instance the recellularized organ might be better at filtration than other functions since so many of the cells infused seem to end up in the glomerulus.
8 EXAMPLE #2 HUMAN KIDNEY EXTRACELLULAR MATRIX SEEDED WITH AMNIONIC FLUID STEM CELLS
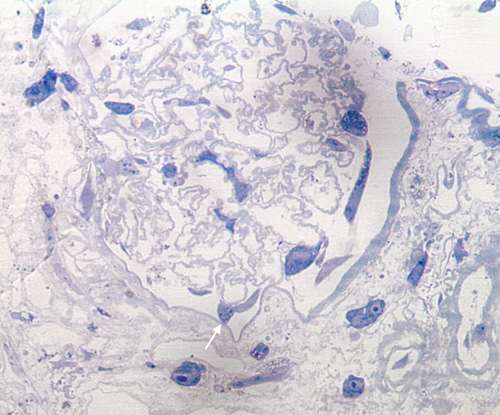
It is of natural interest to see what can be accomplished with human cells infused into human tissues and so the studies of Petrosyan and Perin29 are worth of comment. These authors infuse human amnionic fluid stem cells into decellularized discarded human kidney scaffolds and then allowed culture periods of up to six weeks. The results suggest that “podocytes wandering in the interstitium” is not just a feature of rodent models, but will also be seen with human constructs (Figure 2).
9 A PLAN FOR ACTION
At the 2017 Banff meeting in Barcelona, consideration of TEP matters was added permanently to the mandate of the individual organ chairs for the Banff Transplant Pathology meetings. TEP will be the subject of the day-long pre-meeting that will open the 2019 Banff Meeting in Pittsburgh, September 23-29, 2019. We anticipate the Banff Foundation in the years 2021-2025 will transition to include this new discipline with specific funding from regenerative medicine funding agencies and other sources. Regenerative medicine sessions will be included in the Banff meetings and regenerative medicine experts on the Banff Foundation board, to foster collaboration with regenerative medicine organizations such as TERMIS, CTRMS, and the Regenerative Medicine Community of Practice of the AST. We expect the Banff Classification of Tissue Engineering Pathology to be fully evolved and operational by 2027.
- Are the new blood vessels sufficient to sustain the parenchyma? (This seems to be a major obstacle in many organs.)
- Are too many missing cells and misshapen structures for the organ to function adequately? (Figure 1A.)
- Are there too many cells in the wrong places (e.g. podocytes in the interstitium) (Figure 1B).
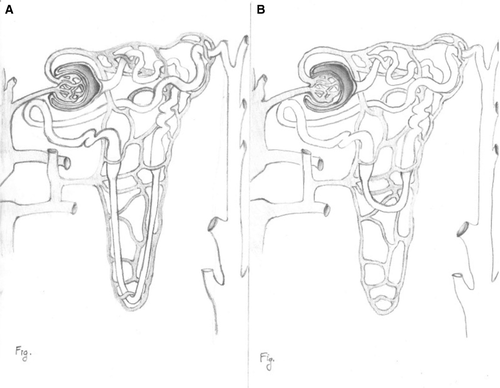
- Are there missing structural elements that represent a risk to the patient? (missing long loops of Henle that could cause lethal polyuria through inability to concentrate the urine, absence of a biliary drainage system in the liver) (Figure 3).
- Is there too much endothelial disruption for the organ to be properly perfused?
- Are there conventional morphological clues that portend neoplastic transformation?
- Are podocytes able to produce glomerular basement membrane in cooperation with glomerular endothelial cells and is the basement membrane normal enough to allow for satisfactory filtration? This is the “holy grail” in tissue engineering of the kidney.
One cannot assume that all important questions can be answered through routine morphologic examination. Some important questions of stem cell–generated tissue and organ suitability for transplantation may be best answered through biomarkers (see Supplementary Material)7 or by using genetic analysis, single cell genomics, intravital microscopy, immunofluorescence, or electron microscopy. It is not possible to predict in advance which modalities of examination will prove to be most important clinically in the practice of the new discipline of TEP. Indeed, we will likely be exploring structure-function relationships in a whole new way in regenerative medicine transplantation.
There are some quantitative concepts that are valuable. The kidney contains more than 26 types of cells.30 In a recent review Petrosyan et al29 asks: Do they all need to be there in a bioengineered kidney? What are the consequences if they are not? The problems with providing a reliable blood supply for bioengineered constructs are not unlike the difficulties encountered with hyperacute rejection in the early days of transplantation.26 The changes were very dramatic, and occurred rapidly, and some people talked of giving up the idea of transplantation. But today one never sees hyperacute rejection. It is possible that the blood supply problems of bioengineered organs may also be overcome by new scientific advances in the near future. The myriad options for cell types, cell delivery, and matrix choices Petrosyan et al have identified29 suggest the need for an artificial intelligence/big data approach to deciding how to construct bioengineered organs.
- Normal, no abnormalities found
- Abnormalities of unknown functional significance
- Abnormalities which will impair the main functions of the organ
- Abnormalities leading to severe organ dysfunction where function may not be great enough to sustain life
- Potential neoplastic abnormalities
Distilled to its essence, an important central idea behind the Banff classification of TEP is determining whether the “right cells are in the right places” in the bioengineered organ, and whether function and intrinsic cellular structure are adequate. Partnering with the Human Cell Atlas Project (HCAP) (see below) will allow one to accurately determine what cell types are normally present in an organ and how the cell population in a bioengineered organ might differ from that.
10 THE LARGER CONTEXT: INTEGRATING TET WITH PROJECTS SUCH AS THE HUMAN CELL ATLAS PROJECT AND “LIQUID BIOPSY” CIRCULATING DNA DETECTION
It would be ideal if the tissue engineering pathology classification we create is not something isolated on its own, idiosyncratic, and based mainly on abnormalities seen in rodent models,24, 25, 27 but fits within a larger human context. A partnership with the newly described human cell atlas project of Aviv Regev and Sarah Teichmann would be highly desirable (see Supplemental Material)32 as would a partnership with “liquid biopsy” systems for detecting circulating DNA in cancer diagnosis.33
The first project of its kind, and as ambitious in scope as the Human Genome Project, … the HCAP aims to chart the types and properties of all human cells, across all tissues and organs, to build a reference map of the … human body.
Single cell analysis can determine type, state, lineage, location, and transitions of cells at a rate of 5000 cells a second for a cost approaching 2.8 cents per cell, with information about DNA, RNA, epigenome, and protein. This project promises to transform research into human development and the progression of diseases and point the way to new diagnostic tools and treatments. Like all new technologies the HCAP approach will be expensive at first but with widespread application costs will come down.
ACKNOWLEDGMENTS
Presented in part in keynote presentations at the 2015 Banff Allograft Pathology meeting in Vancouver and at the 2017 Banff Allograft Pathology meeting in Barcelona, and as an iPoster from the 2016 TERMIS meeting in San Diego https://termis.ipostersessions.com/default.aspx?s=78-58-CE-D4-AB-91-46-64-FF-06-6C-A6-54-AD-8D-D0 and an iPoster at the 2017 CST/CTRMS meeting in Halifax. We are grateful to Drs. Aviv Regev and Sarah Teichmann for advice regarding the Human Cell Atlas collaboration. Supported in part by Roche Organ Transplantation Research Foundation Grant 608390948 to the Banff Foundation for Allograft Pathology. We thank medical student Sina Marzoughi for making suggestions on how to make the paper more accessible and engaging for the general reader.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




