Incidence and outcomes of primary central nervous system lymphoma in solid organ transplant recipients
Abstract
Primary central nervous system lymphoma (PCNSL) risk is greatly increased in immunosuppressed human immunodeficiency virus–infected people. Using data from the US transplant registry linked with 17 cancer registries (1987-2014), we studied PCNSL and systemic non-Hodgkin lymphoma (NHL) in 288 029 solid organ transplant recipients. Transplant recipients had elevated incidence for PCNSL compared with the general population (standardized incidence ratio = 65.1; N = 168), and this elevation was stronger than for systemic NHL (standardized incidence ratio=11.5; N = 2043). Compared to kidney recipients, PCNSL incidence was lower in liver recipients (adjusted incidence rate ratio [aIRR] = 0.52), similar in heart and/or lung recipients, and higher in other/multiple organ recipients (aIRR = 2.45). PCNSL incidence was higher in Asians/Pacific Islanders than non-Hispanic whites (aIRR = 2.09); after induction immunosuppression with alemtuzumab (aIRR = 3.12), monoclonal antibodies (aIRR = 1.83), or polyclonal antibodies (aIRR = 2.03); in recipients who were Epstein-Barr virus–seronegative at the time of transplant and at risk of primary infection (aIRR = 1.95); and within the first 1.5 years after transplant. Compared to other recipients, those with PCNSL had increased risk of death (adjusted hazard ratio [aHR] = 11.79) or graft failure/retransplantation (aHR = 3.24). Recipients with PCNSL also had higher mortality than those with systemic NHL (aHR = 1.48). In conclusion, PCNSL risk is highly elevated among transplant recipients, and it carries a poor prognosis.
Abbreviations
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- aHR
-
- adjusted hazard ratio
-
- aIRR
-
- adjusted incidence rate ratio
-
- CI
-
- confidence interval
-
- CNS
-
- central nervous system
-
- DLBCL
-
- diffuse large B cell lymphoma
-
- EBVE
-
- pstein-Barr virus
-
- HIV
-
- human immunodeficiency virus
-
- ICD-O-3
-
- International Classification of Diseases for Oncology, 3rd edition
-
- NHL
-
- non-Hodgkin lymphoma
-
- PCNSL
-
- primary CNS lymphoma
-
- PTLD
-
- posttransplant lymphoproliferative disorder
-
- SEER
-
- Surveillance, Epidemiology, and End Results
-
- SIR
-
- standardized incidence ratio
-
- SRTR
-
- Scientific Registry of Transplant Recipients
-
- TCM
-
- transplant cancer match
1 INTRODUCTION
Approximately 30 000 solid organ transplants are performed every year in the United States.1 Prolonged immunosuppressive therapy administered to transplant recipients to prevent graft rejection carries an increased risk of cancer in the posttransplant period.2 Non-Hodgkin lymphomas (NHLs) are some of the frequent malignancies in transplant recipients and comprise part of the spectrum of posttransplant lymphoproliferative disorder (PTLD).3
Extranodal involvement in NHLs among transplant recipients is common, but primary involvement of the central nervous system (CNS) is rare.3-6 Primary CNS lymphomas (PCNSLs) affect the brain, leptomeninges, or spinal cord without evidence of any systemic involvement.4, 6 Among individuals infected with human immunodeficiency virus (HIV), PCNSL is a condition that marks the onset of acquired immunodeficiency syndrome (AIDS), and its risk is greatly increased in HIV-infected individuals.7 Few cases of PCNSL have also been reported in patients with primary immunodeficiency disorders, such as Wiskott-Aldrich syndrome and severe combined immunodeficiency disorder.8 However, data on PCNSL risk in other immunosuppressed populations, such as transplant recipients, are lacking.
PCNSLs are usually aggressive diffuse large B cell lymphomas (DLBCLs), and tumor cells are positive for Epstein-Barr virus (EBV) in more than 90% of the cases,9, 10 which differs from PCNSLs in immunocompetent individuals where EBV-related PCNSL cases are uncommon.11 The CNS is an immunologically privileged site, and prolonged suppression of T cell–mediated immunity may allow an EBV-driven oncogenic process.5, 6, 12 PCNSLs often carry a poor prognosis and are associated with high mortality.4, 10, 13-15 Treatment for PCNSL in transplant recipients usually includes reducing the dose of immunosuppressive drugs, which may increase the risk of graft failure.9 Studies on prognosis, however, have been limited by small sample sizes, inclusion of other extranodal NHLs, and absence of evaluation of graft failure as an outcome.16-19
Since PCNSL is a rare malignancy, studies in transplant recipients have mostly been descriptive case series.9, 10, 13, 17-22 The Transplant Cancer Match (TCM) Study provides a unique opportunity to study rare cancers that develop in transplant recipients.2 In this study, we utilized TCM data to describe PCNSL incidence in transplant recipients compared to the general population, evaluate various risk factors for its occurrence, and report on outcomes following PCNSL diagnosis. We also compare PCNSL with NHLs at other sites to provide insights regarding etiology and prognosis.
