Banff Histopathological Consensus Criteria for Preimplantation Kidney Biopsies
Abstract
The Banff working group on preimplantation biopsy was established to develop consensus criteria (best practice guidelines) for the interpretation of preimplantation kidney biopsies. Digitally scanned slides were used (i) to evaluate interobserver variability of histopathologic findings, comparing frozen sections with formalin-fixed, paraffin-embedded tissue of wedge and needle core biopsies, and (ii) to correlate consensus histopathologic findings with graft outcome in a cohort of biopsies from international medical centers. Intraclass correlations (ICCs) and univariable and multivariable statistical analyses were performed. Good to fair reproducibility was observed in semiquantitative scores for percentage of glomerulosclerosis, arterial intimal fibrosis and interstitial fibrosis on frozen wedge biopsies. Evaluation of frozen wedge and core biopsies was comparable for number of glomeruli, but needle biopsies showed worse ICCs for glomerulosclerosis, interstitial fibrosis and tubular atrophy. A consensus evaluation form is provided to help standardize the reporting of histopathologic lesions in donor biopsies. It should be recognized that histologic parameters may not correlate with graft outcome in studies based on organs deemed to be acceptable after careful clinical assessment. Significant limitations remain in the assessment of implantation biopsies.
Abbreviations
-
- CI
-
- confidence interval
-
- DGF
-
- delayed graft function
-
- ECD
-
- expanded criteria donor
-
- HCV
-
- hepatitis C virus
-
- ICC
-
- intraclass correlation
-
- KDPI
-
- Kidney Donor Profile Index
-
- OPO
-
- organ procurement organization
-
- OPTN
-
- Organ Procurement and Transplantation Network
-
- SCD
-
- standard criteria donor
-
- SCr
-
- serum creatinine
-
- UNOS
-
- United Network for Organ Sharing
Introduction
Worldwide, the demand for donor kidneys far exceeds the number of available organs; however, the discard rate of recovered kidneys remains >40%. Preimplantation biopsy—also referred to as procurement, harvest or donor biopsy—is often used by transplant centers in addition to clinical data and parameters of machine pump perfusion to make determinations regarding organ acceptance. The Organ Procurement and Transplantation Network (OPTN) policies currently recommend preimplantation biopsy for all kidneys with a Kidney Donor Profile Index (KDPI) >85% or at the request of the accepting surgeon, according to the deceased donor allocation policy implemented in December 2014 within the United States (OPTN Policy 2.12.A) 1. The KDPI is derived from 10 donor factors (age, height, weight, ethnicity, history of diabetes and/or hypertension, cause of death, serum creatinine [SCr], hepatitis C virus [HCV] status and donor after circulatory death status). Under the former allocation system, preimplantation biopsy was previously recommended for all expanded criteria donor (ECD) kidneys, defined as organs from older donors (aged >60 years) and persons aged 50–59 years with hypertension, SCr >1.5 mg/dL or death from cerebrovascular accident 2, 3. These policies recommended that organ procurement organizations (OPOs) provide the receiving transplant program with biopsy information from a wedge biopsy 10 mm long by 5 mm wide and 5 mm deep to be taken from the kidney cortex to capture at least 25 glomeruli. After the implementation of these recommendations, requests for donor biopsy interpretation by hospital pathologists increased significantly. A pathology data form based on the United Network for Organ Sharing (UNOS) guidelines emphasizes the number of glomeruli present in a wedge biopsy and the percentage of global glomerulosclerosis. This form is currently customized by local OPOs. This practice deviates substantially from routine evaluation of postimplantation allograft biopsies for cause (indication biopsies), which are routinely needle biopsies to assess not only the glomeruli but the tubular, interstitial and vascular compartments, following recommendations established by the Banff group. The Banff group previously defined and subsequently refined the pathologic criteria for postimplantation biopsies performed for cause and more recently applied the same criteria for implantation biopsies in centers performing protocol biopsies 4, 5.
A Banff working group on preimplantation biopsy was established in 2010 and initially conducted a survey asking renal pathologists at large for their input through the Renal Pathology Society website. The results of the survey revealed a list of areas to be addressed. These items became the aims of the Banff working group. Preliminary data of the preimplantation renal biopsy working group and a proposal for Banff consensus criteria for histopathologic interpretation were presented at the 11th Banff conference, held in Paris, France, in 2011. The agreed-upon criteria were subsequently applied to a larger cohort of biopsies, and the working group was expanded to include international participants. The results were presented at the 12th Banff conference, held in Brazil in 2013, at which evaluation of the Banff histopathologic consensus criteria with graft outcome was decided. The study concluded with the results of the comparison of histopathologic data with graft outcome at the 13th Banff conference, held in Vancouver, Canada, in 2015. The current investigation is the first Banff study aiming to develop consensus to define the gold standard for interpretation of preimplantation biopsies. The working group's specific aims were defined as follows: (i) Address interobserver variability utilizing frozen sections, (ii) compare wedge and needle core biopsies, (iii) compare frozen and defrosted formalin-fixed tissue sections, (iv) develop histopathologic criteria for the interpretation of preimplantation biopsies, and (v) compare histopathologic and clinical parameters with graft outcome.
Methods
Briefly, the questionnaire included questions on (i) the participants’ experience with the UNOS-recommended preimplantation wedge biopsies, (ii) the perceived accuracy of frozen section interpretation, (iii) possible interobserver variability in the assessment of preimplantation biopsies, and (iv) recommendations for a pathologic gold standard for evaluating ECD kidneys. The survey results strongly recommended assessment of all aspects listed regarding preimplantation biopsy interpretation.
