Graft-Versus-Host Disease After Simultaneous Pancreas–Kidney Transplantation: A Case Report and Review of the Literature
Abstract
Graft-versus-host disease (GVHD) after solid organ transplantation is rare and usually fatal. We present, to our knowledge, the second successfully treated case in a simultaneous pancreas–kidney (SPK) transplant recipient. A 29-year-old female with end-stage renal disease from type 1 diabetes mellitus received an SPK transplant from a male donor, with rabbit-antithymocyte globulin induction. Twelve days posttransplant, she was readmitted with abdominal pain, nausea and vomiting. She developed leukopenia, abnormal liver enzymes, fever and a skin rash. Skin biopsy showed interface dermatitis consistent with allergic reaction versus GVHD. Fluorescence in situ hybridization of the skin biopsy showed 28% of cells had a Y chromosome confirming GVHD. Short tandem repeats (STR) enriched for CD3+ cells from peripheral blood showed a mixed chimerism. She was successfully treated with a single plasmapheresis to remove antithymocyte globulin, high-dose steroids, photopheresis and high tacrolimus levels (12–15 ng/mL). Five months after transplantation, she has normal renal function and white blood cell count, normal hemoglobin A1C and no evidence of peripheral blood donor chimerism. In conclusion, early diagnosis of GVHD after SPK transplantation may allow successful treatment. STR enriched for CD3+ may be useful to evaluate the response to therapy.
Abbreviations
-
- CMV
-
- cytomegalovirus
-
- EBV
-
- Epstein–Barr virus
-
- ECP
-
- extracorporeal photopheresis
-
- ESRD
-
- end-stage renal disease
-
- FISH
-
- fluorescence in situ hybridization
-
- GVHD
-
- graft-versus-host disease
-
- HBA1c
-
- hemoglobin A1c
-
- HSCT
-
- hematopoietic stem cell transplantation
-
- MPA
-
- mycophenolic acid
-
- PCR
-
- polymerase chain reaction
-
- POD
-
- postoperative day
-
- PRBC
-
- packed red blood cells
-
- rATG
-
- rabbit-antithymocyte globulin
-
- SCr
-
- serum creatinine
-
- SOT
-
- solid organ transplant(s)
-
- SPK
-
- simultaneous pancreas–kidney
-
- STR
-
- short tandem repeats
-
- WBC
-
- white blood cell
Introduction
Graft-versus-host disease (GVHD) is a common complication after allogeneic hematopoietic stem cell transplantation (HSCT). This T cell–mediated disorder consists of donor immune cells (the graft) recognizing the transplant recipient (the host) as foreign, thereby initiating an immune reaction against the host. Common manifestations include fever, maculopapular rash, vomiting, diarrhea and abnormal liver enzymes 1.
Solid organ transplants (SOT) have less lymphoid tissue than HSCT and therefore GVHD is rare 2. The incidence of GVHD after small bowel and liver transplantation is estimated at 5–10% and 1–2%, respectively 3, 4. To date, 12 cases of GVHD following pancreas transplantation have been reported. Five were simultaneous pancreas–kidney (SPK) transplants, and only one survived 5-12. We present the second successfully treated case of GVHD in an SPK recipient and discuss the use of serial short tandem repeats (STR) DNA analyses for early diagnosis and monitoring response to therapy.
Case Report
A 29-year-old African American female with end-stage renal disease (ESRD) presumed secondary to type 1 diabetes mellitus and possible hereditary nephritis underwent an SPK transplant. She was diagnosed with type 1 diabetes mellitus at the age of 13. Additional past medical history included bilateral hearing loss requiring hearing aids, hypertension, anemia and menorrhagia. She underwent hemodialysis thrice weekly through a left upper extremity arterio-venous fistula. Family history was significant for hearing loss in her mother and sister. Her mother died at the age of 36 from heart disease. Her sister also had type 1 diabetes mellitus and ESRD.
