Baseline Donor Chronic Renal Injury Confers the Same Transplant Survival Disadvantage for DCD and DBD Kidneys
Abstract
Histological assessment of baseline chronic kidney injury may discriminate kidneys that are suitable for transplantation, but has not been validated for appraisal of donation after circulatory death (DCD) kidneys. ‘Time-zero' biopsies for 371 consecutive, solitary, deceased-donor kidneys transplanted at our center between 2006 and 2010 (65.5% DCD, 34.5% donation after brain death [DBD]) were reviewed and baseline chronic degenerative injury scored using Remuzzi's classification. High scores correlated with donor age and extended criteria donors (42% of donors), but the spectrum of scores was similar for DCD and DBD kidneys. Transplant outcomes for kidneys scoring from 0 to 4 were comparable (1 and 3 year graft survival 95% and 92%), but were much poorer for kidneys scoring ≥5, with 1 year graft survival only 73%, and 12.5% suffering primary nonfunction. Critically, high Remuzzi scores conferred the same survival disadvantage for DCD and DBD kidneys. On multi-variable regression analysis, time-zero biopsy score was the only independent predictor for graft survival, whereas one-year graft estimated glomerular filtration rate (eGFR) correlated with donor age and biopsy score. In conclusion, the relationship between severity of chronic kidney injury and transplant outcome is similar for DCD and DBD kidneys. Kidneys with Remuzzi scores of ≤4 can be implanted singly with acceptable results.
Abbreviations
-
- CI
-
- confidence interval
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after circulatory death
-
- ECD
-
- extended criteria donors
-
- eGFR
-
- estimated glomerular filtration rate
Introduction
Heightened demand for kidney transplantation has prompted expansion of the deceased donor pool by using increasing numbers of kidneys from so-called ‘marginal' donors. This includes elderly donors or those with significant cardiovascular morbidity (extended criteria donors) and donors with unfavorable donor procedure characteristics, such as donation after circulatory death (DCD) donors. The warm ischaemic insult integral to DCD has been generally considered to impact unfavorably on kidney transplant outcomes. Nevertheless, DCD kidney transplantation in the United Kingdom has expanded dramatically in recent years, with a threefold increase in DCD transplant numbers since 2006. This has been achieved despite marked variation between transplant centers in their use of DCD kidneys, particularly those from extended criteria donors 1, 2. Such center variation persists 2, despite increasing evidence that outcomes for DCD and conventional donation after brain death (DBD) kidney transplants are similar 3, 4. The ongoing concern regarding selection of DCD kidneys for transplantation is apparent in analysis of discard rates of deceased donor kidneys in the United Kingdom; for donors over 60 years, approximately three quarters of offered DBD kidneys are implanted, whereas less than a third of offered DCD kidneys are implanted 5.
One practical approach that may enable more marginal donor kidneys to be transplanted safely is adoption of preimplantation histological assessment of chronic kidney injury. Established by Remmuzi and colleagues, preimplantation biopsies are scored according to the presence of: glomerular sclerosis; tubular atrophy; interstitial fibrosis; and atherosclerosis, giving a score of 0–3 for each of these components. Kidneys with a summed score of >6 should be discarded, those with a score of 0–3 used singly, and those with scores of 4–6 used as a dual kidney transplant 6. Using this strategy, Remuzzi reported an overall improvement in graft survival of kidneys from elderly donors that were first biopsied compared to those that were not biopsied, with a 24% increase in the number of kidneys transplanted 7. While this approach has been shown to be effective for kidneys from DBD donors, its applicability to DCD kidney transplantation is not clear, because the few studies that stratify outcome for DCD kidney transplantation according to time-zero Remuzzi score contain only small numbers of biopsies 8. Hence, preimplantation histological assessment of donor chronic kidney injury has not been widely implemented in the UK and the potential of this approach to further increase the number of kidney transplants from marginal DCD donors has not been reported.
We have previously reported that DCD and DBD kidneys from donors with similar chronic kidney injury (equivalent biopsy scores) have comparable survival 9. However, our DCD kidney transplant programme was in its initial stages and we acknowledged that this finding was perhaps only applicable to relatively young donors that were unlikely to have significant kidney chronic degenerative changes 9. Since then, our DCD transplant activity has increased substantially and we now perform twice as many DCD as DBD kidney transplants and routinely use DCD kidneys from elderly donors (>60 years old) 5. Here we report a single-center, retrospective, observational, cohort analysis of our more recent experience on the impact of donor chronic renal injury on outcomes following deceased donor kidney transplantation. We show that the presence of significant donor chronic kidney injury, as determined by histological assessment of kidney biopsies, confers poorer graft survival, but the detrimental impact is similar regardless of donor type.