A total of 124 biopsies were used. Of these, 40 biopsies were scanned using the Aperio system (Leica Biosystems, Wetzlar, Germany) at Washington University in St. Louis and at the University of Alberta. Slides were scanned on an Aperio ScanScope XT at ×20 magnification, corresponding to 0.50 μm/pixel.
The study was approved by the Washington University Kidney Translational Research Core and the institutional review board at Washington University in St. Louis (IRB 05-2009). Four groups were created as follows: frozen core biopsies (n = 5), defrosted paraffin core biopsies (n = 5), frozen wedge biopsies (n = 15), and defrosted paraffin wedge biopsies (n = 15).
All cases submitted for scoring by intraclass correlation (ICC) analysis were implanted. Kidneys were from 20 unique donors; both kidneys of these donors were implanted. Donor demographic data of 19 donors implanted at the Washington University kidney transplant center in Saint Louis, Missouri, were retrieved (Table 1). We were unable to retrieve the demographics of one donor for whom both kidneys were implanted. Briefly, median age was 49.2 years (range 46.4–56.0 years), and nine donors were male. Causes of death were anoxia in 16%, cerebrovascular in 58%, “natural causes” in 6% and trauma in 16%. Regarding race, 89% were white, 5% were black and 5% were Hispanic. Calculated terminal creatinine is shown in Table 1.
| Donor | Age, y | Sex | Race | Cause of death | Terminal creatinine, mg/dL1 |
|---|---|---|---|---|---|
| 1 | 60 | Female | White | CVA | 0.7 |
| 2 | 25 | Female | White | Anoxia | 1 |
| 3 | 62 | Male | White | CVA | 1.1 |
| 4 | 59 | Male | White | CVA | 1.8 |
| 5 | 48 | Male | White | Head trauma | 2.9 |
| 6 | 56 | Female | White | CVA | |
| 7 | 50 | Male | White | Motor vehicle accident | 0.8 |
| 8 | 41 | Female | White | Natural causes | |
| 9 | 46 | Male | White | Head trauma | 1.4 |
| 10 | 58 | Male | White | CVA | 1.4 |
| 11 | 46 | Male | Black | Anoxia | 1.1 |
| 12 | 48 | Female | White | CVA | 1.1 |
| 13 | 50 | Female | White | CVA | 0.8 |
| 14 | 51 | Male | White | CVA | 1.2 |
| 15 | 50 | Female | White | Anoxia | 0.4 |
| 16 | 46 | Female | White | CVA | 1.3 |
| 17 | 19 | Male | White | Sepsis | 0.6 |
| 18 | 46 | Male | Hispanic | CVA | 0.9 |
| 19 | 49 | Female | White | Unknown | 1.1 |
- CVA, cerebrovascular accident.
- 1Calculated with median terminal creatinine due to missing data.
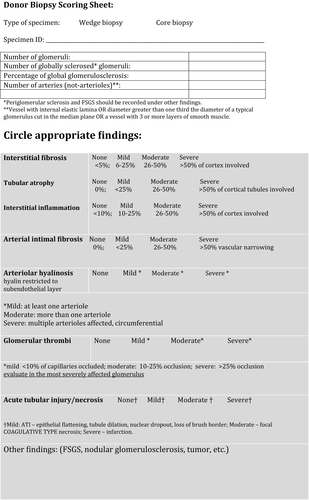
A representative image of the online images is shown in Figure 1. The virtual slide histopathologic data form included designation as wedge or core biopsy and the following parameters: number of glomeruli, number of globally sclerosed glomeruli, percentage of globally sclerosed, number of arteries, interstitial inflammation, interstitial fibrosis, tubular atrophy, arterial intimal thickening, arteriolar hyalinosis, glomerular thrombi, acute tubular injury, and other lesions. A simplified scoring scheme was chosen as follows: none (<5% of area involved), mild (6–25% of area involved), moderate (26–50% of area involved), severe (>50% of area involved). Arteriolar hyalinosis was scored as mild when at least one arteriole was involved, as moderate when more than one arteriole was involved, and as severe when multiple arterioles were affected. The simplified scoring sheet, as described, is shown in Figure 2.
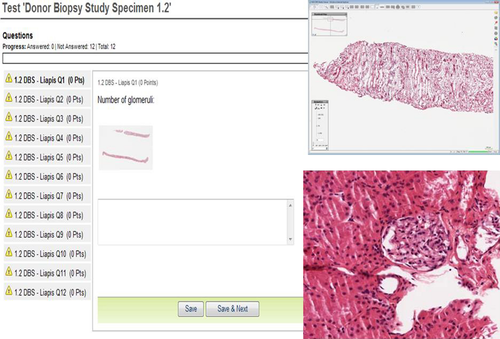
Slides were evaluated by 32 expert renal pathologists in a blinded fashion with no knowledge of donor demographic data. All biopsies were scored by the same group of pathologists, who were assumed to be a random subset of all pathologists. Perfect correlation is indicated as 1, with excellent >0.75, good as 0.5–0.75, fair as 0.25–0.5 and poor as <0.25.
