BET Proteins: An Approach to Future Therapies in Transplantation
Abstract
In order to develop new efficient therapies for organ transplantation, it is essential to acquire a comprehensive knowledge of the molecular mechanisms and processes, such as immune activation, chronic inflammation, and fibrosis, which lead to rejection and long-term graft loss. Recent efforts have shed some light on the epigenetic regulation associated with these processes. In this context, the bromo and extraterminal (BET) family of bromodomain proteins (BRD2, BRD3, BRD4, and BRDT) have emerged as major epigenetic players, connecting chromatin structure with gene expression changes. These proteins recognize acetylated lysines in histones and master transcription factors to recruit regulatory complex and, finally, modify the transcriptional program. Recent studies indicate that BET proteins are essential in the NF-kB-mediated inflammatory response, during the activation and differentiation of Th17-immune cells, and in profibrotic processes. Here, we review this new body of data and highlight the efficiency of BET inhibitors in several models of diseases. The promising results obtained from these preclinical models indicate that it may be time to translate these outcomes to the transplantation field, where epigenetics will be of increasing value in the coming years.
Abbreviations
-
- BET
-
- bromo and extraterminal
-
- BMT
-
- bone marrow transplant
-
- BRD
-
- bromodomain
-
- CTD
-
- carboxy-terminal domain
-
- EGFR
-
- epidermal growth factor receptor
-
- ET
-
- extraterminal
-
- PAI-1
-
- plasminogen activator inhibitor-1
-
- PDGFR
-
- platelet-derived growth factor receptor
-
- P-TEFb
-
- positive transcription elongation factor b
-
- TF
-
- transcription factor
-
- TGF-β1
-
- transforming growth factor-β1
-
- Treg
-
- regulatory T cell
Introduction
Epigenetic regulation refers to changes in gene expression that do not entail any alteration of the DNA sequence 1. At the molecular level, these changes are mediated by a variety of mechanisms, including DNA methylation, histone modifications, noncoding RNAs, and chromatin remodeling complexes. Epigenetic patterns are mainly established during development and, because of their stability and heritability, can be maintained in differentiated cells and after cell division. However, altered expression or abnormal function of proteins associated with chromatin remodeling can lead to epigenetic aberrations that are recognized as key players in several human diseases 2.
Immediately after transplantation, the immune response is almost completely constrained by the use of current immunosuppressive treatments. However, as the transplantation progresses, a set of immune and nonimmune cells and signaling cascades are activated, inducing a barely detectable, low-grade inflammation that, in some cases, leads to irrevocable damage of the allograft. Changes in the chromatin structure mediated by epigenetic modifications play a critical role in gene expression regulation during activation, proliferation, and differentiation of all these cells 3. Thus, the epigenetic mechanisms, especially DNA methylation and histone modifications, provide a novel therapeutic target, whose importance has now been realized 4, 5. Histone acetylation at specific lysine residues is one of the best characterized epigenetic mechanisms and is usually associated with active transcription. The addition of an acetyl group to histones is catalyzed by histone acetyltransferases (“writer” proteins), whereas histone deacetylases (“eraser” proteins) are responsible for removing these epigenetic marks 6. Additionally, “reader” proteins are able to recognize and bind to these epigenetic modifications. Of these, the bromodomain and extraterminal domain family proteins (BETs) bind to acetylated lysine residues in histones and other nonhistone proteins and, concurrently, can recruit other transcription factors to the chromatin and the basal transcriptional machinery 7. BET proteins have long been associated with cancer and development but, in recent years, important studies have demonstrated their essential contribution to inflammatory and autoimmune processes 8-10. The development of potent and selective small molecules (e.g. JQ1 and I-BET 762) that inhibit binding of BETs to chromatin have led to new therapeutic strategies, some of which have already demonstrated their value in several settings, especially in oncology 11, 12. Here, we review the contribution of BETs to immune responses and key nonimmune processes related to rejection and long-term allograft outcome (Table 1).
