The Resurgence of Xenotransplantation
Abstract
There has been an upsurge of interest in xenotransplantation in recent years. This resurgence can attributed to a combination of factors. First, there has been a dramatic improvement in efficacy in several preclinical models, with maximum xenograft survival times increasing to 950 days for islets, 945 days for hearts, and 310 days for kidneys. Second, the rapid development of genome editing technology (particularly the advent of clustered regularly interspaced short palindromic repeats/Cas9) has revolutionized the capacity to generate new donor pigs with multiple protective genetic modifications; what once took many years to achieve can now be performed in months, with much greater precision and scope. Third, the specter of porcine endogenous retrovirus (PERV) has receded significantly. There has been no evidence of PERV transmission in clinical trials and preclinical models, and improved screening methods and new options for the treatment or even elimination of PERV are now available. Balancing these positive developments are several remaining challenges, notably the heavy and often clinically inapplicable immunosuppression required to prevent xenograft rejection. Nonetheless, the potential for xenotransplantation as a solution to the shortage of human organs and tissues for transplantation continues to grow.
Abbreviations
-
- ASGR
-
- asialoglycoprotein receptor
-
- ATG
-
- anti–thymocyte globulin
-
- CRISPR
-
- clustered regularly interspaced short palindromic repeats
-
- CVF
-
- cobra venom factor
-
- GTKO
-
- GGTA1 knockout
-
- h
-
- human
-
- IBMIR
-
- instant blood-mediated inflammatory reaction
-
- IEQ
-
- islet equivalents
-
- MST
-
- median survival time
-
- NHP
-
- nonhuman primate
-
- PCMV
-
- porcine cytomegalovirus
-
- PCV
-
- porcine circovirus
-
- PCXD
-
- perioperative cardiac xenograft dysfunction
-
- PERV
-
- porcine endogenous retrovirus
-
- PLHV
-
- porcine lymphotropic herpesvirus
Introduction
Xenotransplantation has always offered great promise to address the widening gap between demand and supply in transplantation; however, enthusiasm for xenotransplantation waned in the early 2000s due to slow progress in pig-to-nonhuman primate (NHP) preclinical models and fears about zoonosis, particularly in relation to porcine endogenous retrovirus (PERV). This review will examine the recent resurgence of activity and interest in xenotransplantation and the underlying factors involved. We will begin by discussing the promise and potential impact of genome editing, which will enable genetic manipulation of the donor pig on a previously unimaginable scale. We will then discuss progress in clinical trials and NHP studies of solid organ and tissue xenotransplantation within the past 5 years. We will conclude by revisiting the question of infectious risk in light of recent developments.
Genome Editing
Genetic modification is a key technique aimed at creating pigs whose organs and tissues have decreased antigenicity and increased physiological compatibility with humans. Until recently, this goal had been hampered by technological limitations. The generation of a homozygous single-gene knockout pig using traditional homologous recombination might take 3 years from start to finish. This process has been revolutionized by the development of genome editing: first, zinc finger nucleases and transcription activator-like effector nucleases, and more recently, the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system. CRISPR/Cas9 is increasingly favored for its ease of use and versatility, including its capacity to simultaneously modify multiple genes in a single reaction 1. The power of CRISPR/Cas9 is illustrated by its use to produce pigs with homozygous triple knockouts, either of SLA class I genes 2 or of genes encoding carbohydrate xenoantigens 3, in a process taking months rather than years. Furthermore, CRISPR/Cas9 can bypass the requirement for the inefficient and technically challenging technique of somatic cell nuclear transfer to generate knockout pigs; the CRISPR/Cas9 components can simply be injected into porcine zygotes, which are subsequently transferred into foster mothers 4. CRISPR/Cas9 also offers the opportunity to precisely knock transgenes into the pig genome 5.
The potential of genome editing can be glimpsed in a study examining the effect of CRISPR-mediated deletion of multiple xenoantigens on the histocompatibility of pig cells with human serum 3. CRISPR/Cas9 was used to generate GGTA1/CMAH/B4GALNT2 triple-knockout pigs, which lack expression of the nonhuman carbohydrates galactose-α1,3-galactose and N-glycolylneuraminic acid as well as an SDa antigen-like glycan. Crossmatching human serum with porcine peripheral blood mononuclear cells revealed that a significant proportion of renal transplant waitlisted patients had a negative IgG crossmatch to these pigs, and a sizeable percentage had a negative IgM and IgG crossmatch. These promising results suggest that the threat posed by preexisting antibodies in some patients may be reduced by genome editing of the donor pig. The news for highly sensitized (calculated panel-reactive antibody >80) patients was not as good, as many of those patients had a positive crossmatch to the triple-knockout pigs, probably due to cross-reaction of anti-HLA antibodies with SLA class I molecules 3. However, with the precision afforded by CRISPR/Cas9, rational modification of SLA class I genes may eventually provide a solution to even this problem.