2 MATERIALS AND METHODS
2.1 Study subjects and ascertainment of NHL
The TCM Study links the US Scientific Registry of Transplant Recipients (SRTR) with population-based cancer registries.2 The SRTR provides information on all US solid organ transplant recipients beginning in 1987, including demographics, medical characteristics, transplanted organs, and induction and baseline maintenance immunosuppressive medications. Record linkages were completed between the SRTR and 17 cancer registries covering approximately 51% of the US transplant population (see Table 1 note). We included transplant recipients residing in the geographic areas covered by the participating cancer registries, and excluded those who had unknown race/ethnicity (N = 1878), a cancer registry diagnosis of NHL before transplantation (N = 609), or SRTR diagnosis of HIV infection (N = 530). This study was approved by human subjects review committees at the National Cancer Institute and, as required, participating cancer registries.
| Characteristics | Number of transplants (%) |
|---|---|
| Total | 288 029 (100) |
| Age at transplantation, y | |
| 0-17 | 21 121 (7.3) |
| 18-34 | 44 001 (15.3) |
| 35-49 | 86 250 (29.9) |
| 50-64 | 108 506 (37.7) |
| 65+ | 28 151 (9.8) |
| Sex | |
| Male | 176 853 (61.4) |
| Female | 111 176 (38.6) |
| Race/ethnicity | |
| Non-Hispanic white | 179 699 (62.4) |
| Non-Hispanic black | 50 073 (17.4) |
| Hispanic | 42 684 (14.8) |
| Asians/Pacific Islander | 15 573 (5.4) |
| Organ transplanted | |
| Kidney | 167 207 (58.0) |
| Liver | 62 405 (21.7) |
| Heart and/or Lung | 41 496 (14.4) |
| Other/multiple | 16 921 (5.9) |
| Year of transplantation | |
| 1987-1994 | 44 128 (15.3) |
| 1995-1999 | 59 657 (20.7) |
| 2000-2004 | 73 225 (25.4) |
| 2005-2009 | 82 152 (28.5) |
| 2010-2014 | 28 867 (10.0) |
- Participating registries with years of coverage: California (1988-2012), Colorado (1988-2009), Connecticut (1973-2009), Florida (1981-2009), Georgia (1995-2010), Hawaii (1973-2007), Illinois (1986-2013), Iowa (1973-2009), Kentucky (1995-2011), Michigan (1985-2009), New Jersey (1979-2010), New York (1976-2010), North Carolina (1990-2010), Pennsylvania (1985-2013), Seattle-Puget sound area of Washington (1974-2014), Texas (1995-2010), and Utah (1973-2008).
Incident NHLs were identified from the linked cancer registries using the International Classification of Diseases for Oncology, 3rd edition codes (ICD-O-3).23 Since most PCNSLs are DLBCL, we assessed only NHLs that were recorded as DLBCL or unspecified NHL (most of which would have been DLBCLs) using ICD-O-3 morphology codes (DLBCL: 9678-9680, 9684, 9688, 9735, 9737; unspecified NHL: 9590-9596, 9675, 9820, 9970). PCNSL cases were then defined using topography codes for the CNS (C70.0-C72.9), while systemic NHLs were the remaining NHLs at other sites.
2.2 Statistical analyses
The follow-up began at transplantation or the start of cancer registry coverage (whichever came later), and ended at the earliest of death, graft failure or retransplantation, loss to follow-up, or the last date of cancer registry coverage. We compared incidence of PCNSL and systemic NHL in transplant recipients to the general population using standardized incidence ratios (SIRs), obtained by dividing the observed number of cases by the expected number in the general population. Since the AIDS epidemic has strongly affected contemporaneous general population rates for PCNSL since 1980,24 we calculated the expected number of cases by applying general population cancer incidence rates for DLBCL and unspecified NHL obtained from the Surveillance, Epidemiology, and End Results (SEER) program for 1973-1979 to person-time at risk among transplant recipients, stratified by sex and age (5-year groups). The comparison to pre-1980 expected counts allows the SIR to better capture the impact of transplantation on increasing risk above that seen in a largely immunocompetent population. We present exact confidence intervals (CI) for the SIRs.