| Genes | BET | Cell type | Disease model | Ref |
|---|---|---|---|---|
| Inflammation | ||||
| VCAM1, SELE, CCL2, CSF2, LTB, TNFAIP3, IRAK2, CSF2RB, CXCR7, CXCL1, ICOSLG | BRD4 | Human umbilical vein endothelial cells | Atherosclerosis (hypercholesterolemic mice) | 22 |
| TNFA, IL1B, IL6, CCL2 (MCP-1), IL10 |
BRD2 BRD3 BRD4 |
Murine bone marrow–derived macrophages | Endotoxemic (LPS-induced “cytokine storm”) | 44 |
| CCL2, CCL5 (RANTES), IL6, CSF2, CCL20, LTB, ICOSLG, IL8 | BRD4 | Human tubular epithelial cells (HK2 cell line) |
Unilateral ureteral obstruction Systemic infusion of angiotensin II Nephrotoxic serum nephritis |
48 |
| IL6, TIMP1, NOX4, COL8A1, CCL21A, CTGF | BRD4 | Neonatal rat ventricular cardiomyocytes (NRVM) | Transverse aortic constriction (TAC) (cardiac hypertrophy) | 6 |
| Th-17 cell differentiation and activation | ||||
| IL17A, IL21, IL22, IL23R, RORc, RORγt, GMCSF, BATF |
BRD2 BRD4 |
Human Th-17 differentiated cells |
Collagen-induced arthritis (CIA) Experimental autoimmune encephalomyelitis (EAE) |
35 |
| Fibrosis | ||||
| IL6, ACTA2, PAI1, COL1A1, FN1 |
BRD2 BRD4 |
Human primary lung fibroblasts | Bleomycin-induced pulmonary fibrosis | |
| COL1A1, ACTA2, COL1A2, DES, PDGFRB, CCND1, FBN1, FN1, TIMP1, TGFB1 | BRD4 |
Human activated hepatic stellate cells (HSCs) (LX-2 cell line) Primary mouse HSCs |
Carbon tetrachloride (CCl4) mouse model of liver injury | 59 |
| TS2, HNF4A, JUNB, FOXP1, CDH2 | BRD4 | Human lung cancer A549 cell line | 60 | |
| ACTA2, COL1A1, P53, FBN1, SMAD7, CCL2, c-MYC | BRD4 | Renal interstitial fibroblast cells (NRK-49F) | Unilateral ureteral obstruction | 61 |
- BET, bromo and extraterminal; BRD, bromodomain; LPS, lipopolysaccharide.
Structure and Function of BET Proteins
In mammals, the BET family comprises four protein members (BRD2, BRD3, BRD4, and BRDT) with two N-terminal tandem bromodomains (BRDs) that allow the interaction with acetylated lysines in histone and nonhistone proteins, and a C-terminal region that includes a highly conserved extraterminal (ET) domain for protein interactions that are not mediated by BRDs 13. With the single exception of BRDT, which is restricted to testis, most BETs are ubiquitously expressed and carry out a broad range of cellular functions. One of most important of these is to act as a scaffold at the chromatin level, recruiting regulatory proteins to chromatin regions with acetylated histones. Therefore, these proteins are epigenetic “readers” connecting histone acetylation to chromatin function through the recruitment of several epigenetic protein complexes, such as the catalytic subunit of the NuRD remodeling complex, the histone demethylase JMJD6, and the SWI/SNF nucleosome remodeling complex or the methyltransferase NSD3 (Figure 1) 14, 15. BETs can also interact with acetylated transcription factors (TFs), such as NF-kB, in order to facilitate chromatin binding 16.