Islet Xenotransplantation
Of all types of xenotransplantation, transplanting porcine islets to treat type 1 diabetes is generally viewed as the most likely to reach the clinic first. The form that this takes—intraportal delivery of “naked” islets, as practiced in clinical allotransplantation, or an alternative transplant site and/or some means of encapsulation—will ultimately depend on which method provides the optimal balance of efficacy, safety, and cost. Clinical trials of microencapsulated WT neonatal porcine islets transplanted in the absence of immunosuppression have been performed in New Zealand and Argentina 6, 7. Alginate microcapsules containing 5000 to 20 000 islet equivalents per kilogram of body weight (IEQ/kg) were transplanted intraperitoneally either as a single dose in 14 diabetic patients 6 or as two equal doses 3 mo apart in eight diabetic patients 7. Although there was no significant reduction in insulin dose in any group, patients receiving two transplants of 10 000 IEQ/kg showed a significant long-term (>600 days) decrease in hemoglobin A1c and a reduction in serious unaware hypoglycemic events 7. The procedure was safe, with minimal adverse events and no evidence of zoonosis 6, 8, but improved efficacy is clearly required to warrant clinical application.
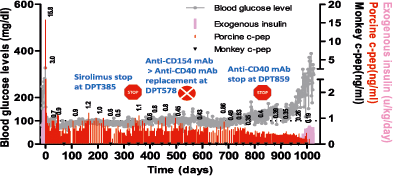
Intraportal islet xenotransplantation, in contrast, has demonstrated convincing efficacy in preclinical NHP models. A key recent study showed that WT adult pig islet xenografts maintained insulin-independent normoglycemia for a median of 303 days (maximum 950 days; Figure 1) in five consecutive diabetic monkey recipients 9, 10. A drawback of the procedure, however, was the relatively high dose of islets required (80 000–100 000 IEQ/kg). The study also highlighted that control of the inflammatory/immune response is a greater issue for porcine islets than for allogeneic islets. Recipients were treated with a complex protocol, including a cocktail of agents to minimize the instant blood-mediated inflammatory reaction (IBMIR) and long-term immunosuppression to inhibit cellular and humoral immune responses 9, that is not feasible for most prospective islet graft recipients. Several strategies to tackle the problems of IBMIR and chronic immunosuppression are being pursued. Studies in baboons 11 and monkeys 12 suggest that IBMIR can be reduced by using genetically modified (GGTA1 knockout [GTKO] with or without human CD55 [hCD55] to hCD59) neonatal pig islets. Shin et al 9, 10 attempted to induce tolerance in porcine islet xenografts in a pig-to-monkey model by adoptively transferring ex vivo expanded regulatory T cells, but grafts were rejected when maintenance immunosuppression was discontinued. Another option is genetic modification to engineer local production of immunosuppressive agents by the xenograft itself 13-15, although the efficacy of this strategy has not been directly tested in NHP preclinical models.
Heart Xenotransplantation
The heterotopic pig-to-NHP cardiac xenotransplantation model has been another area in which there have been major recent advances. Mohiuddin et al achieved a median survival time (MST) of 298 days (maximum 945 days) in five consecutive baboons transplanted with hearts from GTKO pigs transgenic for hCD46 and thrombomodulin 16. The immunosuppressive regimen was induction with anti–thymocyte globulin (ATG), anti-CD20, and cobra venom factor (CVF), and maintenance with anti-CD40, mycophenolate mofetil, steroids, and continuous heparin. Intensive and ongoing treatment with anti-CD40 was required for long-term graft survival. It was suggested that the expression of human thrombomodulin may be important to prevent the development of dysregulated coagulation, although this was not formally demonstrated. At a practical level, there was no indication that a pig cardiac xenograft would retain its natural rate of development and thus “outgrow” the abdominal space of the recipient 16. This remains an open question because Abicht et al reported significant enlargement of porcine intrathoracic cardiac xenografts in baboon recipients, although it was not possible to distinguish between physiological growth and pathological changes due to humoral rejection 17. Furthermore, Tanabe et al recently reported hypertrophy of porcine kidney xenografts in baboons and cautioned that the growth of donor organs—due, at least in part, to intrinsic factors—should be taken into account when transplanting life-supporting xenografts into a limited space 18.