We used Poisson regression to compare PCNSL and systemic NHL incidence among subgroups of transplant recipients, defined by demographic characteristics, transplanted organ, induction and baseline immunosuppressive medications, EBV serostatus at the time of transplantation, and time since transplantation. Factors significantly associated with PCNSL incidence in univariate Poisson models, or otherwise believed to be clinically important, were included in multivariable models for PCNSL and systemic NHL. Although EBV serostatus was not significantly associated with PCNSL in univariate analyses (Table S1), it is an important risk factor for NHL in transplant recipients. Therefore, we included this variable in multivariable analyses. To determine whether risk factors differed for the two NHL types, we compared PCNSL and systemic NHL in a multivariable logistic regression model that included the predictors from the Poisson model.
We evaluated the impact of PCNSL or systemic NHL on transplant outcomes in 2 additional analyses. First, we evaluated whether PCNSL or systemic NHL in transplant recipients is associated with risk of death, graft failure/retransplantation, or the composite outcome using Cox regression. The time scale was time since transplantation, and diagnosis of PCNSL or systemic NHL was included as a time-dependent variable. Adjusted hazard ratios (aHRs) are presented incorporating adjustment for age at transplantation, sex, transplanted organ, year of transplantation, and race/ethnicity. As some transplants could have occurred before the start of cancer registry coverage, we allowed for delayed entry. Second, we compared risk of death among transplant recipients with PCNSL and those with systemic NHL using Cox regression with time since NHL diagnosis as the time scale. This analysis included only the NHL cases. Follow-up continued through any graft failure or retransplantation events and ended at death, loss to follow-up, or the last date of the cancer registry coverage (whichever occurred earlier). We conducted an additional survival analysis adjusted for cancer treatment. The proportional hazards assumption was tested by introducing interaction terms of PCNSL or systemic NHL diagnosis with follow-up time and was met for all models. We plot Kaplan-Meier survival curves to characterize the probability of death following PCNSL or systemic NHL diagnosis. Some cancer registries did not provide complete information on survival or cancer treatment and were excluded from the relevant survival analyses (see Table 3 and Table S2 notes).
We also ascertained EBV status of the NHL tumors. Cancer registries do not collect this information, but it is collected by the SRTR for PTLD cases. We therefore searched for SRTR records on PTLD and, if available, used the EBV data closest in time to the corresponding matched NHL diagnosis in the cancer registry.
3 RESULTS
Among the 288 029 transplants included in the study, the kidney was the most frequently transplanted organ (58.0%), followed by liver (21.7%), heart and/or lung (14.4%), and other/multiple organs (5.9%) (Table 1). A large proportion of transplants occurred between the ages 50-64 years (37.7%), in males (61.4%), and in non-Hispanic whites (62.4%). The median follow-up time for all included transplants was 4.0 years (interquartile range, 1.5-7.7 years).
During follow-up, 168 cases of PCNSL and 2043 cases of systemic NHL were diagnosed (incidence 11.5 and 140.0 per 100 000 person-years, respectively). PCNSL and systemic NHL occurred at a median of 1.7 years and 3.3 years after transplant, respectively. Compared to the general population, incidence was substantially elevated for both PCNSL (SIR = 65.1; 95% CI = 55.6-75.7) and systemic NHL (SIR = 11.5; 95% CI = 11.0-12.0).
As shown in Table 2, PCNSL incidence appeared to increase with age but the differences were not significant. PCNSL incidence did not differ by sex, while Asians/Pacific Islanders had 2-fold higher incidence (adjusted incidence rate ratio [aIRR] = 2.09; 95% CI = 1.24-3.52) than non-Hispanic whites. Compared with kidney recipients, risk was lower for liver recipients (aIRR = 0.52; 95% CI = 0.31-0.92), not different for heart and/or lung recipients, and higher for other/multiple organ recipients (aIRR = 2.45; 95% CI = 1.54-3.90). Regarding drugs used for induction immunosuppression, PCNSL incidence was higher with administration of alemtuzumab (aIRR = 3.12; 95% CI = 1.57-6.22), monoclonal antibodies (aIRR = 1.83; 95% CI = 1.03-3.28), or polyclonal antibodies (aIRR = 2.03; 95% CI = 1.35-3.06), compared to no induction drugs. Transplant recipients who were EBV-seronegative at the time of transplant had higher PCNSL incidence (aIRR = 1.95; 95% CI = 1.09-3.48) than EBV-seropositive recipients. PCNSL incidence was greatest within the first 1.5 years after transplant (Figure 1), then gradually decreased over time. No significant differences in incidence were observed with respect to body mass index, donor type (for kidney recipients), baseline maintenance immunosuppressive drugs, cytomegalovirus serostatus, or human leukocyte antigen (HLA) mismatches (Table S1).