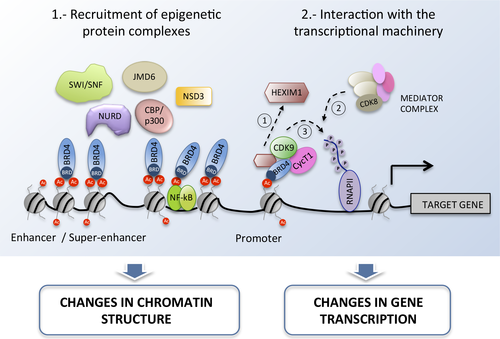
BET proteins also play an important regulatory role by directly interacting with the transcription machinery. BRD4 is able to recruit the positive transcription elongation factor b (P-TEFb) of the RNA polymerase II complex to the chromatin (Figure 1) 17. P-TEFb is made up of the cyclin-dependent kinase Cdk9 and a cyclin subunit (T1, T2, or K). After recruitment, the effect of BRD4 on the P-TEFb complex is twofold: first, BRD4 mediates the release of the 7SK snRNA/HEXIM1 inhibitory complex, which allows polymerase II pause release and the generation of the transcription-initiation complex 14; second, BRD4 exerts a kinase activity that phosphorylates the serine 2 of the carboxy-terminal domain (CTD) of the polymerase II and, thus, contributes to the formation of the transcription-elongation complex 18. In addition, BRD4 also interacts with the mediator complex, a large and flexible protein complex that functions as a transcriptional coactivator of the RNA polymerase II, drawing attention to the broad regulatory role of BRD4 (Figure 1) 19. It is important to mention that the functional interaction between BETs and polymerases is not restricted to BRD4, since BRD2 and BRD3 are also known to facilitate RNA polymerase II elongation due to their ability to bind to hyperacetylated chromatin 20. Therefore, BETs directly interact with promoters and gene sequences to guide transcription. Nonetheless, BETs can also affect transcription through the interaction with distant regulatory regions. In fact, BRD4 is highly enriched in some large enhancer regions, often called superenhancers, in combination with the mediator complex. The binding of BRD4 to these superenhancers seems to be particularly critical for the expression of some pro-oncogenic genes such as c-MYC, and during NF-kB activation 21, 22. Finally, the degree of functional redundancy between BET family members and their isoforms is not yet known. Although interactions with other proteins seem to differ between BETs, it is well documented that BRD2, BRD3, and BRD4 co-localize at approximately two thirds of their chromatin binding sites, suggesting that they share some regulatory functions 23.
BET Proteins in Cell Cycle: A Potential Therapeutic Target in Transplantation
Proliferation signals play a central role in the immune response since they are closely associated with plasticity and activation processes. In organ transplantation, mTOR inhibitors are highly efficient immunosuppressive agents due to their ability to reduce lymphocyte activation by blocking proliferation signals in response to alloantigen 24.
BET proteins have been identified as being postmitotic bookmarks; BRD2 and BRD4 remain associated with the chromatin throughout the cell cycle, including in mitotic chromosomes. Upon chromosome decondensation, BETs promote the rapid transcription of many genes associated with cell progression 25. For instance, BRD3 is a transcriptional regulator of cyclin D1, which is essential for G1/S phase transition, and BRD2 regulates the expression of cyclins D1, A and E 21. On the other hand, BRD4 is also an important transcription regulator of cell cycle–associated genes (e.g. CCND1, CCND2, ORC2, MCM2), stimulating G1 transcription and promoting S phase transition 26.
Although BETi have not been tested in the transplantation context, they are well known to exert a strong cytostatic effect that can interfere with immune functions. BRD2 regulates cyclin A expression, which is required for B cell proliferation, and BETi efficiently block in vitro the proliferation of lipopolysaccharide (LPS)-stimulated B lymphocytes 27. In addition, it has been reported that these inhibitors can suppress cell cycle–related genes, such as c-MYC, in CD4+ T cells, blocking proliferation without exerting a strong cytotoxic effect 28. Moreover, a combined therapy of BETi and rapamycin has been shown to synergistically inhibit the growth and survival of human osteosarcoma cells in vitro and in mouse xenografts 29. This implies that inhibition of BETs might reproduce or potentiate some of the immunomodulatory effects of mTOR inhibitors, or at least those associated with cell cycle arrest. It is important to point out that the antiproliferative effect of these BETi is very weak in some cell types, such as fetal fibroblasts, and some cancer cell lines tested in vitro 30, 31. Therefore, it is not clear how these drugs will affect nonimmune cells and further studies need to be carried out in animal models to clarify this matter.