Methods
Study population
This is a single-center, retrospective, observational, cohort study that examined the impact of donor chronic kidney injury on graft outcomes. The study population comprised 371 recipients of a single kidney transplant between June 2006 and August 2010 (census date January 31, 2012). All DCD kidneys were procured from controlled, Maastricht category 3 10, donors who incurred irrecoverable brain injury, but did not meet the criteria for diagnosis of brainstem death. Kidney procurement was performed as previously described 1 and donation was pursued for a minimum of 4 h after withdrawal of life-supporting treatment. Kidneys from DBD donors were allocated nationally according to an algorithm that incorporates HLA matching, time on the waiting list, level of sensitization to HLA and donor-recipient age difference 11. National sharing has yet to be implemented in the United Kingdom for DCD kidneys, and they were allocated locally using a similar algorithm to that used nationally for DBD kidneys. Extended criteria donors (ECD) were defined as those ≥60 years or those aged 50–59 years with two of the following three features: hypertension; terminal serum creatinine > 115 mmol/L; or death from cerebrovascular accident 12. Donors with acute kidney injury (high terminal creatinine) were considered for kidney donation only when recent tests indicated satisfactory baseline renal function.
Histopathological evaluation of kidney biopsies
Baseline donor chronic kidney injury was assessed routinely (as recommended by Banff 13) by performing a surgical wedge biopsy of kidney grafts following revascularization in the recipient (time-zero biopsy). Occasionally a wedge biopsy of the kidney was performed prior to implantation and analysis of this biopsy was used to inform suitability for transplantation. The decision to perform such preimplantation biopsies was based on donor information such as age and co-morbidities, as well as macroscopic kidney appearance (including perfusion characteristics) at the time of organ procurement. In the absence of a preimplantation or time-zero kidney biopsy, baseline donor chronic kidney injury was assessed by analysis of core needle biopsies that had been performed, when clinically indicated, in the first two weeks after transplantation. This approach was deemed justifiable because our histopathologists (VB, ST and MHG) were confident that donor baseline chronic kidney injury was not influenced by recipient factors in such a short time frame 14 and that patterns of baseline chronic kidney injury in core needle biopsies performed within the first two weeks from transplantation and in wedge biopsies performed at time zero on the same kidneys were similar. All available kidney biopsies were reexamined by an experienced histopathologist, blinded to the donor characteristics, and scored for chronic histopathological changes according to the classification system introduced by Remuzzi and colleagues (also known as the Pirani score) 6. In brief, the Remuzzi total biopsy score comprises four components: glomerular, tubular, interstitial and vascular; each component may score from 0 (normal) to 3 and, therefore, the total score may range from 0 to 12. The average number of glomeruli sampled on biopsy was 42 (SD: 32). A baseline donor kidney biopsy was not available for 51 cases and this was mainly due to biopsy sampling errors, insufficient tissue for histological assessment and missing samples that were not available for reevaluation.
Clinical variables
Delayed graft function was defined as the provision of dialysis in the first week after transplantation and primary nonfunction was defined as a graft that never achieved sufficient function to allow discontinuation of dialysis, excluding acute vascular thrombosis 15. Estimated Glomerular Filtration Rate (eGFR) was calculated using the four variable Modification of Diet in Renal Disease formula and is expressed in milliliters per minute, adjusted for body surface area 16. Immunosuppression was administered according to standard protocols, as described previously 9.
Statistical analysis
Differences in demographic and clinical characteristics between recipients of DCD and DBD kidneys were examined with t-test for continuous variables or with Chi-squared test for discrete variables. The effect of risk factors on kidney transplant survival was assessed using Cox regression. Results are expressed as hazard ratios with calculated 95% confidence interval (CI); p-values were derived from likelihood ratio tests. Kaplan–Meier curves were plotted to depict kidney transplant survival and curve comparison was performed with the log-rank test. Greenwood's formula was used to analyze the survival curves at fixed time points (1 year and 3 years). Kidney transplant function at 1 year (eGFR) was analyzed using linear regression. Incomplete data entries were assumed to be missing completely at random and were not included in our analyses. Graft survival was censored for patient death with a functioning graft (conclusions from graft survival analysis performed without censoring for patient death were essentially unchanged and are, therefore, not reported).