The following three comparisons were performed: (i) frozen versus paraffin wedge biopsies, (ii) frozen versus paraffin core biopsies, (iii) frozen core versus wedge. Paraffin sections were prepared from the defrosted formalin-fixed and paraffin-embedded wedge or core biopsies.
Data from an additional 74 biopsies were collected for graft outcome submitted by the following transplant centers: Washington University in St. Louis; University of Pittsburgh Medical Center; Johns Hopkins University; Institute for Clinical and Experimental Medicine (IKEM; Prague, Czech Republic); Mayo Clinic (Arizona); Transplantation Institute, Medical University of Warsaw (Poland); INCUCAI (Argentinian national institute for procurement and implants) and CUCAIBA (Buenos Aires institute of procurement and implants; Buenos Aires, Argentina); University Hospital, University of São Paulo (Brazil); and Santa Casa de Misericordia de Porto Alegre Hospital and Universidad Federal de Ciências da Saúde de Porto Alegre (Rio Grande do Sul, Brazil).
A code was assigned to each center, and a worksheet was distributed to participating pathologists. The following demographic and clinicopathologic data of the donors and recipients were obtained: donor age; donor SCr; donor cause of death; cold ischemia time (in minutes); donor type (ECD vs. standard criteria donor [SCD]); recipient age; race; date of transplant; age at transplantation; cause of renal failure before transplantation; delayed graft function (DGF; defined as DGF requiring dialysis); slow graft function (persistent elevation in SCr, does not require dialysis); presence or absence of proteinuria at any time; acute cellular rejection (yes or no, grade and number of events); and SCr at 1, 3, 6, and 12 mo after transplant. Posttransplant biopsy findings were also reported if available. The various variables including sex, race, graft function, graft failure and proteinuria were assigned numeric codes to perform statistical analysis. All patients were anonymized.
Statistics
ICCs were used to measure reproducibility between pathologists. ICC is a number between 0 and 1, with a larger number indicating greater reliability 6. ICCs and associated 95% confidence intervals (CIs) were calculated using a two-way random-effects model. Because the model requires balanced data, only data from pathologists rating all participants were used in the analysis. For most of these measurements, data were discrete in nature, so κ statistics were also calculated (not shown) when the measurement had four possible values (e.g. scores of 0, 1, 2 or 3). Results of the κ statistics were at least relatively similar in every case when comparing ratings of 0 or 1 against ratings of 2 or 3 (usually within 0.1 and always within 0.25); therefore, for consistency, and because the CIs for κ statistics are not available with standard methods in a multiple rater case, we showed the ICCs and associated CIs in all cases. The CIs quantify the variability of the estimated ICCs by providing the middle 95% of the range of ICCs that we would expect to observe if we repeated the experiment 100 times. An ICC of ≥0.5 was considered to represent adequate agreement.
Descriptive statistics of demographic and clinical data of biopsies used for graft outcome comparison were summarized. Unadjusted ordinal and standard logistic regression were used to evaluate associations between interstitial fibrosis, arterial intimal fibrosis, and glomerular thrombi and graft failure. Early graft function was modeled using unadjusted ordinal logistic and multinomial regression. Both characteristics of donor and recipient and some of the Kidney Donor Risk Index criteria were considered. Odds ratios for logistic regression and relative risk ratios for multinomial regression were presented, along with p-values from likelihood ratio tests. Relationships between graft function and recipient race and donor SCr were also evaluated adjusting for interstitial and arterial intimal fibrosis levels. Finally, associations between the same predictors and creatinine at different time points were modeled by linear regression. All analyses were conducted using Stata version 14 (StataCorp, College Station, TX).
Results
Interobserver concordance
ICCs measure reproducibility between pathologists (Table 2). Results were calculated to assess scoring reliability (pathologist/biopsy) using digital slides. The final score was the mean of all observers. Frozen core biopsies were scored by 23 pathologists, paraffin core biopsies were scored by 32 pathologists, frozen wedge biopsies were scored by 29 pathologists, and paraffin wedge biopsies were scored by 31 pathologists (Table 2). The total number of glomeruli showed excellent correlation between pathologists (ICC 0.778). The following showed good correlation: number of globally sclerosed glomeruli (ICC 0.563), percentage of globally sclerosed glomeruli (ICC 0.626) and interstitial fibrosis (ICC 0.528). Arteriolar hyalinosis, tubular atrophy, arterial intimal fibrosis, glomerular thrombi and inflammation in nonscarred areas all showed fair correlation (<0.5). The parameters with poor correlation were acute tubular injury (ICC 0.172) (Table 2). Acute tubular injury had poor correlation (ICC 0.172).
| Variable | ICC |
|---|---|
| Number of glomeruli | 0.7782 |
| Number of globally sclerosed | 0.5632 |
| Percentage of globally sclerosed | 0.6262 |
| Number of arteries | 0.449 |
| Interstitial inflammation, total includes scarred areas | 0.443 |
| Interstitial fibrosis | 0.5282 |
| Tubular atrophy | 0.455 |
| Arterial intimal fibrosis | 0.414 |
| Arteriolar hyalinosis | 0.317 |
| Glomerular thrombi | 0.415 |
| Acute tubular injury | 0.172 |
| Inflammation in nonscarred areas (Banff i score) | 0.262 |
- ICC measures reliability between pathologists: <0.25, poor; 0.25–0.5, fair; 0.5–0.75, good; >0.75, excellent. ICC, intraclass correlation.