The patient received a 1A, 1B, 1DR HLA-mismatched, cytomegalovirus (CMV) donor seropositive into recipient seronegative, Epstein–Barr virus recipient seropositive SPK transplant from a male donor. Induction immunosuppression included methylprednisolone 500 mg intraoperatively and rabbit-antithymocyte globulin (rATG) 7 mg/kg given over 4 days. She was transitioned to maintenance prednisone 20 mg daily, mycophenolic acid (MPA) 360 mg twice daily and tacrolimus 1 mg twice daily, with target tacrolimus levels of 8–10 ng/mL. Infectious disease prophylaxis consisted of sulfamethoxazole/trimethoprim 800/160 mg daily, valganciclovir 900 mg daily and fluconazole 100 mg daily. She had prompt graft function and was discharged on postoperative day (POD) 8 with serum creatinine (SCr) of 1.3 mg/dL and normal glucose, amylase and lipase.
On POD 12 she was readmitted with lower abdominal pain, nausea and nonbloody emesis. White blood cell (WBC) count was 16.9/mm3, amylase 201 U/L (normal 28–100 U/L) and lipase 197 U/L (normal 20–50 U/L). SCr, glucose and liver function tests were normal. An abdomen and pelvis computed tomography showed closed-loop small bowel obstruction. She underwent an exploratory laparotomy with adhesiolysis; however, a yellow-whitish fluid was noted throughout her abdominal cavity and empiric vancomycin, meropenem and micafungin were started. No allograft biopsy was performed during laparotomy. Antibiotics were discontinued after 48 h of negative cultures. Two days later, she developed a fever of 39.4°C and antibiotics were restarted. Whole blood CMV polymerase chain reaction, blood, urine and sputum cultures were rechecked and remained negative. A nasopharyngeal swab for common viral infections was positive for rhinovirus. To evaluate for an occult infection or an early posttransplant lymphoproliferative disorder, the patient underwent a whole body positron emission tomography scan which showed increased fluorodeoxyglucose uptake in the gastric lumen. A follow-up upper endoscopy revealed gastritis but gastric mucosa biopsies were unremarkable. She received one unit of packed red blood cells (PRBC) on two different occasions given a hemoglobin <7.5 g/dL.
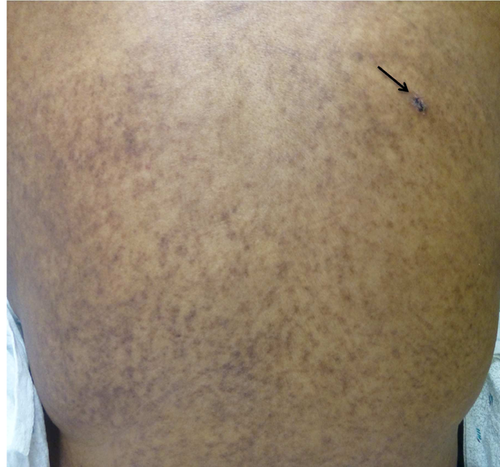
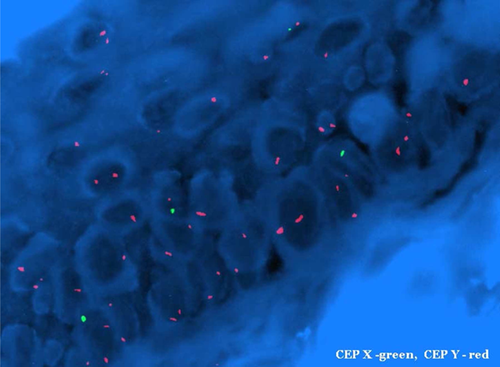
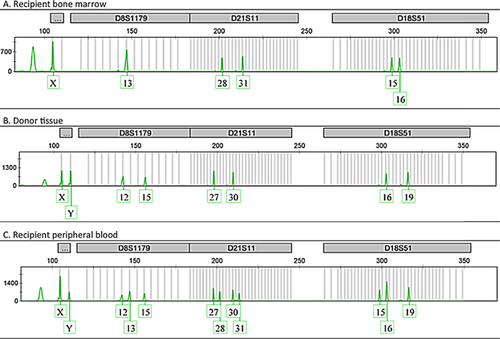
Two weeks later, fevers persisted and she developed diarrhea and a diffuse, pruritic, purplish, mottled rash over her trunk and upper extremities (Figure 1). The WBC decreased to 2.5/mm3 (absolute lymphocyte count 0.1/mm3, absolute neutrophil count 2.4/mm3) and liver enzymes were mildly elevated (alanine transferase 477 U/L [normal 7–53 U/L], aspartate transferase 452 U/L [normal 11–47 U/L], alkaline phosphatase 385 U/L [normal 38–126 U/L], bilirubin 0.7 [normal 0.3–1.1 mg/dL]). Serum amylase, lipase and glucose remained normal. A skin biopsy