| Characteristics | Primary CNS lymphoma | Systemic Non-Hodgkin lymphoma | P valuec | ||||
|---|---|---|---|---|---|---|---|
| N | Incidence ratea | aIRRb (95% CI) | N | Incidence ratea | aIRRb (95% CI) | ||
| Total | 168 | 11.5 | 2043 | 140.0 | |||
| Age at transplantation, y | .010 | ||||||
| 0-17 | 10 | 8.4 | 0.83 (0.42-1.66) | 264 | 222.0 | 1.76 (1.50-2.05) | |
| 18-34 | 21 | 8.9 | 0.70 (0.43-1.16) | 293 | 124.2 | 1.24 (1.07-1.44) | |
| 35-49 | 57 | 12.0 | Reference | 485 | 102.5 | Reference | |
| 50-64 | 65 | 12.4 | 1.19 (0.82-1.72) | 789 | 151.1 | 1.43 (1.27-1.60) | |
| 65+ | 15 | 13.7 | 1.22 (0.68-2.19) | 212 | 193.8 | 2.02 (1.72-2.39) | |
| Sex | .137 | ||||||
| Male | 99 | 11.2 | Reference | 1349 | 152.6 | Reference | |
| Female | 69 | 12.0 | 1.07 (0.79-1.46) | 694 | 120.5 | 0.84 (0.76-0.92) | |
| Race/ethnicity | .024 | ||||||
| Non-Hispanic white | 105 | 11.0 | Reference | 1546 | 162.2 | Reference | |
| Non-Hispanic black | 23 | 10.4 | 0.85 (0.54-1.35) | 181 | 81.9 | 0.61 (0.52-0.71) | |
| Hispanic | 23 | 11.1 | 1.05 (0.67-1.67) | 227 | 109.5 | 0.80 (0.69-0.92) | |
| Asians/Pacific Islander | 17 | 21.8 | 2.09 (1.24-3.52) | 89 | 114.2 | 0.88 (0.71-1.09) | |
| Organ transplanted | <.001 | ||||||
| Kidney | 105 | 12.5 | Reference | 863 | 102.5 | Reference | |
| Liver | 17 | 5.3 | 0.52 (0.31-0.92) | 494 | 152.9 | 1.35 (1.19-1.52) | |
| Heart and/or Lung | 22 | 10.1 | 0.93 (0.58-1.51) | 542 | 248.6 | 2.05 (1.83-2.29) | |
| Other/multipled | 24 | 31.4 | 2.45 (1.54-3.90) | 144 | 188.1 | 1.88 (1.56-2.25) | |
| Year of transplantation | .024 | ||||||
| 1987-1994 | 25 | 7.9 | 1.02 (0.57-1.81) | 589 | 185.7 | 1.53 (1.32-1.78) | |
| 1995-1999 | 52 | 12.6 | 1.40 (0.87-2.24) | 562 | 135.9 | 1.11 (0.96-1.28) | |
| 2000-2004 | 42 | 10.0 | Reference | 519 | 123.6 | Reference | |
| 2005-2009 | 45 | 16.7 | 1.16 (0.75-1.82) | 318 | 117.8 | 0.82 (0.71-0.95) | |
| 2010-2014 | 4 | 10.2 | 0.51 (0.18-1.46) | 55 | 140.3 | 0.74 (0.55-0.98) | |
| Induction therapy | .017 | ||||||
| No induction therapy | 69 | 7.9 | Reference | 1298 | 148.5 | Reference | |
| Alemtuzumab only | 12 | 31.3 | 3.12 (1.57-6.22) | 59 | 154.0 | 1.56 (1.18-2.07) | |
| IL-2 receptor antagonists only | 21 | 10.0 | 1.07 (0.63-1.82) | 233 | 111.2 | 0.94 (0.81-1.10) | |
| Monoclonal antibody only | 15 | 18.9 | 1.83 (1.03-3.28) | 146 | 184.0 | 1.28 (1.08-1.53) | |
| Polyclonal antibody only | 47 | 19.8 | 2.03 (1.35-3.06) | 291 | 122.4 | 1.06 (0.92-1.21) | |
| Multiple induction drugs | 4 | 19.1 | 1.72 (0.61-4.84) | 16 | 76.3 | 0.66 (0.40-1.08) | |
| EBV serology statuse | .082 | ||||||
| Positive | 45 | 11.1 | Reference | 401 | 98.7 | Reference | |
| Negative | 16 | 18.5 | 1.95 (1.09-3.48) | 270 | 311.5 | 3.01 (2.57-3.52) | |
| Missing | 107 | 11.1 | 1.23 (0.80-1.89) | 1372 | 141.9 | 1.19 (1.03-1.37) | |
| Time since transplantation, y | .001 | ||||||
| 0.01-0.50 | 16 | 12.7 | Reference | 283 | 223.9 | Reference | |
| 0.51-1.00 | 35 | 30.2 | 2.36 (1.31-4.26) | 331 | 285.3 | 1.27 (1.09-1.49) | |
| 1.01-1.49 | 30 | 27.8 | 2.17 (1.18-3.98) | 144 | 133.7 | 0.59 (0.48-0.72) | |