- 1Frozen core biopsies: 23 pathologists. Paraffin core biopsies: 32 pathologists. Frozen wedge biopsies: 29 pathologists. Paraffin wedge biopsies: 31 pathologists.
- 2Indicates good or excellent ICC.
Comparisons of core frozen versus paraffin and wedge frozen versus paraffin
ICC was excellent in the frozen core biopsies for number of glomeruli only and poor correlation for all remaining parameters (Table 3). Paraffin core biopsies showed excellent and good correlation for number of glomeruli and number of globally sclerosed glomeruli, respectively; arterial intimal fibrosis had good ICC (0.571). All other histologic parameters had fair or poor correlation (ICC <0.5), including interstitial fibrosis and tubular atrophy.
| Variable | Core | Wedge | ||
|---|---|---|---|---|
| Frozen (n = 5) | Paraffin (n = 5) | Frozen (n = 15) | Paraffin (n = 5) | |
| Number of glomeruli | 0.8242 (0.612–0.975) | 0.9362 (0.835–0.992) | 0.7102 (0.556–0.862) | 0.8132 (0.599–0.973) |
| Number of globally sclerosed | 0.323 (0.120–0.810) | 0.8252 (0.618–0.975) | 0.6042 (0.440–0.795) | 0.6652 (0.402–0.944) |
| Number of arteries | 0.275 (0.099–0.772) | 0.401 (0.177–0.851) | 0.349 (0.209–0.583) | 0.488 (0.239–0.890) |
| Interstitial fibrosis | −0.013 (−0.031 to 0.147) | 0.044 (−0.003 to 0.373) | 0.306 (0.177–0.535) | 0.342 (0.142–0.817) |
| Tubular atrophy | −0.031 (−0.040 to 0.053) | 0.023 (−0.010 to 0.288) | 0.262 (0.147–0.483) | 0.381 (0.165–0.841) |
| Interstitial inflammation | 0.041 (−0.015 to 0.403) | 0.150 (0.040–0.629) | 0.5652 (0.398–0.769) | 0.7272 (0.474–0.957) |
| Arterial intimal fibrosis | 0.325 (0.123–0.811) | 0.5712 (0.307–0.918) | 0.453 (0.296–0.680) | 0.379 (0.163,0.841) |
| Arteriolar hyalinosis | 0.063 (<0.001–0.450) | 0.081 (0.012–0.484) | 0.133 (0.062–0.303) | 0.377 (0.163–0.839) |
| Glomerular thrombi | −0.019 (−0.035 to 0.126) | −0.008 (−0.024 to 0.140) | −0.009 (−0.021 to 0.029) | 0.058 (0.005–0.411) |
| Tubular injury | −0.005 (−0.030 to 0.213) | −0.030 (−0.031 to −0.011) | 0.107 (0.047–0.255) | −0.0002 (−0.007 to 0.073) |
- ICC measures reliability between pathologists: <0.25, poor; 0.25–0.5, fair; 0.5–0.75, good; >0.75, excellent. ICC, intraclass correlation.
- 1Confidence intervals are in parentheses. They quantify the variability of the estimated ICC by providing the middle 95% of the range of ICCs that we would expect to observe if we repeated the experiment 100 times.
- 2Indicates good or excellent ICC 6.
On paraffin-defrosted wedge biopsies, ICC scores were excellent for number of glomeruli and number of globally sclerosed and interstitial inflammation. The remaining histologic parameters had fair ICC values (range 0.342–0.488) on the paraffin slides. Comparing frozen sections of wedge kidney biopsies with corresponding paraffin sections showed comparable reproducibility (Table 3). Arteriolar hyalinosis was the only variable that showed a significant discrepancy between the two preparations; frozen section evaluation of arteriolar hyalinosis showed poor correlation (ICC 0.133) compared with fair correlation using paraffin (ICC 0.377). There were insufficient cases with glomerular thrombi to adequately evaluate reproducibility of this feature. Acute tubular injury showed poor correlation regardless of the tissue-preparation technique. Lack of correlation in the latter may be due to freezing artifact of tubular epithelial cells that can be misinterpreted as acute tubular injury. CIs were calculated as shown in parentheses in Table 3.
Frozen wedge biopsies showed greater concordance for number of glomeruli, number of globally sclerosed glomeruli and interstitial inflammation compared with frozen core biopsies. Evaluation of number of arteries, arterial intimal fibrosis, arteriolar hyalinosis and tubular injury were comparable but showed poor concordance between wedge and core frozen-section biopsies. Interstitial fibrosis (0.306 vs. −0.013) and tubular atrophy (0.262 vs. −0.032) showed better concordance in the wedge biopsies.
Correlation of donor clinical and histopathologic characteristics with early graft function
The mean ages (Tables 4 and 5) of the donors and recipients were 50 and 51 years, respectively. Regarding race, 78% of recipients were white and 22% were black. Overall, 62% were men. Moreover, 53% of the donor kidneys were from SCDs and 47% were from ECDs. The majority of the biopsies were wedge (74%).