Results
Between June 2006 and August 2010, 371 deceased donor renal transplants were implanted as single transplants at the Cambridge Transplant Centre. Of these, 243 (65%) recipients were transplanted with kidneys from controlled DCD donors, and 128 (35%) from DBD donors. Donors from both groups had similar demographic and clinical characteristics (Table 1) and 42% of transplanted kidneys were procured from donors fulfilling ECD criteria 12. Acute kidney injury did not preclude donation and the proportion of donors with elevated terminal creatinine >150 µmol/L was comparable in the two groups (Table 1). Compared with recipients of DCD kidneys, recipients of DBD kidneys were more likely to have preformed HLA specific antibodies and to have received kidneys that were better HLA-matched (reflecting national allocation), but had longer cold ischaemic times.
| Variable | DBD (n = 128) | DCD (n = 243) | p-Value |
|---|---|---|---|
| Recipient age (years), mean (SD) | 47.9 (13.5) | 50.3 (13.5) | 0.105 |
| Recipient sex, n (%) | |||
| Female | 50 (39%) | 74 (31%) | 0.120 |
| Male | 78 (61%) | 169 (70%) | – |
| Recipient ethnicity, n (%) | |||
| White | 115 (90%) | 213 (88%) | 0.367 |
| Non-white | 13 (10%) | 30 (12%) | – |
| Graft number, n (%) | |||
| First | 101 (79%) | 224 (92%) | 0.001 |
| Second/third | 27 (21%) | 19 (8%) | – |
| HLA level1, n (%) | |||
| 1 | 31 (24%) | 9 (3%) | <0.001 |
| 2 | 42 (33%) | 99 (41%) | – |
| 3 | 52 (41%) | 128 (53%) | – |
| 4 | 3 (2%) | 7 (3%) | – |
| Cold ischaemic time2 (hours) | |||
| Mean (SD) | 14.8 (3.71) | 13.6 (4.25) | 0.010 |
| Calculated reaction frequency3, n (%) | |||
| ≤15% | 73 (57%) | 190 (78%) | <0.001 |
| >15% | 55 (43%) | 53 (22%) | – |
| Remuzzi biopsy score2, n (%) | 106 | 214 | 0.061 |
| 0 | 27 (26%) | 41 (19%) | – |
| 1 | 15 (14%) | 17 (8%) | – |
| 2 | 12 (11%) | 41 (19%) | – |
| 3 | 16 (15% | 45 (21%) | – |
| 4 | 21 (20%) | 37 (17%) | – |
| 5 | 5 (5%) | 21 (10%) | – |
| ≥6 | 10 (9%) | 12 (6%) | – |
| Donor age (years), mean (SD) | 48.2 (16.5) | 50.2 (16.2) | 0.256 |
| Donor sex, n (%) | |||
| Female | 67 (52%) | 103 (42%) | 0.085 |
| Male | 61 (48%) | 140 (58%) | – |
| Donor ethnicity2, n (%) | |||
| White | 119 (94%) | 232 (95%) | 0.319 |
| Non-white | 8 (6%) | 11 (5%) | – |
| Extended criteria donor status (ECD)2, n (%) | |||
| ECD | 56 (44%) | 103 (46%) | 0.968 |
| Not ECD | 70 (56%) | 122 (54%) | – |
| Donor cause of death | |||
| Intracranial haemorrhage/thrombosis | 79 (62%) | 87 (36%) | <0.001 |
| Hypoxic brain damage | 16 (12.5%) | 54 (22%) | – |
| Intracranial other (tumour etc) | 13 (10%) | 7 (3%) | – |
| Trauma | 11 (8.5%) | 41 (17%) | – |
| Cardiovascular | 1 (1%) | 6 (2%) | – |
| Other | 8 (6%) | 48 (20%) | – |
| Agonal phase duration (minutes)4,2 | |||
| Median (SD) | – | 18 (120) | N/A |
| Donor terminal creatinine2 (µmol/L) | |||
| Mean (SD) | 97.4 (62) | 99.7 (70) | 0.767 |
| Donor terminal creatinine2 >150µmol/L n (%) | 15 (14%) | 28 (12%) | 0.749 |
- 1 HLA mismatch level was defined according to UK allocation policy for kidneys from brain-death donors and was based on the mismatch between donor and recipient at the HLA-A, HLA-B and HLA-DR loci: level 1 was a 000 HLA-A, HLA-B and HLA-DR mismatch; level 2 was a 0 HLA-DR plus 0/1 HLA-B mismatch; level 3 was a 0 HLA-DR plus 2 HLA-B mismatch or a 1 HLA-DR plus 0/1 HLA-B mismatch; and level 4 was a 2 HLA-DR or a 1 HLA-DR plus 2 HLA-B mismatch.