Contribution to Th17 Cell-Mediated Rejection
The immunological response of the recipient toward the transplanted organ determines graft rejection and long-term transplantation outcome. This immune response is regulated by T cell subsets, mainly by helper T cells (Th: Th1, Th2, and Th17) and regulatory T cells (Treg). It is well known that epigenetic mechanisms play a critical role during the differentiation of T lymphocytes to Th and Treg subsets 32, 33. On exposure to transforming growth factor-β1 (TGF-β1) and IL-6, naive CD4+ T lymphocytes initiate a differentiation process toward the Th17 phenotype, which is orchestrated by a subset of transcriptional factors (RORγt, RORα, IRF4, AHR, and NF-kB) that regulate the expression of cytokines secreted by these cells (IL-17A, IL-17F, IL-21, IL-22, and granulocyte-macrophage–colony-stimulating factor [GM-CSF]) 34. Th17 cells have often been associated with inflammatory responses and graft rejection, making them a suitable therapeutic target. Interestingly, treatment with the specific BETi JQ1 reduces the level of expression of IL-17A, IL-21, and IL-22 cytokines, and of RORγt and RORα TFs in Th17 differentiated cells 35 (Figure 2). However, no effect was observed in the IFNG, IL-4, and FOXP3 genes expressed by other T cell lineages, showing their specificity for this cell type.
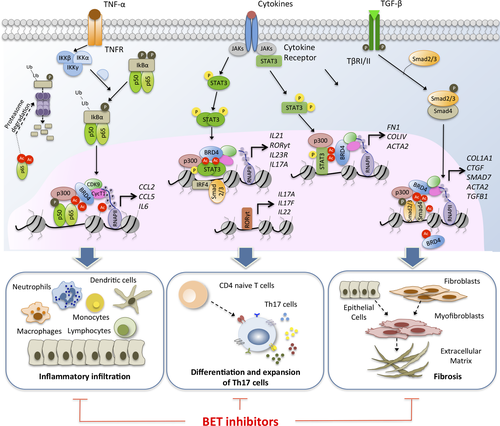
Mechanistically, BETs control IL-21 mRNA transcription, which activates STAT-3 and RORγt. IL-21 acts as an autocrine factor, establishing an amplification loop essential for Th17 differentiation. It has been observed that in vivo administration of JQ1 in collagen-induced arthritis and experimental autoimmune encephalomyelitis animal models impairs the function of pathogenic Th17 cells 35. Moreover, in vitro administration of BETi (I-BET 762) to CD4+ T cells also downregulates the production of some chemoattractants for myeloid cells and neutrophils, such as GM-CSF and IP-10, and increases the expression of inhibitory cell-surface receptors (Lag3, PD-1, and Tim3) of anergic T cells 9. Elevated levels of the chemokine IP-10 (CXCL10) early after kidney transplantation compromise the long-term graft function 36, whereas induction of anergic T cells could facilitate a suboptimal antigen-driven signaling of alloreactive T cells.
One essential aspect is the regulation of the BATF transcription factor by BRD4 37. BATF is required for the differentiation of Th17, follicular helper T cells (Tfh), and effector CD8+ T cells. Accordingly, it has recently been reported that inhibition of BATF increases the allograft survival in cardiac transplantation by modulating the Th17-mediated alloimmune response 38. BATF also regulates the expression of IL-21, an essential cytokine for the development of Tfh, which contributes to an exacerbated humoral response and antibody production in patients with chronic renal allograft rejection 39. In CD8+ T cells, deficiency of BATF blocks the expression of key TFs (T-bet, Runx3, and STATs), contributing to profound defects in the differentiation to effector T cells 40. These results are very relevant to adoptive immunotherapy treatments for cancer patients, in which ex vivo CD8+ T cells cultured with JQ1 show impaired differentiation toward effector T cells, favoring the expansion of long-lived memory T cells (stem cell–like memory and central memory), with superior in vivo persistence and antitumor effects 37. Thus, BETi during the initial period after transplantation could contribute to modulate the long-term immune response, rendering T cells unable to induce an alloimmune response but without altering the functions of existing memory T cells.
Regulation of NF-kB-Mediated Inflammation
The control of inflammatory reactions immediately after transplantation is crucial to improving long-term graft survival. The process can be triggered by diverse stimuli or damage signals produced by rejection, infections, or the ischemia/reperfusion (I/R) injury inherent to the transplantation procedure.
Specifically, there is clear experimental evidence of the role of TF NF-kB in the development of an inflammatory response, but the system is highly complex and several therapies aimed at modulating its activation have so far proved inefficient. Initially, several extracellular stimuli induce the activation of the classic (RelA/p50) or alternative (RelB/p52) NF-kB pathways that work jointly with a wide range of coactivators, corepressors, and posttranslational modifiers (phosphorylation and acetylation) of NF-kB proteins. Hence, phosphorylation of serines 276 and 536 of the RelA subunit is essential for recruiting the lysine acetyltransferase (KAT) p300/CBP that subsequently acetylates RelA subunit 41. BRD4 interacts directly with the acetylated lysine-310 of the RelA subunit, favoring its stability (Figure 2) 42, 43. This interaction mainly takes place in superenhancer regions in which BETs act as coactivators of the NF-kB-mediated regulatory functions once these regions have been occupied by this transcription factor 22.