| Outcome | Predictor (p-value) | ||
|---|---|---|---|
| Ordinal logistic regression1,2 | Multinomial logistic regression1,3 | ||
| Slow versus normal EGF | Delayed versus normal EGF | ||
| Donor age (n = 73) | 1.01 (0.44) | 1.00 (0.78) | 1.01 (0.43) |
| Donor serum creatinine, mg/dL (n = 74) | 1.58 (0.05) | 2.34 (0.07) | 2.25 (0.06) |
| Donor type (n = 73) | |||
| ECD versus baseline of SCD | 1.91 (0.15) | 3.69 (0.08) | 2.24 (0.13) |
| Recipient age (n = 74) | 1.02 (0.22) | 1.03 (0.27) | 1.02 (0.22) |
| Recipient race (n = 74) | |||
| Black versus white/other | 4.65 (<0.01) | 0.76 (0.82) | 4.76 (0.03) |
| Recipient sex (n = 74) | |||
| Female versus male | 0.79 (0.61) | 0.67 (0.57) | 0.76 (0.61) |
| Cold ischemia time, min (n = 74) | |||
| (range 924.6, 1380) versus <924.6 | 1.08 | 0.80 | 1.10 |
| (range 1380, 3345) versus <924.6 | 1.44 (0.77) | 0.75 (0.84) | 1.50 (0.84) |
| Continuous cold ischemia time, min (n = 74) | 1.00 (0.63) | 1.00 (0.50) | 1.00 (0.60) |
| ACR (n = 74) | |||
| Yes versus no ACR | 1.17 (0.72) | 0.60 (0.51) | 1.17 (0.77) |
| CVA (n = 74) | |||
| Yes versus no CVA | 2.17 (0.08) | 1.29 (0.72) | 2.44 (0.09) |
| Donor age (n = 73) | |||
| >50 years (vs. <50 years) | 1.31 (0.55) | 1.00 (0.81) | 1.36 (0.81) |
| Globally sclerosed glomeruli, %5 (n = 74) | 1.04 (0.05) | 1.10 (0.03) | 1.08 (0.04) |
| Interstitial fibrosis5 (n = 74) | |||
| 6–25% versus 0–5% | 1.77 | ||
| 26–50% versus 0–5% (none were >50%) | 4.13 (0.17) | NA4 | NA4 |
| Arterial fibrosis5 (n = 70) | |||
| 1–25% versus none | 1.46 | ||
| 26–50% versus none | 1.40 | ||
| >50% versus none | 3.79 (0.79) | NA4 | NA4 |
| Glomerular thrombi5 (n = 74) | |||
| Present (vs. absent) | 1.93 (0.41) | 5.36 (0.19) | 3.01 (0.36) |
- ACR, acute cellular rejection; CVA, cerebrovascular accident; ECD, expanded criteria donor; EGF, early graft function; NA, not assessed; SCD, standard criteria donor.
- 1The p-values for both models use the likelihood ratio test for the overall significance of the variable as a whole.
- 2The ordinal logistic model shows the proportional odds ratio associated with the level, or 1-U increase, of a given predictor variable and the odds of slow versus normal graft function or delayed versus slow graft function. The ordinal logistic model assumes that these odds ratios are equal between the adjacent ordered outcome levels.
- 3The multinomial logistic model fits separate models for slow versus normal and delayed versus normal graft function. The multinomial model gives a relative risk ratio associated with the level, or 1-U increase, of a given predictor variable and slow versus normal graft function or delayed versus normal graft function.
- 4Model coefficients could not be fit because of sparse data.
- 5The four main variables of interest were adjusted for recipient race (as the only significant characteristic) in a multivariable model; the other p-values are from the unadjusted ordinal or multinomial model.
| Outcome | Predictor Creatinine, mg/dL (p-value)1 | ||||
|---|---|---|---|---|---|
| 1 mo (n = 74) | 3 mo (n = 72) | 6 mo (n = 66) | 1 year (n = 67) | 2 years (n = 50) | |
| Donor age (n = 73) | 0.007 (0.03) | 0.006 (0.04) | 0.004 (0.21) | 0.007 (0.03) | 0.010 (0.02) |
| Donor SCr (n = 74) | <0.001 (0.98) | 0.005 (0.83) | 0.002 (0.96) | 0.002 (0.97) | 0.038 (0.55) |
| Donor type (n = 73) | |||||
| ECD versus baseline of SCD | 0.123 (0.20) | 0.102 (0.20) | 0.048 (0.61) | 0.182 (0.05) | 0.283 (0.01) |
| Recipient age (n = 74) | 0.004 (0.32) | 0.005 (0.08) | 0.003 (0.46) | 0.006 (0.12) | >0.001 (0.99) |
| Recipient race (n = 74) | |||||
| Black versus white/other | −0.433 (p < 0.01) | −0.171 (0.08) | −0.255 (0.02) | −0.037 (0.75) | −0.243 (0.21) |
| Recipient sex (n = 74) | |||||
| Female versus male | −0.177 (0.07) | −0.159 (0.05) | −0.188 (0.05) | −0.116 (0.22) | −0.160 (0.17) |
| Cold ischemia time, min | |||||
| (range 924.6, 1380) versus <924.6 | <0.001 | 0.002 | −0.125 | 0.070 | 0.002 |
| (range 1380, 3345) versus <924.6 | −0.019 (0.98) | 0.022 (0.97) | −0.033 (0.63) | 0.015 (0.81) | 0.123 (0.59) |
| Continuous cold ischemia time, min (n = 74) | <0.001 (0.75) | <0.001 (0.68) | <0.001 (0.98) | <0.001 (0.57) | <0.001 (0.74) |
| ACR (n = 74) | |||||
| Yes (vs. no ACR) | 0.097 (0.32) | 0.129 (0.11) | 0.148 (0.13) | 0.191 (0.04) | 0.480 (p < 0.01) |
| CVA (n = 74) | |||||
| Yes (vs. no CVA) | 0.005 (0.96) | 0.014 (0.86) | −0.008 (0.93) | −0.088 (0.34) | −0.083 (0.48) |
| Donor age (n = 73) | |||||
| >50 years (vs. <50 years) | 0.266 (p < 0.01) | 0.194 (0.01) | 0.182 (0.05) | 0.262 (p < 0.01) | 0.326 (p < 0.01) |
| Globally sclerosed glomeruli, %2 (n = 74) | 0.002 (0.74) | 0.001 (0.80) | 0.009 (0.12) | 0.006 (0.35) | 0.004 (0.45) |
| Interstitial fibrosis2 | |||||
| 6–25% versus 0–5% | 0.089 | 0.058 | 0.124 | 0.105 | −0.005 |