- 2 Variable contains missing data. Cold ischaemic time was not available for three DBD cases and five DCD cases; biopsy scores were not available for 22 DBD cases and 29 DCD cases; donor ethnicity was not available for one DBD case; ECD status was not available for two DBD cases and 20 DCD cases; agonal phase duration was not available for 71 DCD cases; donor terminal creatinine was not available for 19 DBD cases and 56 DCD cases.
- 3 Recipient sensitization was defined as HLA antibody reaction frequency, which is calculated by comparison of unacceptable HLA specificities with HLA types of donors of identical ABO blood group in a pool of 10 000 donors on the UK transplant database.
- 4 Agonal phase was defined as the time from withdrawal of donor life-supporting treatment to donor circulatory arrest.
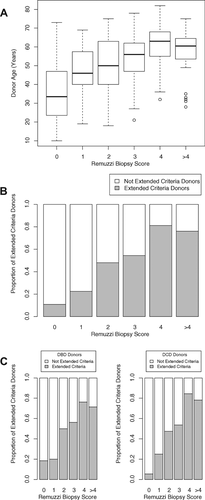
Implanted kidneys were assessed histologically according to the Remuzzi scoring system (Table 1). Kidney biopsies were available for 320 (86%) transplants; of these, 51 (16%) were obtained and analyzed before implantation, 237 (74%) at reperfusion of the graft and the remaining 32 (10%) were core biopsies obtained within 2 weeks following transplantation. Preimplantation biopsies were more likely to be performed for kidneys procured from elderly, ECD and donors with elevated preterminal creatinine (data not shown). Severity of donor chronic renal injury correlated with donor age (Figure 1A) and with ECD status (Figure 1B). The correlation with donor age was, however, relatively weak (Pearson's correlation coefficient: 0.49, p < 0.001, Figure 1A), and kidneys from elderly donors frequently had only minimal chronic injury whereas some kidneys from younger donors scored less favorably. Approximately one quarter (33%) of histologically assessed kidneys scored over three (Table 1); a score at which Remuzzi advocates that kidneys should be implanted as double transplants 6. This reflects that approximately 80% of the available biopsies were analyzed routinely after the transplant and that the biopsy score was therefore not known before implantation.
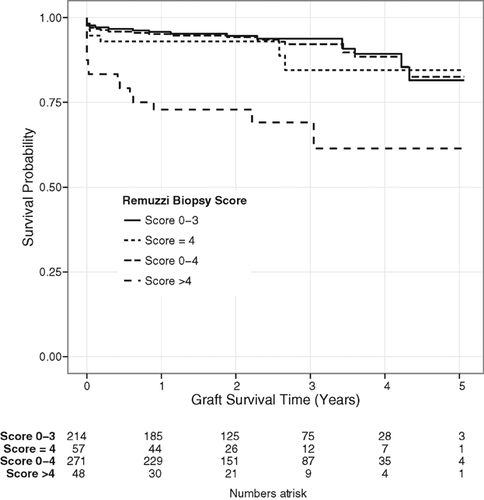
The impact of donor chronic kidney injury on outcomes following transplantation was assessed initially by Kaplan–Meier analysis. Survival at 1 year of kidney transplants with a biopsy score of 3 or less was significantly better than kidneys that scored greater than 3 (1 year graft survival of 95.8% vs. 83.7% respectively, p = 0.008). The use of a biopsy score value of 3 to discriminate between single or dual transplantation, as proposed by Remuzzi, has recently been questioned 17. Because in our series time-zero biopsies were not routinely analyzed until after transplantation, this enabled analysis of outcomes for kidneys transplanted singly despite scoring over 3 on biopsy. We therefore repeated the Kaplan–Meier analysis, but considered kidneys with Remuzzi score of 4 as a separate group (Figure 2). Survival of kidneys in the group that scored 4 was similar to that of kidneys in the group that scored 0–3, whereas survival of kidneys scoring 5 and over was significantly poorer (Figure 2). Analysis of the survival curves (Figure 2) highlights that the difference in longer term outcomes for kidneys that scored ≤4 and ≥5 is apparent within the first year of transplantation; 1 year graft survival for kidneys scoring ≤4 was 95%, whereas only 73% of kidneys scoring ≥5 were still functioning at 1 year (p = 0.012). The increased failure within the first year in the latter group partly reflects a higher incidence of primary nonfunction [6 of 48, (12.5%) vs. 7 of 272 (2.6%) for kidneys scoring ≤4, p < 0.001], but also that 3 of the remaining 12 kidneys that failed within the first year in this group never achieved good graft function (eGFR persistently less than 20 mL/min/m2). On multivariable logistic regression analysis (Table 2), chronic kidney injury, as assessed by Remuzzi biopsy score, was the only significant independent predictor of kidney transplant primary nonfunction (OR: 4.78, 95% CI: 1.31–17.38, p = 0.018 for kidney transplants with biopsy score ≥5). Thus, our analysis suggests that kidneys with a biopsy score of up to 4 can be safely transplanted singly, but that caution should be exercised using kidneys with higher scores as single transplants (Figure 2).