| 1.50-2.00 | 11 | 10.9 | 0.85 (0.39-1.83) | 71 | 70.4 | 0.31 (0.24-0.40) | |
| 2.01-3.00 | 11 | 6.1 | 0.47 (0.22-1.02) | 139 | 76.8 | 0.33 (0.27-0.41) | |
| 3.01-5.00 | 17 | 5.9 | 0.47 (0.23-0.92) | 273 | 95.1 | 0.40 (0.34-0.48) | |
| 5.01-10.00 | 38 | 9.8 | 0.81 (0.44-1.47) | 509 | 131.6 | 0.52 (0.45-0.61) | |
| ≥ 10.01 | 10 | 6.5 | 0.57 (0.25-1.29) | 293 | 190.1 | 0.66 (0.55-0.79) | |
- Underlined values were considered to be statistically significant at P < .05.
- aIRR, adjusted incidence rate ratio; CI, confidence intervals; CNS, central nervous system; EBV, Epstein-Barr virus; NHL, non-Hodgkin lymphoma
- a Incidence rates are reported as events per 100 000 person-years of follow-up.
- b aIRRs were derived from multivariable Poisson regression models that included all the variables in the table.
- c P values were obtained from a multivariable logistic regression model comparing primary CNS lymphoma to systemic NHLs.
- d Other/multiple transplants included pancreas, intestine, kidney-heart, kidney-liver, kidney-pancreas, liver-intestine, pancreas-intestine, and pancreas-liver-intestine transplants.
- e This represent the patients' EBV serostatus at the time of transplantation, and not the EBV status of the lymphomas that occurred in the posttransplant period.
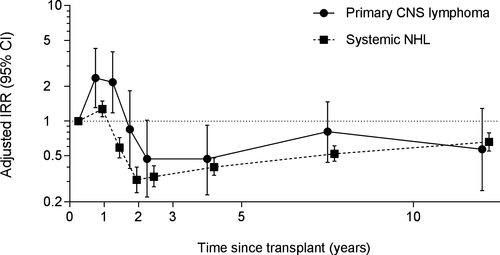
The pattern for systemic NHLs differed from that for PCNSL for some, but not all variables (Table 2). Systemic NHL incidence was high in young transplant recipients (age at transplantation, 0-17 years), decreased with increasing age until 35-49 years, and then increased again, with highest incidence in people ≥65 years at transplantation (Table 2). Incidence of systemic NHLs was lower among females compared to males, and among non-Hispanic blacks and Hispanics compared to non-Hispanic whites. In contrast with PCNSL, a high incidence of systemic NHLs was observed among liver (aIRR = 1.35; 95% CI = 1.19-1.52), heart and/or lung (aIRR = 2.05; 95% CI = 1.83-2.29), and other/multiple organ recipients (aIRR = 1.88; 95% CI = 1.56-2.25), compared to kidney recipients. Incidence declined with calendar year of transplant (aIRR = 1.53 for 1987-1994 and aIRR = 0.74 for 2010-2014, vs. 2000-2004). Risk of systemic NHLs was elevated with induction immunosuppression using alemtuzumab (aIRR = 1.56; 95% CI = 1.18-2.07) or monoclonal antibody (aIRR = 1.28; 95% CI = 1.08-1.53) but not with polyclonal antibody or IL-2 receptor antagonists, compared to no induction drugs. Similar to PCNSL, higher incidence of systemic NHLs was observed in EBV-seronegative recipients (aIRR = 3.01; 95% CI = 2.57-3.52). The pattern with respect to time since transplant was similar between PCNSL and systemic NHL (Figure 1), although the magnitude of the peak in the early posttransplant period was lower in systemic NHL. The patterns for PCNSL and systemic NHL differed for age at transplantation (P = .010), race/ethnicity (P = .024), transplanted organs (P < .001), year of transplantation (P = .024), induction immunosuppression (P = .017), and time since transplantation (P = .001) (Table 2).