A major challenge for the cardiac field is to translate these exciting results to an orthotopic life-supporting setting. Maximum survival in the extremely challenging pig-to-NHP orthotopic model is just 57 days 19. Results to date suggest that a phenomenon termed perioperative cardiac xenograft dysfunction (PCXD), and not rejection, is the current barrier to long-term survival 20. PCXD occurs in 40–60% of cases and can be reversible in the first two perioperative weeks. Histological analysis of cardiac xenografts that failed secondary to PCXD showed no signs of rejection but rather a picture more similar to cardiac stunning or ischemia/reperfusion injury. Until the underlying mechanism is delineated, it will be difficult to design therapies or genetic engineering strategies to prevent PCXD.
Kidney Xenotransplantation
The generation of CRISPR-modified pigs with improved histocompatibility with renal transplant waitlisted patients 3 has coincided with significant progress in other areas of kidney xenotransplantation. Two groups have achieved >7-mo survival (maximum 310 days) in pig-to-NHP preclinical models 21-23, demonstrating that prolonged life-supporting function is achievable. Of note, the recipients in these studies did not exhibit proteinuria, which had been identified in earlier studies as a major obstacle in kidney xenotransplantation. It remains unclear whether proteinuria in the context of renal xenografts is secondary to antibody-mediated rejection (as is the case for renal allografts) or is due to a physiological incompatibility and unrelated to rejection 24.
Both groups stressed the potential importance of (i) the donor pig genotype common to the studies (GTKO plus one or two human complement regulatory proteins) and (ii) effective costimulation blockade. In addition, Higginbotham et al 21 found that long-term graft survival was associated with low levels of preexisting donor-specific antibodies, whereas Iwase et al 22 proposed the potential importance of anti-inflammatory therapy and the expression of an additional human transgene for the anticoagulant, anti-inflammatory endothelial protein C receptor. There were several differences between the models, including the recipient species (baboon 22 or rhesus monkey 21) and the immunosuppressive regimen (ATG/anti-CD20/anti-CD40/rapamycin 22 or anti-CD4/anti-CD8/anti-CD154/mycophenolate mofetil 21). This makes it difficult to identify the factors responsible for the major prolongation of maximum survival from the previous record of 3 mo 23. Nevertheless, if routine prolonged survival using clinically available immunosuppression can be demonstrated in the NHP model, then progression to a clinical trial with recipients with a negative crossmatch may be justified.
The NHP kidney xenograft model has been a critical test bed for the study of xenogeneic tolerance by Yamada, Sachs, and colleagues, who have investigated “thymokidney” and mixed chimerism strategies. Most recently, refinements in the latter have prolonged the duration of macrochimerism and the survival of life-supporting GTKO kidney xenografts 25.
Liver Xenotransplantation
Until recently, survival in pig-to-NHP liver xenotransplantation was limited to 9 days by a lethal thrombocytopenic coagulopathy that developed in the early posttransplant period 26. Different approaches have been taken to tackle this problem. One strategy is to identify the cause of coagulopathy and attempt to correct it by genetic modification of the donor pig. Pig liver sinusoidal endothelial cells were found to phagocytose human and NHP platelets by a mechanism involving the asialoglycoprotein receptor (ASGR) 27. ASGR1 knockout pigs were generated, and their livers were shown to phagocytose significantly fewer human platelets than WT livers in an ex vivo perfusion circuit 28. Unexpectedly, livers from GGTA1/CMAH double-knockout pigs also sequestered fewer human platelets than WT or GTKO livers ex vivo 29. However, the in vivo impact of these genetic modifications has not yet been tested, and the GGTA1/CMAH double-knockout pigs cannot be fully tested in the NHP model because Old World primates, unlike humans, have a functional CMAH gene.
Another approach to preventing liver xenograft-induced coagulopathy has been to treat the recipient with recombinant human coagulation factors, based on the observation of marginal coagulation factor production in baboon recipients. In the first of two studies using GTKO pig donors, baboons were immunosuppressed with ATG, CVF, tacrolimus, and steroids and treated with Octaplex (human coagulation factors II, VII, IX, X, protein C, and protein S; Octapharma, Lachen, Switzerland) or NovoSeven (factor VIIa; Novo Nordisk, Plainsboro, NJ) 30. The coagulation factor treatment rendered posttransplant thrombocytopenia manageable and eliminated the need for platelet transfusions; however, survival of the six recipients was limited to a maximum of 7 days, mainly due to thrombosis or intra-abdominal bacterial infection. This highlighted the fine line between bleeding and clotting that hemostasis requires, as well as the infectious risks of surgical reexploration in the early postoperative period. In the second study, the same group transplanted a single baboon with the Octaplex protocol, adding belatacept to the maintenance therapy and avoiding reexploration 31. This recipient developed a transient thrombocytopenia that spontaneously recovered within 2 weeks and survived for 25 days without evidence of rejection or thrombosis. Although these results are preliminary, they suggest that a pig liver may be capable of maintaining life long enough to be used as a bridge to transplant, with the potential for further advances using donors that have been genetically modified to avoid thrombocytopenia 28, 29.