First reports have shown that BETs are essential for macrophage-mediated inflammatory responses 44. Blockage of BETs downregulates the expression of TNF-α, IL-6, IL-1β cytokines and the MCP-1 chemokine by altering NF-kB activity, and ameliorates the “cytokine storm” produced by LPS-induced macrophages 45. Moreover, BETi are able to disrupt the normal maturation of dendritic cells (DCs), impairing the development of an adaptive immune response 46. Treatments in vitro with JQ1 decrease the transcriptional activity of STAT5, resulting in the reduced expression of costimulatory molecules (CD80, CD83, and CD86) and cytokines (IL-12 and IL-10) required for the maturation of human monocyte-derived DCs. These JQ1-treated DCs are not capable of inducing the differentiation of inflammatory T cells (Th1 cells). A relevant study carried out in mice undergoing MHC-disparate allogeneic bone marrow transplant (BMT) showed that BETi (I-BET 151), through disruption of BRD4-acetylated RealA interaction, decreased the expression of surface molecules on DCs, impairing T cell priming and further activation 47. Early treatment during BMT reduced graft-versus-host disease severity and improved the mortality without disturbing the graft-versus-tumor effect.
We have recently shown that BETs are active players in renal inflammation 48. BRD4 contributes to the inflammatory response through direct binding to acetylated histone H3 in the promoter of pro-inflammatory genes such as IL6, CCL2 (MCP-1), and CCL5 (RANTES) promoting gene transcription, and through interaction with the acetylated-RelA subunit, which is required for the further activation of the NF-kB complex. Administration of JQ1 in diverse models of experimental renal damage not only diminishes the presence of infiltrating inflammatory cells (monocytes/macrophages, neutrophils, dendritic cells) and Th17 lymphocytes but also decreases the expression of biomarkers of renal damage such as Ngal and Kim-1. It is of particular note that blockage of BETs restores the renal function (creatinine and albumin levels) in these in vivo models, highlighting the important role of BETs as possible therapeutic targets for the treatment of inflammatory renal diseases.
On the other hand, inflammation can be the result of I/R injury caused by the tissue hypoxia inherent to transplantation. The cellular response to hypoxia is mainly mediated by the hypoxia-inducible factor 1 α (HIF-1α), a transcriptional complex that regulates the transcription of numerous genes. Upon hypoxia, HIF-1α is acetylated at lysine 709 by p300, enhancing protein stability 49. However, this is not enough to stimulate gene transcription, since BRD4 needs to be recruited to facilitate the binding of the CDK8-mediator and p-TEFb elongation complexes 50, whose activity is essential to stimulate RNAPII elongation and transcription. In this context, histone deacetylase (HDAC) inhibitors preserve renal function in a renal transplantation–based cold ischemia model 51, although the BETi effect remains undetermined.
BRD4 as a Key Target in the Fibrotic Gene Network
Fibrosis, or the excessive deposition of extracellular matrix around injured tissues, is one of the most common causes of loss of function and graft failure. Despite being a widespread public health problem, few therapeutic options are available and new antifibrotic therapies need to be developed.
Mechanistically, signaling pathways mediated by platelet-derived growth factor receptors (PDGFR), epidermal growth factor receptors (EGFRs), and TGF-β1 receptors are the main contributors to the migration and activation of fibroblasts. It has been extensively reported that epigenetic mechanisms, and in particular histone acetylation, orchestrate the fibroblast activation that leads to fibrosis. After TGF-β1 stimulation, the KAT p300 acetylates STAT3 and Smad2/3, enabling their nuclear accumulation and inducing the expression of extracellular matrix proteins such as fibronectin and collagen 52. Overexpression of p300 has been associated with profibrotic responses in angiotensin II-induced renal damage and diabetic nephropathy 53, 54. Moreover, HDAC inhibitors, such as MS-275 or tubastatin A, suppress TGF-β-induced histone H4 acetylation and phosphorylation of Smad3 in collagen type I promoter, inhibiting the renal fibrosis induced in renal damage mouse models 55, 56.