| 26–50% versus 0–5% (none were >50%) | 0.144 (0.58) | 0.056 (0.80) | 0.150 (0.48) | 0.070 (0.58) | −0.051 (0.96) |
| Arterial fibrosis2 | |||||
| 1–25% versus none | 0.047 | 0.095 | 0.189 | 0.109 | −0.030 |
| 26–50% versus none | −0.085 | −0.100 | −0.101 | −0.073 | −0.170 |
| >50% versus none | 0.070 (0.74) | 0.332 (0.12) | −0.136 (0.11) | −0.046 (0.55) | −0.223 (0.54) |
| Glomerular thrombi2 | |||||
| Present (vs. absent) | 0.140 (0.39) | −0.007 (0.96) | 0.075 (0.74) | 0.018 (0.92) | −0.007 (0.97) |
- ACR, acute cellular rejection; CVA, cerebrovascular accident; ECD, expanded criteria donor; SCD, standard criteria donor; SCr, serum creatinine.
- 1All p-values were based on the F-test and coefficients (which give the mean difference in creatinine between categories of the predictor, or for a 1-U change) were based on the linear regression model. Creatinine values were log-transformed to better achieve normality.
- 2The four main variables were adjusted for donor age, donor type, recipient race and ACR (as the significant characteristics) in a multivariable model; the other p-values are from the unadjusted linear regression model.
Logistic and multinomial regression analysis showed that donor SCr and black race affected early graft function by both ordinal logistic regression and multinomial regression analysis (Table 4). Percentage of global glomerulosclerosis correlated with worse SCr at 6 mo (p = 0.04), 1 year (p = 0.04) and 2 years (p = 0.06; Table 5). Donor age was divided into patients aged <50 and >50 years. Divided in this manner, donor age uniformly affected all SCr time points from 1 mo to 1 year. None of the histopathologic characteristics in this study had any correlation with outcome in this small data set in which all kidneys analyzed had mostly mild pathology and were deemed to be of a quality acceptable for transplantation.
| Good wedge biopsies not restricted to the subcapsular cortex can be superior to needle biopsies. |
| Histopathologic parameters with good or fair reproducibility include number of glomeruli, number of globally sclerosed glomeruli, percentage of globally sclerosed glomeruli, interstitial fibrosis and arteriosclerosis. Although only percentage of glomerulosclerosis was identified as a statistically significant parameter that associated with graft function, other studies noted that significant interstitial fibrosis and arteriosclerosis can also adversely affect graft function. Rigidly defined histologic cutoffs such as 20% glomerulosclerosis should not be used in isolation to discard kidneys. |
| Comprehensive clinical evaluation such as that required in calculation of KDPI is an important part of donor evaluation; however, the C-statistic (ability to predict graft failure) for KDPI is only 0.6, and further studies seeking to rigorously evaluate the incremental value of biopsy readings over clinical assessment alone need to be performed. |
|
Training of general pathologists to read donor biopsies using consistent criteria is recommended. Adoption of rapid formalin-fixation and paraffin-embedding protocols that have the potential to eliminate problems associated with interpreting frozen sections need to be studied further. |
- KDPI, Kidney Donor Profile Index.
Discussion
Numerous studies have addressed various issues related to preimplantation kidney biopsies and recommended a set of histopathologic parameters to be evaluated based on their predictive value for graft outcome 7-13. Other investigators included clinical and additional histopathologic parameters in evaluation of donor kidneys 14-17. Histopathologic scoring systems were suggested to assist decision making by transplant teams regarding organ acceptability for transplantation; however, none of the previously proposed scoring systems were as rigorously tested for reproducibility as in the current study. Furthermore, there is no widely accepted histopathologic scoring system for donor kidney biopsies (recently reviewed by Wang et al) 18. Reproducibility in our study is modest. In this regard, future studies should evaluate new histopathology metrics and tissue-preparation techniques.
The significant disagreements among previous studies exist in part because of different methodologies applied to analysis, procurement and processing of donor biopsies 19-21. The significance of arterial intimal fibrosis was acknowledged both by Mazzucco et al and Bosmans et al 20, 21. A likely reason why different studies find different histologic lesions to be critical is that chronicity in any anatomic compartment is prognostic, but sampling considerations lead to different lesions being overrepresented in different biopsy sets. Thrombi were rare in our cohort; however, a recent study showed that thrombi can be missed due to sampling error even when kidney involvement is extensive 22.