performed 19 days after readmission (and 4 days after the skin rash presented) showed interface dermatitis consistent with allergic reaction versus GVHD. Fluorescence in situ hybridization (FISH) of the skin biopsy revealed a Y-chromosome in 28% of cells (donor was male) confirming the diagnosis of GVHD (Figure 2). STR enriched for CD3+ cells from peripheral blood showed a mixed chimerism with 52% donor cells and 48% recipient cells (Figure 3). A bone marrow biopsy showed hypocellularity but no chimerism.
With a clear diagnosis of GVHD, treatment was initiated and antibiotics were discontinued. Due to the high risk for CMV primary infection, valganciclovir prophylaxis was continued despite leukopenia (WBC count 1.3/mm3). She was treated with methylprednisolone 2 mg/kg for 7 days followed by a slow prednisone taper from 100 to 5 mg over 4 months (Figure 4). Tacrolimus target levels were increased to 12–15 ng/mL (mean measured level 12.9 ng/mL, range 5.8–19.3 ng/mL), MPA was held given leukopenia and rhinovirus infection, and topical steroids were started. She underwent one treatment of plasmapheresis (1.5 plasma volume pheresis using albumin 5% as replacement) to remove circulating rATG that could be contributing to host T cell depletion 13. Over 1 month, she received seven treatments of extracorporeal photopheresis (ECP) with resolution of pruritus after the second treatment. She developed significant anemia (nadir hemoglobin 7.5 g/dL) requiring transfusion of two units of PRBC. Pruritus resolved and further photopheresis treatments were discontinued. Under this regimen fevers resolved and WBC (5.9/mm3) and liver enzymes normalized.
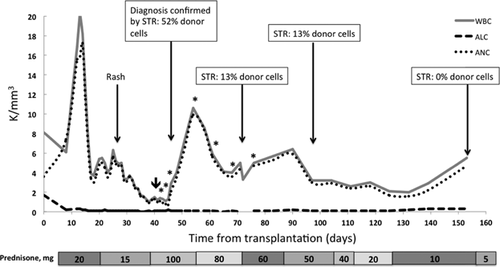
Five weeks after initiation of treatment, repeat peripheral blood STR enriched for CD3+ cells showed improved chimerism, with only 13% donor cells. Unfortunately, while on the high dose steroids, she developed Cushingoid features including facial swelling, hyperglycemia and proximal myopathy. Six weeks after the diagnosis of GVHD, the patient had fasting glucose levels between 150 and 170 mg/dL with a hemoglobin A1c (HBA1c) of 5.8%. Despite initiation of sitagliptin 50 mg daily and tapering steroids, 5 weeks later HBA1c increased to 8% with a C-peptide of 4.3 ng/mL and glucose of 102 mg/dL. Amylase, lipase and SCr remained normal. Sitagliptin was increased to 100 mg daily and prednisone was decreased to 10 mg/day. Five months after transplantation, she has normal renal function (SCr 1.3 mg/dL) and WBC count (5.5/mm3), and the anemia has improved (last hemoglobin 11.9 g/dL). Furthermore, peripheral blood donor chimerism became undetectable, prednisone was decreased to 5 mg daily and HBA1c decreased to 5.4%. With a lower prednisone dose, we were able to discontinue sitagliptin and she has maintained normal fasting glucose levels (range 76–96 mg/dL).