| Variable | Odds ratio | 95% CI | p-Value |
|---|---|---|---|
| Donor type | |||
| DBD | Reference | – | – |
| DCD | 2.06 | 0.41–10.19 | 0.377 |
| Remuzzi biopsy score1 | |||
| 0–4 | Reference | – | – |
| >4 | 4.78 | 1.31–17.38 | 0.018 |
| Graft number | |||
| First | Reference | – | – |
| Second/third | 6.29 | 0.87–45.36 | 0.068 |
| Cold ischaemic time (per hour) | 0.98 | 0.85–1.14 | 0.825 |
| Recipient sensitization | |||
| Non-sensitized | Reference | – | – |
| Sensitized# | 1.14 | 0.24–5.47 | 0.871 |
| Donor age (per decade) | 1.38 | 0.52–3.66 | 0.522 |
| Extended criteria donor (ECD) | |||
| Not ECD | Reference | – | – |
| ECD | 4.64 | 0.22–97.69 | 0.324 |
| Donor terminal creatinine (per unit increase) | 1.01 | 1.00–1.02 | 0.142 |
- # HLA sensitization was defined as recipient calculated reaction frequency >15%.
- 1 Biopsy score was not available for three kidneys with primary nonfunction.
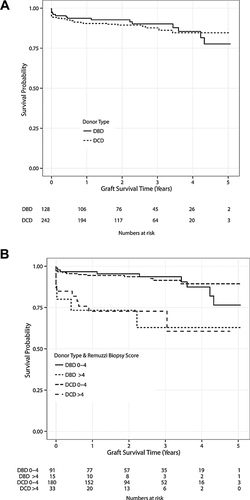
Previous reports detailing histological assessment of chronic kidney injury have focused largely on kidneys from DBD donors. Our study differs in that approximately twice as many DCD as DBD kidney transplants were performed, providing sufficient numbers to assess the impact of donor chronic kidney injury on outcomes of DCD kidneys. In agreement with recently published UK registry data 3, 4, outcomes for DCD and DBD kidney transplants in our series were comparable (Figure 3A). Discard rates for DCD kidneys are currently much higher than discard rates for DBD kidneys in the United Kingdom 5, but the comparable results for DCD and DBD kidneys achieved in our series do not reflect a selection bias to using more optimal DCD kidneys, because DCD and DBD donor characteristics were similar (Table 1) and, notably, the spectrum of baseline kidney injury in both groups was comparable (Figure 1C). The implication from these observations that high histology scores confer the same graft survival disadvantage for DCD and DBD kidneys is further supported by analysis of Kaplan–Meier survival data, stratified for DCD and DBD kidney transplants by biopsy score (Figure 3B).
We then examined the utility of time-zero biopsy assessment of baseline donor chronic kidney injury compared to other donor and recipient factors in predicting kidney graft outcomes. Univariable Cox regression analysis showed that established chronic kidney injury and ECD status were associated with higher risk of graft failure (Table 3). However, on multivariable analysis (Table 4), chronic kidney injury, as assessed by Remuzzi biopsy score, was the only statistically significant independent predictor of kidney transplant survival (HR: 3.88, 95% CI: 1.78–8.44, p < 0.001). Notably, donor terminal creatinine, a marker of acute kidney injury, was not associated with allograft survival. Similarly, for the DCD cohort, agonal phase duration did not influence kidney transplant outcome (HR: 0.99, 95% CI: 0.99–1.00, p = 0.632). Contrary to most large registry analyses 3, 4, 18, donor age was also not associated with transplant survival (Table 4), perhaps reflecting the smaller transplant numbers and relatively shorter follow-up of patients in our single-center study. However, on multiple linear regression modelling, donor age was the strongest predictor of graft function (eGFR) at 1 year, with every additional year in donor age associated with 0.25 mL/min/m2 decrease in 1 year eGFR (Table 5A). In this analysis, baseline donor chronic kidney injury had only a marginal impact on eGFR. This likely reflects the relatively large proportion of kidneys with a Remuzzi score ≥5 that failed within the first year (27%) and which were therefore excluded from eGFR analysis. In support, when the analysis was repeated, but including the recipients of those kidneys that failed within the first year by assigning them an arbitrary eGFR value of 15 mL/min/m2, the impact of baseline donor chronic kidney injury on 1 year graft function became more apparent (Table 5B).