Such discrepant results have caused ambiguity. It is unclear whether donor kidney biopsies are able to minimize inappropriate discard of marginal organs. Many investigators are seeking additional prognostic information beyond histopathology. Reese et al, for example, explored the role of urine biomarkers and showed an association of high urinary neutrophil gelatinase-associated lipocalin and liver-type fatty acid binding protein concentrations with lower recipient estimated glomerular filtration rate at 6 mo and good correlation with donor acute kidney injury but found no predictive value for DGF or early allograft dysfunction after transplantation 23. It appears that the fundamental question of how best to predict deceased donor kidney function after transplantation remains elusive. Our study shows that histopathologic parameters in the donor biopsy may be useful but do not all correlate with graft outcome; however, the study is limited by selection bias. Only allografts that are clinically acceptable for transplantation have outcome data. Nonetheless, our results are in agreement with previously published studies 20, 21, 24, 25. Perhaps the difficulty lies in discriminating between changes related to normal aging without significant kidney dysfunction and changes, possibly related to aging, that are predictive of graft outcome. This line of questioning is an ongoing investigation 26.
The working group developed a consensus scoring sheet for donor biopsies, as shown in Figure 2. Following extensive debate, a simplified scoring schema was determined, distinct from Banff scoring for indication biopsies. During this study, we found nonuniformity across multiple institutions with regard to scoring parameters in actual procurement practices. We found, for example, that most centers use wedge biopsies because of perceived higher risk for bleeding associated with needle core biopsies; however, others found needle biopsies acceptable 27. Our study shows some superiority of frozen wedge versus needle core biopsies. This may be related to the lack of standardization of needle biopsies. Different needle gauges lead to different degrees of sampling that are not apparent in standardized wedge biopsies. Banff participants expressed the opinion that there are technical difficulties in cutting 18-gauge needle core biopsies, resulting in inadequate material for frozen section interpretation (data not shown). Needle biopsies are perceived to be more likely to damage larger caliber medullary vessels. The possibility of using punch biopsies instead was suggested, but most in the working group thought that an ample wedge biopsy with good cortical sampling is more likely to yield sufficient number of glomeruli and is likely superior to needle or punch biopsies. We also found that wedge biopsies were often subcapsular and not deep enough to capture vessels (data not shown).
The value of having an experienced renal pathologist versus a general surgical pathologist with no training in reading donor biopsies was also addressed 28. The authors found that trained renal pathologists improved histopathology scores compared with nonrenally trained pathologists 28, 29. Notably, the study by Azancott et al used formalin-fixed (not defrosted), paraffin-embedded biopsies. One may predict that discordance may be higher if frozen sections are used, but none of the participating centers in our study reported using formalin-fixed, paraffin-embedded tissue for initial evaluation of preimplantation biopsies. Our study found frozen and defrosted formalin-fixed, paraffin-embedded wedge biopsies to be comparable. Our study did not compare interpretation by renal pathologists versus general pathologists, but in our opinion, there is a need for training of pathologists assigned to donor biopsy interpretation. There is also need for closer collaboration and communication of pathologists with the transplant teams, particularly on the issue of appropriate sampling of the donor kidney. To this end, the next step of the Banff working group is to assist in preparing educational material for sample processing and interpretation of donor biopsies. Our study used digitally scanned slides accessed remotely from sites on three continents. A digital system for donor biopsy interpretation has already been implemented in some centers in the United States and Europe 30.
Histopathologic assessment of preimplantation biopsies is one component of donor organ assessment and is not an exclusive determinant to discard or transplant donor kidneys. The positive or negative predictive value of studies such as this one is inherently limited because of organ usage bias (in our study, all implanted kidneys were good kidneys), recipient characteristics and posttransplant variables affecting graft outcome. Our outcome data can be considered preliminary. Nevertheless, knowledge of the structural integrity of the kidney is intuitively an important parameter to consider in organ allocation. Importantly, other lesions in addition to those shown to be reproducible in this study need to be mentioned when observed in biopsy assessment. Examples include severe arteriolar hyalinosis, cholesterol emboli, coagulative acute tubular necrosis, extensive thrombosis, myoglobin cast nephropathy, focal segmental glomerulosclerosis, overt proliferative glomerulonephritis, Kimmelstiel–Wilson nodules of diabetic nephropathy, and bile cast nephropathy associated with end-stage liver disease due to HCV infection. Working group preliminary data (not shown) and personal experience of the participants attest to the existence of lesions in donor biopsies, as described earlier. In addition, published studies are confirmatory 31. The donor biopsy can potentially be incorporated into the now widely used KDPI to better judge the impact of medical factors such as black ethnicity, deceased donor status, hypertension, diabetes mellitus and HCV infection. Comprehensive clinical evaluation such as that required in calculation of KDPI is an important part of the donor evaluation; however, the C-statistic (ability to predict graft failure) for KDPI is only 0.6, and further studies seeking to rigorously evaluate the incremental value of biopsy readings over clinical assessment alone need to be performed. Examples of specific scenarios that warrant investigation include hypertension or diabetes mellitus of unknown duration and donors of black race with normal renal function. In these cases, high KDPI can be misleading. Currently, these donor parameters automatically result in the assignment of a substantial negative clinical score without regard to either duration or severity of clinical symptoms and without an attempt to determine whether the donor kidney is actually affected by any of these factors (Table 6).
The caveats and current problems in this study include the small cohort size and freeze artifact. Freeze artifact appears to be a particularly significant factor in a pathologist's ability to assess arteriolar hyalinosis and interstitial fibrosis. The issue of rapid protocols for formalin fixation and paraffin embedding, now technically much improved and increasingly adapted in clinical practice, was not evaluated in this study but needs to be investigated.