Discussion
GVHD is a rare but usually fatal complication after SOT 2. The common clinical manifestations of diarrhea, rash and elevated liver enzymes occur when immunocompetent donor T cells transferred with the graft are activated by alloantigens presented by host antigen presenting cells, resulting in an inflammatory response against host organs 14. Cytopenias resulting from donor immune-mediated attack on the recipient bone marrow is also a common feature of GVHD following SOT, and a frequent cause of death 2. Here, we describe the second successfully treated case after SPK transplantation. Moreover, we demonstrate the presence of donor chimerism and its resolution with clinical improvement.
Our patient presented with many typical features. However, these clinical and histologic features are not specific and the diagnosis is often delayed as similar findings are seen in drug reactions and infections, both common entities in the transplant population 6. A delay in diagnosis contributes to the high mortality rate of greater than 80% 14. In most cases, death results from sepsis, bone marrow failure and multi-organ failure 15, 16. In our case, we believe that the relatively early diagnosis of GVHD followed by aggressive treatment led to a favorable outcome. A skin rash, although characteristic of GVHD, can be a late presentation 7. In the appropriate context, the work up of fever of unknown origin early after transplantation should entertain checking peripheral blood STR even in the absence of a skin rash.
The diagnosis of GVHD can be made on clinical indications and biopsy 17 but is facilitated by demonstrating donor lymphoid chimerism. A value greater than 20% more than 1-week posttransplant is highly specific 2. Various methods are used to diagnose donor lymphocyte chimerism. FISH analysis of tissue biopsy can be done using fluorescent probes against Y or X chromosome DNA to distinguish donor lymphocytes from recipients when a gender mismatch exists 18. In our case, the skin biopsy FISH showed a Y chromosome in 28% of cells, confirming GVHD. STR analysis of peripheral blood can be helpful for diagnosis and monitoring clinical response to treatment 19. As many of these patients are profoundly lymphopenic, the key for reliable STR testing is to perform enrichment for CD3+ cells. Without this extra step, STR can be falsely negative for chimerism. At the time of diagnosis, STR analysis of the peripheral blood lymphocytes demonstrated 52% of donor cells, followed by 13% at weeks 5 and 12, and 0% by week 16 after treatment. In our case and other cases reported, the degree of chimerism tended to parallel the clinical course of GVHD 14. As the degree of chimerism decreased, the patient's signs and symptoms abated, allowing us to tailor the prednisone taper to clinical symptoms and cytogenetic improvement. Monitoring STR is a strength and novel finding of our report.
Compared to the case presented by Chang et al 8, a distinct characteristic of our patient is the lack of prior immunosuppression. A risk factor for GVHD is an immunocompromised host, who is more susceptible to competent donor T cells 15. Many cases of GVHD after kidney and/or pancreas transplantation occurred in hosts that were receiving their second, third and sometimes fourth transplants and have therefore been on chronic immunosuppression for a long time 7, 8, 11, 12 (Table 1).