EBV status of the tumors could be ascertained for 48 (28.6%) PCNSLs and 771 (37.7%) systemic NHLs. Of these, 43 (90%) PCNSL cases were EBV positive compared to 462 (60%) systemic NHL cases (P < .0001).
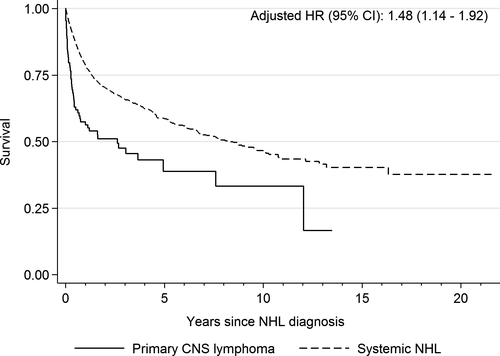
PCNSL was associated with increased risk of death (aHR = 11.79; 95% CI = 9.37-14.83), graft failure/retransplantation (aHR = 3.24; 95% CI = 2.19-4.78), and the combined endpoint of death/graft failure/retransplantation (aHR = 7.01; 95% CI = 5.75-8.55), compared to transplant recipients without any NHL. An elevated risk of these outcomes was also observed after diagnosis of systemic NHLs, but the increase was smaller (Table 3). In an analysis that ignored graft failure and continued to follow NHL patients across multiple transplants, recipients with PCNSL had increased risk of death compared to those with systemic NHLs (aHR = 1.48; 95% CI = 1.14-1.92) (Figure 2). We further evaluated the receipt of treatment after NHL diagnosis and its effect on survival in an analysis restricted to registries that provided treatment information (Table S2). Of the 117 PCNSL cases, 19 (16.2%) received neither chemotherapy nor radiation, 17 (14.5%) received only radiation, 31 (26.5%) received only chemotherapy, 24 (20.5%) received both, and treatment information on 26 (22.2%) cases was unknown. After additional adjustment for cancer treatment, PCNSL remained associated with increased risk of death compared to systemic NHLs (aHR = 1.41; 95% CI = 1.08-1.85). The median survival times after PCNSL and systemic NHL diagnosis were 1.1 and 2.7 years, respectively, while the 5-year overall survival rates for PCNSL and systemic NHL were 35% (95% CI = 24-47%) and 43% (95% CI = 40-46%), respectively.
| Risk factor | Transplantsa | Death | Graft failure/retransplantation | Death/graft failure/retransplantation | |||
|---|---|---|---|---|---|---|---|
| Events (%) | Adjusted HRb (95% CI) | Events (%) | Adjusted HRb (95% CI) | Events (%) | Adjusted HRb (95% CI) | ||
| No NHL | 283 254 | 50 076 (21.4) | Reference | 41 896 (17.9) | Reference | 91 972 (39.3) | Reference |
| Primary CNS lymphoma | 137 | 73 (53.3) | 11.79 (9.37-14.83) | 25 (18.3) | 3.24 (2.19-4.78) | 98 (71.5) | 7.01 (5.75-8.55) |
| Systemic NHL | 1705 | 915 (53.7) | 4.80 (4.50-5.13) | 198 (11.6) | 1.67 (1.45-1.92) | 1113 (65.3) | 3.53 (3.33-3.75) |
- CI, confidence intervals; CNS, central nervous system; HR, hazard ratio; NHL, non-Hodgkin lymphoma
- a Data from 3 cancer registries were excluded since they provided incomplete information on survival for NHL cases.
- b Hazard ratios were adjusted for age at transplantation, sex, race, organ transplanted, and year of transplantation.
4 DISCUSSION
Solid organ transplant recipients have an increased risk of cancer, including NHLs, largely due to iatrogenic immunosuppression.2 In a comprehensive analysis of a large cohort of solid organ transplant recipients, we demonstrate that PCNSL incidence is substantially elevated in transplant recipients compared to the general population. We identified that incidence is higher among transplant recipients who receive certain induction immunosuppression regimens and those who are EBV-seronegative at the time of transplant. In addition, a large fraction of PCNSL tumors were EBV-positive. These results highlight the likely etiologic roles of iatrogenic immunosuppression and EBV infection in the posttransplant period in contributing to the development of PCNSL. We also demonstrated that both systemic NHL and PCNSL adversely affect survival in transplant recipients, but PCNSL carries a worse prognosis.