Lung Xenotransplantation
Lungs present perhaps the greatest challenge in solid organ xenotransplantation. Despite the testing of numerous genetic modifications and treatments to inhibit the innate and adaptive immune responses, complement, coagulation, and inflammation, maximum survival of life-supporting lung xenografts in the NHP model remains limited to 8 days 32.
Corneal and Tissue Xenotransplantation
Corneal xenotransplantation faces unique anatomical and surgical challenges, although recent progress in NHP models suggest that these are not insurmountable (reviewed by Kim and Hara 33). The survival of WT pig full-thickness corneal xenografts in monkeys treated with anti-CD154–based immunosuppression (MST 318 days; maximum >933 days) was significantly greater than in monkeys treated with steroids alone (MST 28 days; maximum 29 days) 34. In vitro studies suggest that the immunogenicity of pig corneas can be reduced by genetic modification 35, but whether this will permit a reduction in immunosuppression remains to be determined.
Intracerebral transplantation of porcine neural tissue or choroid plexus, which secretes neurotrophic and neuroprotective factors, has been proposed as a treatment for Parkinson disease. In a study in which parkinsonian monkeys were transplanted with porcine embryonic neuroblasts, maturation of the xenografts and significant improvement in clinical symptoms were observed at 6 mo after transplant in immunosuppressed recipients 36. Improved neurological scores were also observed at 6 mo in parkinsonian monkeys transplanted with microencapsulated porcine neonatal choroid plexus cells in the absence of immunosuppression 37. The microcapsules are currently being tested in a phase IIb clinical trial in New Zealand.
Infectious Risk
The risk posed by the xenograft as a potential carrier of bacterial, fungal, or viral pathogens can be minimized by careful screening of donor pigs and their maintenance in specific or designated pathogen-free facilities (reviewed recently by Denner and Mueller 38). The definition of pathogen-free is somewhat fluid, and the screening strategy may differ depending on multiple factors such as the location and relative isolation of the facility 6, 39, 40. Microbiological safety data have been gathered from preclinical and clinical trials using encapsulated islets from “high health status” pigs. Six nonimmunosuppressed monkey recipients showed no evidence of transmission of viruses of possible concern (porcine circovirus [PCV], porcine lymphotropic herpesvirus [PLHV], porcine reproductive and respiratory syndrome virus, Porcine cytomegalovirus [PCMV], and PERV) at 1 year and up to 4 years after transplant 39. Similarly, no transmission of PCV, PLHV, PCMV, or PERV was detected in 14 nonimmunosuppressed human recipients up to 1 year after transplant 6. Although the potential risk is increased with the use of immunosuppression, which will likely be required for most forms of xenotransplantation, these initial results are nevertheless encouraging.
From a theoretical standpoint, PERV represents the most serious challenge because it is present at multiple copies in the pig genome and thus, unlike other viral pathogens, cannot be eliminated by breeding. Although there has been no evidence to date of PERV transmission from pigs to humans or NHPs 38, the International Xenotransplantation Association advocates a cautious approach, including screening of donor pigs for low PERV expression levels and monitoring of xenograft recipients 41. The absence of PERV transmission in the 14 patients mentioned earlier and in eight subsequent recipients of encapsulated porcine islets confirmed PERV-negative by polymerase chain reaction and serology at 1 year after transplant 8 supports this approach. In the unlikely event of PERV infection of recipients, recent in vitro studies suggest that several licensed retroviral inhibitors used to treat human immunodeficiency virus will also be efficacious against PERV 42, 43. Furthermore, it may be possible to completely eliminate the risk of PERV by gene editing of the donor pig. Yang et al 44 used CRISPR/Cas9 to knock out all 62 copies of PERV in a pig cell line, reducing PERV transmission to human cells in vitro >1000-fold. It remains to be determined whether a healthy pig with a PERV-free genome can be generated.
Summary
Clinical xenotransplantation remains a daunting challenge, particularly for sensitive organs such as the lung, but the pace of progress in several areas suggests that clinical trials may no longer be a distant prospect.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. P.J.C. is a member of the Scientific Advisory Board of Hunan Xeno Life Sciences Inc. A.J.T. is the founder of Xenobridge LLC and has patents pending.