| Refs. | Transplant type | Transplant number | Chimerism confirmed (% donor cells) | Treatment | Outcome |
|---|---|---|---|---|---|
| Deierhoi et al 12 | Pancreas and spleen | Second | Yes (80–100%) | Splenic irradiation followed by transplant splenectomy, CyA stopped, high dose CS and ATG | Death on POD 60 |
| Kimball et al 5 | SPK | First | Yes (% NA) | Decreased IS (OKT3 and AZA stopped, CyA reduced); GCSF used | Death on POD 22 |
| Gulbahce et al 10 | PAK | Second | No | NA | Death on POD 76 |
| SPK (living kidney) | First | Yes (20%) | Pancreas and kidney allograft removal | Death on POD ≥77 | |
| Weinstein et al 7 | PAK | Second | Yes (11%) | MPA, FK stopped. Increased CS, ATG, CyA | Death on POD 73 |
| SPK (living kidney) | First | Yes (7%) | CS and FK stopped initially. ATG, CS, CyA | Death on POD 55 | |
| Weng et al 11 | PAK | Second | Yes (100%) | CS; GCSF | Death on POD 145 |
| Osband et al 9 | SPK | First | Yes (99%) | MMF stopped, ATG, increased FK, CS; GCSF used | Death on POD 52 |
| Sharma et al 6 | Pancreas | First | Yes (38%) | CS, mesenchymal stromal cells | Death on POD ∼165 |
| Chang et al 8 | Pancreas | Second | No | Decreased initially (low dose CS only). Later high dose CS and reinstitution of FK and MMF | Survived working graft |
| SPK | Fourth | No | CS, topical FK | Survived working grafts | |
| PAK | Second | No | High dose CS, sirolimus, ATG, daclizumab, rituximab | Survived working grafts | |
| Current case | SPK | First | Yes (52%) | High dose CS, increased FK, photopheresis | Survived working grafts |
- ATG, antitymocyte globulin; AZA, azathioprine; CS, corticosteroids; CyA, cyclosporine; FK, tacrolimus; GCSF, granulocyte colony-stimulating factor; GVHD, graft-versus-host disease; IS, immunosuppression; MMF, mycophenolate mofetil; MPA, mycophenolic acid; NA, data not available; PAK, pancreas after kidney; POD, postoperative day; SPK, simultaneous pancreas–kidney.
The optimal treatment of GVHD after solid organ transplantation remains unclear. The literature describes both enhanced and reduced immunosuppression 2, 10, 20. Additionally, most reported cases have been fatal making the interpretation of the available data difficult. The most recent report of three cases after pancreas transplantation describes successful treatment with high dose corticosteroids and rATG. This contrasts with the more-established treatment of GVHD after HSCT, where methylprednisolone at 2 mg/kg/day followed by a slow prednisone taper is the standard of care 21. Our patient responded to this more traditional regimen, as well as targeting higher tacrolimus levels of 12–15 ng/mL. Additionally, with the thinking that the patient's immune system might be able to “reject” the donor T cells, the patient underwent plasmapheresis to remove the residual rATG administered during induction immunosuppression 13. Last, although ECP is often used for steroid-refractory GVHD after HSCT, there have been no reports of photopheresis in SOT with acute GVHD. During this procedure circulating lymphocytes are collected by leukophoresis and exposed ex vivo to psoralen and ultraviolet-A treatment prior to being reinfused into the patient. Through this mechanism ECP induces apoptosis of activated lymphocytes 22. We chose this therapy given the patient's diffuse skin rash and severe pruritus, which resolved after the second ECP treatment. A strength of our report is that this is the first report using ECP to treat GVHD after pancreas transplantation.
A limitation of this simultaneous multi-therapy approach is the inability to relate the improvement to one specific therapy. We are therefore uncertain if any of the therapies implemented were causally linked to the very good outcome. However, given this condition's high mortality rate, we felt the benefit of this multifaceted approach was greater than the potential risks and likely contributed to the patient's overall favorable outcome.
In conclusion, GVHD after SPK transplant is rare. A high index of suspicion is essential for early detection. Diagnosis can be made clinically and by biopsy but is facilitated by demonstration of donor chimerism by FISH and/or STR which can be used for monitoring treatment response. Early diagnosis may allow successful treatment with (1) supportive care aimed at treating or preventing infections and cytopenias and (2) elimination of donor chimerism through high dose steroids, higher calcineurin inhibitors levels, and possibly the use of ECP. Whether ECP allows for a faster steroid taper resulting in fewer side effects remains to be elucidated.
Acknowledgments
We thank Drs. George Burke, Alden Doyle and Peter Westervelt for helpful discussions regarding diagnosis and management and critical review of the manuscript. We thank Dr. TuDung Nguyen for providing the FISH picture.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.