| Variable | Hazard ratio | 95% CI | p-Value |
|---|---|---|---|
| Donor type | |||
| DBD | Reference | – | – |
| DCD | 1.13 | 0.61–2.12 | 0.697 |
| Remuzzi biopsy score | |||
| 0 | Reference | – | – |
| 1 | 0.75 | 0.15–3.87 | 0.730 |
| 2 | 1.37 | 0.40–4.47 | 0.617 |
| 3 | 1.09 | 0.32–3.78 | 0.887 |
| 4 | 1.55 | 0.47–5.08 | 0.472 |
| 5 | 5.05 | 1.65–15.46 | 0.005 |
| ≥6 | 5.50 | 1.74–17.36 | 0.004 |
| Recipient age (per decade) | 1.04 | 0.83–1.29 | 0.743 |
| Recipient gender | |||
| Female | Reference | – | – |
| Male | 0.96 | 0.52–1.80 | 0.909 |
| Recipient ethinicity | |||
| White | Reference | – | – |
| Non-white | 1.64 | 0.73–3.68 | 0.235 |
| Graft number | |||
| First | Reference | – | – |
| Second/third | 1.42 | 0.63–3.19 | 0.394 |
| HLA level | |||
| Level 1 | Reference | – | – |
| Level 2 | 2.06 | 0.47–9.01 | 0.337 |
| Level 3 | 2.76 | 0.65–11.61 | 0.167 |
| Level 4 | 1.00 | 0.00–Inf | 1.000 |
| Cold ischaemic time (per hour) | 1.01 | 0.94–1.09 | 0.713 |
| Recipient sensitization | |||
| Non-sensitized | Reference | – | – |
| Sensitized1 | 1.24 | 0.66–2.35 | 0.505 |
| Donor age (per decade) | 1.19 | 0.97–1.44 | 0.0882 |
| Donor gender | |||
| Female | Reference | – | – |
| Male | 0.76 | 0.42–1.37 | 0.355 |
| Donor ethnicity | |||
| White | Reference | – | – |
| Non-white | 2.30 | 0.82–6.48 | 0.114 |
| Extended criteria donor (ECD) | |||
| Not ECD | Reference | – | – |
| ECD | 2.25 | 1.18–4.27 | 0.013 |
| Donor terminal creatinine (per unit increase) | 0.999 | 0.994–1.004 | 0.718 |
- 1 HLA sensitization was defined as recipient calculated reaction frequency >15%.
| Variable | Hazard ratio | 95% CI | p-Value |
|---|---|---|---|
| Donor type | |||
| DBD | Reference | – | – |
| DCD | 0.95 | 0.42–2.17 | 0.903 |
| Remuzzi biopsy score | |||
| 0–4 | Reference | – | – |
| >4 | 3.88 | 1.78–8.44 | <0.001 |
| Graft number | |||
| First | Reference | – | – |
| Second/third | 1.88 | 0.52–6.75 | 0.336 |
| Cold iscaemic time (per hour) | 1.01 | 0.93–1.10 | 0.823 |
| Recipient sensitization | |||
| Non-sensitized | Reference | – | – |
| Sensitized1 | 1.23 | 0.49–3.04 | 0.657 |
| Donor age (per decade) | 1.15 | 0.69–1.90 | 0.602 |
| Extended criteria donor (ECD) | |||
| Not ECD | Reference | – | – |
| ECD | 1.86 | 0.43–8.01 | 0.406 |
| Donor terminal creatinine (per unit increase) | 1.00 | 0.99–1.01 | 0.510 |
- 1 HLA sensitization was defined as recipient calculated reaction frequency >15%.
| Variable | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Donor type | |||
| DBD | Reference | – | – |
| DCD | 2.27 | −1.91 to 6.44 | 0.289 |
| Remuzzi biopsy score | |||
| 0–4 | Reference | – | – |
| >4 | −5.56 | −11.37 to 0.25 | 0.062 |
| Graft number | |||
| First | Reference | – | – |
| Second/third | 1.03 | −5.67 to 7.72 | 0.764 |
| Cold ischaemic time (per hour) | 0.01 | −0.47 to 0.50 | 0.955 |
| Donor age (per decade) | −2.51 | −4.66 to–0.35 | 0.023 |
| Recipient age (per decade) | −1.61 | −3.36 to 0.13 | 0.071 |
| Extended criteria donor (ECD) | |||
| Not ECD | Reference | – | – |
| ECD | −4.98 | −11.82 to 1.86 | 0.154 |
- Information on kidney transplant function (eGFR) at 1 year following transplantation was not available for four cases.