Several studies have also implicated BETs in the regulation of signaling pathways associated with fibrogenesis, showing the efficacy of BETi in different mouse models of tissue fibrosis. Treatment with BETi reduces migration, proliferation, and cytokine secretion in lung fibroblasts isolated from idiopathic pulmonary fibrosis patients, and prevents the development of pulmonary fibrosis in mice 57, 58. After TFG-β1 stimulation, the increased acetylation of lysine 5 of H4 histone (AcH4K5) facilitates the recruitment of BRD4 to the promoter of IL6, ACTA2 (α-smooth muscle actin, α-SMA), and SERPINE1 (plasminogen activator inhibitor-1) genes 57 (Figure 2). BRD4 has been identified as a potent driver of liver fibrosis in vitro and in vivo 59. In hepatic stellate cell–derived myofibroblasts, BRD4 is highly enriched at enhancer regions of profibrotic genes and colocalizes with transcription factors such as ETS1, SRF, SMAD3, and NF-kB involved in multiple profibrotic pathways. Moreover, BRD4 binds to super-enhancer regions of the three master transcription factors (ETS2, HNF4A, and JUNB) involved in regulating TGF-β-induced epithelial-to-mesenchymal transition 60. Similar results have recently been reported in a mouse model of renal fibrosis induced by unilateral ureteral obstruction 61. Administration of BETi (I-BET 151) attenuates fibrosis by regulating several processes: downregulation of growth factor receptors (EGFR and PDGFR); inhibition of the phosphorylation and acetylation of Smad3, STAT3, and NF-kB; suppression of c-Myc and p53 transcription factors; and arrest of the cell cycle at the G2/M phase of renal epithelial cells. Overall, these results highlight the role of BETs as a novel therapeutic target for the treatment of fibrosis that commonly leads to the loss of long-term function and graft failure.
Concluding Remarks and Future Perspective
The knowledge of the molecular-level processes that contribute to the loss of transplanted grafts will provide novel and promising therapeutic opportunities. Targeting BET proteins with specific inhibitors has brought about a revolution in cancer research and treatment, but recent studies have also highlighted their therapeutic potential in inflammatory and autoimmune diseases. All these results suggest an important role of BETs in transplantation and the next steps should aim to assess the therapeutic potential of BETi in mouse transplantation models in anticipation of their beneficial results in humans. Studies should first attempt to evaluate the effect that the early administration of these inhibitors could have on initial events (I/R injury–mediated inflammation and innate/adaptive immune response) that condition the long-term outcomes. One advantage is that we know when the transplantation procedure happens so we can act in advance to prevent adverse effects. Another important point to be considered is the underlying inflammatory response in transplantation that contributes unnoticed to fibrosis and graft failure. Targeting the epigenetic mechanisms that contribute to the expression of master transcription factors, such as NF-kB and STAT3, could block these responses during the evolution of the transplantation.
The known inhibitors of BETs show little toxicity and are well accepted in vivo models. However, we cannot rule out the off-target molecular and cellular effects of these drugs 62. To ameliorate this problem, novel BET-restricted inhibitors are under development, including bivalent inhibitors, which target the two bromodomains in the BET proteins in order to improve specificity 63, and proteolysis-targeted chimeras that induce specific degradation of some BET members 64. Finally, recent efforts have been made to target the kinase activity of BRD4 65, although additional studies are required to clarify the potential advantages of these new inhibitors. Furthermore, the ongoing development of new epigenetic inhibitors with narrow specificity and studies of these in combination with other epigenetic agents, such as HDAC or HAT inhibitors, will expand our knowledge, enabling its application to transplantation.
Acknowledgments
This work is supported by Plan Nacional de I+D+I 2008–2011 and European Union Fondos Feder; Instituto de Salud Carlos III (grant numbers PI12/02587, PI14/0041, and PI16/01318); REDinREN RD16/0009/0020 and RD16/0009/0007 (Kidney Research Network) and Plan de Ciencia, Tecnología e Innovación 2013-2017 del Principado de Asturias (reference GRUPIN-14-030).
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