In conclusion, the working group found wedge biopsies to be superior to needle core biopsies. This issue was debated repeatedly in the published literature, and some authors suggested the superiority of needle biopsies. Our study did not confirm the latter suggestion. The histopathologic variables that have good reproducibility—number of glomeruli, number of globally sclerosed glomeruli, percentage of globally sclerosed glomeruli—are all superior using wedge kidney biopsies. Reproducibility is fair for interstitial fibrosis, tubular atrophy, interstitial inflammation, arteriolar thrombi and arterial intimal fibrosis. The current study represents the consensus of the Banff working group on scoring donor kidney biopsies; however, our consensus is provisional and subject to future review.
Recommendations and future work
- A thorough examination of the donor biopsy requires attention to all anatomic compartments. Rigidly defined histologic cutoffs such as 20% glomerulosclerosis should not be used in isolation to discard kidneys. Such organs will have a suboptimal half-life but may provide dialysis-free survival for years to appropriately selected recipients. Other parameters including arteriolar hyalinosis, acute tubular injury, thrombotic microangiopathy and diabetic nephropathy must also be included in a histopathologic donor biopsy scoring form as checkmarks.
- Training of general pathologists to read donor biopsies using consistent criteria should be pursued.
- Adoption of rapid formalin-fixation and paraffin-embedding protocols may have the potential to eliminate problems associated with interpreting frozen sections and should be investigated.
Acknowledgments
We acknowledge support from Roche Organ Transplantation Research Foundation Grant 608390948 (to the Banff Foundation for Allograft Pathology for infrastructure support). H.L., J.P.G., P.R. contributed equally in the design, data collection, and data analysis and manuscript preparation. C.K. provided clinical information of the biopsies used for digital evaluation. S.B., E.K., B.F., S.S., M.M., M.H. participated in digital scoring, contributed clinical follow up/graft survival data and or critically reviewed the manuscript. E.H., A.P.P., D.D., H.G., M.S., K.L.P. contributed detailed histopathologic and clinical data of pre and post implantation biopsies and graft/patient survival data. V.P., D.L., T.H., X.G. performed statistics. The following participated in digital scoring and or contributed ideas and suggestions.
| Afrousian | Marjan | University of Texas Medical Branch, Galveston, TX |
| Alexander | Mariam | Mayo Clinic, Rochester, MN |
| Arend | Lois | Johns Hopkins University Hospital, Baltimore, MD |
| Bajema | Ingeborg | Leiden University Medical Center, Leiden, the Netherlands |
| Balasubramanian | Manjula | Albert Einstein Medical Center, Philadelphia, PA |
| Chander | Praveen | New York Medical College |
| Cheunsuchon | Boonyarit | Siriaj Hospital, Mahidol University, Bangkok, Thailand |
| Cornell | Lynn | Mayo Clinic, Rochester, MN |
| de Franco | Marcello | University of San Paulo, Renal Transplant Service, Brazil |
| Farkash | Evan | University of Michigan Med School, Ann Arbor, MI |
| Fogo | Agnes | Vanderbilt University |
| Fyfe | Billie | Robert Wood Johnson Med. School, New Brunswick, NJ |
| Iskander | Samy | Wake Forest University School of Medicine, NC |
| Kemeny | Eva | University Szeged, Szeged, Hungary |
| Lukic | Dusan | McMaster University, Hamilton, Ontario, Canada |
| Mazzucco | Gianna | Universita Degli Studi Di Torino, Italy |
| Monga | Guido | Università del Piemonte Orientale “A. Avogadro,” Novara, Italy |
| Mubarak | Muhammed | University Hospital Basel, Basel, Switzerland |
| Nickeleit | Volker | University of North Carolina, Chapel Hill, NC |
| Nizze | Horst | Universitat Rostock, Germany |
| Papadimitriou | John | University of Maryland, Baltimore, Maryland |
| Picken | Maria | Loyola University Medical Center, Maywood, IL |
| Pullman | James | Montefiore Medical Center, Bronx, NY |
| Racusen | Lorraine | Johns Hopkins University Hospital, Baltimore, MD |
| Sadeghipour | Alireza | Atieh hospital, Tehran Tehran, Iran |
| Saker | Zakaria | National Center of Urology, Georgia (Tbilisi, Georgia) |
| Setty | Suman | University of Illinois College of Medicine, Chicago, IL |
| Sharma | Shree | Nephropath, Little Rock, AR |
| Sheaff | Michael | Barts Health NHS Trust, London, UK |
| Soares | Maria F. | Universidade Federal do Parana, Curitiba, Brazil |
| Solez | Kim | University of Alberta, Edmonton Alberta, Canada |
| Taheri | Diana | Isfahan University of Medical Sciences, Isfahan Isfahan, Iran |
| Tan | Jane | Stanford Health Care, Stanford, CA |
| Troxell | Megan | Oregon Health Science University, Portland, OR |
| Truong | Luan | Weill Medical College of Cornell University, Houston, TX |
| Vasquez Martul | Eduardo | Hospital Universitario A Coruna, A Coruna, Spain |
| Walker | Patrick | Nephropath, Little Rock, AR |
| Wellen | Jason | Dept. of Surgery, Washington University, St. Louis, MO |
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