PCNSL is a rare malignancy and its risk is highly elevated in immunosuppressed individuals such as HIV-infected people.7 Several case series have described primary CNS involvement in PTLDs diagnosed after solid organ transplantation.9, 10, 20, 21, 25-27 However, very few reports in transplant recipients focused on PCNSLs, which are extranodal NHLs and thus comprise a subset of monomorphic PTLDs.17, 18 PCNSL is a distinct clinicopathological disease compared to systemic non-CNS NHLs and the 2008 World Health Classification of lymphoid neoplasms defined “Primary CNS DLBCL” as a new entity.28
In particular, PCNSLs differ in their gene expression and genomic profile compared to systemic NHL.29-33 PCNSL tumor cells are characterized by downregulation or loss of expression of HLA class I and II genes, which may help them escape immune surveillance.29, 32, 33 Along with the vascular endothelial cells in the CNS, these tumor cells also express high levels of interleukin (IL)-4 and STAT6, which support B cell growth.30 A genome-wide gene expression comparison between PCNSL and non-CNS DLBCL identified several signatures unique to PCNSL, including expression of genes related to extracellular matrix and cellular adhesion pathways leading to CNS tropism, B cell migration, lymphoproliferation, and aggressive clinical features.31 In this study, we found some differences between transplant recipients who developed PCNSL and those with systemic NHL in terms of demographic characteristics, transplanted organs, induction immunosuppression, and time since transplantation, further suggesting that PCNSL and systemic NHL are etiologically distinct entities.
For instance, we did not observe an association between age at transplantation and PCNSL incidence independent of recipients' EBV serostatus, which may be due to the small number of PCNSL cases. Nonetheless, a distinct pattern was observed for systemic NHL, where the incidence was high in the youngest (0-17 years) and the oldest (65+ years) age groups. Associations between age and NHL risk in transplant recipients have been described previously,16, 34 and might be related to control of primary EBV infection in children or processes related to aging in older adults. EBV-seronegative transplant recipients have a high risk of acquiring the infection in the posttransplant period.35, 36 Prolonged immunosuppression and lack of EBV-specific T cell immune responses may lead to unchecked monoclonal expansion of EBV-infected B cells and development of lymphoma.37 The elevated incidence of PCNSL among EBV-seronegative recipients in this study, and the increase in incidence within 1.5 years following transplant, support the role of primary EBV infection in contributing to PCNSL. Similar findings have been reported previously for NHLs overall and DLBCL in transplant recipients,22, 34, 38-40 although in our study, the pattern for PCNSL incidence according to time since transplantation was stronger than for systemic NHLs.
To some extent, the pattern in PCNSL incidence also reflects the degree of immunosuppression among transplant recipients. The relatively high incidence associated with “other/multiple” organ transplants and low incidence for liver transplants may be due to differences in the intensity of immunosuppressive regimens. Perhaps surprisingly, however, recipients of a heart and/or lung did not have higher PCNSL incidence than kidney recipients, even though they typically receive intensive immunosuppression. Induction immunosuppressive drugs given immediately posttransplant to prevent acute rejection include anti-CD3 monoclonal antibodies (OKT3), alemtuzumab, IL-2 receptor antagonists, and polyclonal antibodies such as anti-thymocyte globulin.41, 42 We previously reported that alemtuzumab induction was associated with an increased risk of NHL, colorectal cancer, and thyroid cancer among kidney recipients.43 Herein, we demonstrated that PCNSL risk was increased after induction with alemtuzumab, monoclonal antibodies, and polyclonal antibodies. The incidence of systemic NHL was also increased after alemtuzumab and monoclonal antibodies, but not polyclonal antibodies, although it is unclear why this pattern differs between PCNSL and systemic NHL.