| Variable | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Donor type | |||
| DBD | Reference | – | – |
| DCD | 1.77 | −2.41 to 5.95 | 0.406 |
| Remuzzi biopsy score | |||
| 0–4 | Reference | – | – |
| >4 | −7.67 | −8.86 to–6.48 | 0.007 |
| Graft number | |||
| First | Reference | – | – |
| Second/third | −0.95 | −7.65 to 5.75 | 0.777 |
| Cold ischaemic time (per hour) | 0.17 | −0.31 to 0.65 | 0.494 |
| Donor age (per decade) | −2.42 | −4.60 to–0.23 | 0.031 |
| Recipient age (per decade) | −1.70 | −3.43 to 0.04 | 0.056 |
| Extended criteria donor (ECD) | |||
| Not ECD | Reference | – | – |
| ECD | −6.53 | −13.34 to 0.28 | 0.064 |
- Information on kidney transplant function (eGFR) at 1 year following transplantation was not available for four cases.
- # Kidneys that failed within the first year following transplantation (n = 37) were assigned an arbitrary eGFR value of 15 mL/min/m2.
The above analyses included core biopsies performed within the first 2 weeks after transplantation because it is unlikely that the severity of baseline donor chronic kidney injury would be altered by recipient factors in such a short time frame 14. Nevertheless, to account for potential variability created by a different biopsy technique taken at a different time point we repeated the key analyses (Kaplan–Meier, Cox regression) with the core biopsy cohort excluded (n = 32). Results from these analyses were essentially unaltered; baseline donor chronic kidney injury remained the only predictor for graft failure, but the impact of baseline chronic kidney injury was similar for DCD and DBD kidneys, and only evident for kidneys with a Remuzzi score greater than 4 (data not shown).
Discussion
Preimplantation biopsy grading of the severity of baseline chronic kidney injury can, by providing a framework for either discarding deceased donor kidneys or allocating as single or dual transplants, improve outcomes of ‘marginal' or extended criteria kidneys 7, 17, 19. However, the values of the Remuzzi score that determine single or dual implantation were derived from a hypothetical evaluation of the minimum required nephron mass, and the precise cut-off points of the score are still debated and have yet to be validated clinically 20, 21. Similarly, few studies have examined the relevance of the Remuzzi score to assessment of DCD kidneys, and it is possible that acute ischaemic injury related to donor instability during the ‘agonal' or asystolic phases, which would not be apparent on time-zero biopsy, may have a greater impact on long-term DCD kidney outcomes than the presence of chronic baseline renal degenerative changes. This is particularly pertinent to deceased donor kidney transplantation in the United Kingdom, because circulatory death donors have increased approximately threefold in the last 6 years and, although national DCD and DBD rates are reaching parity, marked geographical variation in DCD kidney usage persists 2. Our study addresses these two concerns, and we demonstrate for the first time that acceptable transplant results are achieved by implanting kidneys singly that score 4 or less, and critically, that baseline chronic renal injury confers the same survival disadvantage for DCD as DBD kidney transplants. Our results, therefore, suggest that more widespread use of preimplantation biopsy analysis may be a means for reducing the currently high rate of discard of DCD kidneys.
The strength of association between the severity of baseline chronic kidney injury, as assessed by time-zero biopsy, and long term kidney transplant outcome was surprising. Our multivariable analysis revealed that the Remuzzi score was the only independent predictor for kidney graft survival, emphasizing that chronic renal injury acquired in the donor is a major determinant of kidney transplant outcome, but further suggesting that factors such as donor age and ECD status, which are commonly considered to impact upon transplant outcome 3, 22-24 and which were included in our analysis, may instead be surrogate markers for the presence of baseline chronic degenerative changes.
Our study cohort includes a sizeable proportion of DCD kidneys and, to our knowledge, our analysis represents the largest reported series to date examining the utility of histological assessment of baseline donor chronic kidney injury in the context of DCD kidney transplantation. Snoeijs et al have previously reported an association between chronic baseline donor kidney injury and outcome after DCD kidney transplantation, but this series was limited to only 49 biopsies and it was consequently not possible to evaluate the precise threshold of the Remuzzi score above which results for single kidney transplantation are poor 8. Furthermore, a comparison of the impact of chronic renal injury on outcomes following DCD and DBD kidney transplantation was not performed. Thus, our demonstration that baseline chronic kidney injury impacts similarly upon outcomes for DBD and DCD kidney transplantation is probably our most important finding. That baseline chronic kidney injury does not synergize with DCD status in conferring poorer transplant survival presumably explains the equivalent results for DCD and DBD kidney transplants in our series, but also suggests that the comparable outcomes reported for DCD and DBD kidneys in UK registry analyses 3, 4 does not reflect bias in selecting only the optimal donors from the potential DCD pool. A further corollary of our findings is that the ischaemic injury to which DCD, but not DBD, kidneys are subjected during procurement does not impact significantly on long term transplant outcome. This supports our previous report that agonal phase characteristics do not correlate with DCD kidney survival or function 1. A number of kidney transplants in our series were retrieved from donors with prolonged agonal phases, because we routinely wait a minimum of 4 h for cardiorespiratory arrest to occur following withdrawal of life supporting treatment before abandoning DCD retrieval. Hence, although prolonged agonal phase is associated with donor haemodynamic instability 1, our analysis suggests, within the constraints of the four-hour wait period of controlled DCD donation, that additional acute ischaemic injury does not compromise long-term DCD kidney transplant outcomes; these are instead influenced by preexisting chronic kidney injury. This argument may similarly explain why DCD kidneys from donors with elevated terminal creatinine with an established acute injury profile did not have poorer outcomes.
The presence of baseline chronic renal injury as indicated by unfavorable time-zero biopsy score was associated with donor age and ECD status, and on the basis of our analysis, we have now adopted a strategy of performing more routine preimplantation biopsy on elderly or ECD donors (irrespective of DCD or DBD status) and implanting those kidneys which score 4 or less as a single transplant and those that score from 5 to 7 as dual transplants into a single recipient. This policy could be criticized in that performing double transplants into one single recipient potentially loses a kidney from the donor pool, and although marginal, this kidney may still confer a survival advantage compared to remaining on dialysis when transplanted singly into another recipient. However, the short-term outcomes for kidneys that scored 5 or more are undoubtedly poor; approximately one quarter failed within the first year, with a primary nonfunction rate of 12.5%, and we feel these outcomes preclude use of such kidneys as single transplants. Moreover, the majority of kidneys that scored 5 or more were retrieved from elderly DCD donors (reflecting the current preponderance of DCD donors in our deceased donor pool), and given that kidneys from such donors are generally not considered for transplantation in the United Kingdom, one could argue that their transplantation as a dual transplant represents a kidney gained, rather than a kidney lost.
Hence, the most relevant application of our findings is perhaps as a means for determining, from the large numbers of ‘marginal' DCD kidneys that are currently either not procured or discarded in the United Kingdom, those which are suitable for transplantation. Preimplantation biopsy analysis of these kidneys would be expected to reveal a relatively large proportion with unfavorable chronic baseline changes; for example, 21% of DCD kidneys from donors ≥60 years in our series scored 5 or greater, which, from our analysis, would be associated with prohibitively poor outcomes. However, the greatest limitation of our study is its retrospective nature, which, when coupled with the fact that the majority of our biopsies were only analyzed after transplantation, prohibits definitive conclusion as to whether routine histological assessment of time-zero biopsies can decrease kidney discard rates. Addressing this important question is difficult, because a randomized study is impractical, but at the very least would require prospective evaluation in which transplant outcomes and discard rates are compared, perhaps, between centers with access to preimplantation biopsy analysis and those without.
In summary, our study includes for the first time reasonable numbers of DCD kidneys, as well as kidneys that were implanted singly despite baseline biopsy scores generally considered to be unfavorable. This has enabled us to clarify that a Remuzzi biopsy score of 5 is the cut-off below which deceased-donor kidneys can be implanted singly, with reasonable expectation of satisfactory transplant outcomes; kidneys that score from 5 to 7 should be implanted as dual transplants into a single recipient. Importantly, and contrary to what may have been expected, our study also confirms that baseline chronic kidney injury confers the same survival disadvantage for DCD and DBD kidney transplants and suggests that these baseline changes are more important determinants of DCD kidney transplant outcome than the acute ischaemic injury inherent to DCD procurement. We propose that more routine use of preimplantation biopsy analysis may prove valuable in identifying those kidneys, from the large pool of elderly DCD donors that are currently discarded, that could be transplanted safely.
Acknowledgments
This study was supported by the NIHR Cambridge Biomedical Research Centre. Yining Chen is supported by the Engineering and Physical Sciences Research Council Grant EP/J017213/1.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