We also showed that Asians/Pacific Islanders had more than twice the incidence of PCNSL compared with non-Hispanic whites, while the incidence of systemic NHL was highest among non-Hispanic whites. The reason for this difference is unknown. A previous analysis of SEER data found that PCNSL incidence was similar for non-Hispanic whites and Asians/Pacific Islanders in adults over 50 years of age in the US general population (a largely immunocompetent population among whom the prevalence of HIV-infected individuals and transplant recipients is low).44 Similar analyses for younger individuals in the US population (0-49 years) found a higher PCNSL risk in non-Hispanic blacks, which may be attributed to the higher prevalence of HIV infection in this group.44, 45
We found that PCNSL increased the risk of death and graft failure/retransplantation in transplant recipients compared to recipients without any NHL. The risk was also increased after systemic NHL, but the magnitude was much lower. Furthermore, when we restricted the analysis to NHL cases, transplant recipients with PCNSL had a 1.5-fold increased risk of dying compared to recipients with systemic NHL. PCNSL has a low 5-year survival rate of 30% in immunocompetent individuals.15 The 5-year survival rate after PCNSL was similar among transplant recipients in our study as has been reported for immunocompetent individuals, but lower than for recipients with systemic NHL. PCNSL treatment is centered around the use of high-dose methotrexate-based chemotherapy, and whole-brain radiotherapy (considered to be a standard-of-care until the early 1990s) is often used as a salvage therapy.46 Rituximab, an anti-CD20 monoclonal antibody that has been the mainstay of treatment of systemic NHLs, has poor CNS penetration.47, 48 Furthermore, patients with renal insufficiency, older age, or poor performance status—factors that are often present in transplant recipients—may not be candidates for high-dose methotrexate therapy and may receive palliative whole-brain radiotherapy or supportive care.46 Mortality in PCNSL patients may be caused by involvement of the CNS itself, particularly the deep structures within the brain,49 or delayed neurotoxic effects of therapy.50 Management of PCNSL in transplant recipients has an added complexity as it occurs on the background of iatrogenic immunosuppression. Often, the dose of immunosuppressive drugs may be reduced or drugs may be changed as part of the treatment for PCNSL,9 which can increase the risk of graft failure. We observed a 3-fold elevated risk of graft failure/retransplantation following PCNSL, which contributes to the high mortality.
A strength of our study was the use of a population-based cohort that was representative of US transplant recipients, and the linkage to cancer registries allowed for largely complete and unbiased ascertainment of NHL diagnoses. The large sample size allowed us to evaluate the PCNSL incidence for different patient subgroups. We were also able to compare incidence and outcomes for PCNSL and systemic NHLs. However, our study had some limitations. Due to the rarity of PCNSL, the number of cases was modest, which affected the precision of estimates for some subgroups. EBV serostatus was not available for many transplant recipients, and EBV status of the tumors could not be directly ascertained and was frequently missing, which precluded us from evaluating the role of this virus in detail. We evaluated the risk of NHLs and hence focused on monomorphic PTLD cases; a fraction of PTLD cases affecting the CNS are described as polymorphic PTLD,10 which we did not capture. Also, information on only the initial maintenance immunosuppressive therapy was available, so we could not evaluate the effect of changes in the medication regimen over time.
In conclusion, PCNSL risk is highly elevated in solid organ transplant recipients, particularly within the first 1.5 years after transplant. Although it is a rare malignancy, physicians managing the care of transplant recipients should be aware about this heightened risk, particularly for recipients who are EBV-seronegative at the time of transplant, and those who are treated with alemtuzumab, monoclonal antibodies, or polyclonal antibodies for induction immunosuppression. As some risk factors for PCNSL differ from that of systemic NHL, the possibility of etiologic heterogeneity should be explored in other populations. The prognosis of PCNSL in transplant recipients is poor, pointing to a need to develop improved therapies for this malignancy.
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program of the National Cancer Institute. The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California (Tina Clarke), Colorado (Jack Finch), Connecticut (Lou Gonsalves), Florida (Brad Wohler), Georgia (Rana Bayakly), Hawaii (Brenda Hernandez), Iowa, Illinois (Lori Koch), Kentucky (Jaclyn Nee), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Leticia Nogueria), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Matthew Chaloux, Michael Curry, Ruth Parsons). The SRTR is currently operated under contract number HHSH250201500009C (Health Resources and Services Administration) by the Minneapolis Medical Research Foundation, Minneapolis, MN. Previously the SRTR was managed under contracts HHSH250201000018C and HHSH234200537009C. The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201300019I), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (HSN261201000032C and N01-PC-35143), New Jersey (HHSN261201300021I, N01-PC-2013-00021), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN2612013000171). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP003875-01), Illinois (5U58DP003883-03), Maryland (U58DP12-1205 3919-03), Michigan (5U58DP003921-03), New Jersey (5U58/DP003931-05-00), New York (U58DP003879), North Carolina (U58DP000832), and Texas (5U58DP000824-04). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, Massachusetts (Massachusetts Cancer Prevention and Control Cooperative Agreement 5458DP003920), New Jersey, New York (including the Cancer Surveillance Improvement Initiative), Texas, Utah, and Washington, as well as the University of Utah and Fred Hutchinson Cancer Research Center in Seattle, WA.
DISCLAIMER
The views expressed in this article are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




