Morbidity and Mortality of Live Lung Donation: Results From the RELIVE Study
Abstract
The Renal and Lung Living Donors Evaluation Study assesses outcomes of live lung (lobectomy) donors. This is a retrospective cohort study at University of Southern California (USC) and Washington University (WASHU) Medical Centers (1993–2006), using medical records to assess morbidity and national databases to ascertain postdonation survival and lung transplantation. Serious complications were defined as those that required significant treatment, were potentially life-threatening or led to prolonged hospitalization. The 369 live lung donors (287 USC, 82 WASHU) were predominantly white, non-Hispanic and male; 72% had a biological relationship to the recipient, and 30% were recipient parents. Serious complications occurred in 18% of donors; 2.2% underwent reoperation and 6.5% had an early rehospitalization. The two centers had significantly different incidences of serious complications (p < 0.001). No deaths occurred and no donors underwent lung transplantation during 4000+ person-years of follow-up (death: minimum 4, maximum 17 years; transplant: minimum 5, maximum 19). Live lung donation remains a potential option for recipients when using deceased donor lungs lacks feasibility. However, the use of two live donors for each recipient and the risk of morbidity associated with live lung donation do not justify this approach when deceased lung donors remain available. Center effects and long-term live donor outcomes require further evaluation.
Abbreviations
-
- DCC
-
- data coordinating center
-
- HRSA
-
- Health Resources and Services Administration
-
- LAS
-
- lung allocation score
-
- MMRF
-
- Minneapolis Medical Research Foundation
-
- NCHS
-
- National Center for Health Statistics
-
- NCI
-
- National Cancer Institute
-
- NHLBI
-
- National Heart, Lung and Blood Institute
-
- NIAID
-
- National Institute of Allergy and Infectious Diseases
-
- NIH
-
- National Institutes of Health
-
- OPTN
-
- Organ Procurement and Transplantation Network
-
- RELIVE
-
- Renal and Lung Living Donors Evaluation Study
-
- SRTR
-
- Scientific Registry of Transplant Recipients
-
- SSA
-
- Social Security Administration
-
- SSDMF
-
- Social Security Death Master File
-
- SSN
-
- Social Security Number
-
- UNOS
-
- United Network for Organ Sharing
-
- USC
-
- University of Southern California
-
- WASHU
-
- Washington University
Introduction
Inadequate donor lung allograft supply and deaths of candidates awaiting lung transplantation led to implementation of living lobar lung donation 1 and consequent broadening of the pool of donor organs which historically came from deceased donors. Though the number of live lung donations has minimally impacted the number of lung transplants performed in the United States 2, 3, live lung donation has had a greater effect in countries like Japan that have had low deceased donor rates 4-6. After a transient rise in frequency in the 1990s and early 2000s, live lung donation decreased in the United States due to changes in the lung allocation system and effects of medical management on transplant urgency 2. However, live lung donation grew in frequency in some areas outside the United States due to lack of brain death laws, continued unavailability or acceptance of deceased donation, and altered lung allocation policies 4-9.
Live donation confronts society with complex ethical and medical issues regarding short- and long-term donor risks 10-12. Since each live lung recipient typically has two donors, the overall donation/transplant scenario puts three people at risk for death and morbidity, while only one of the three (i.e. the recipient) has a likelihood of medically benefiting from the procedure. Relationships between donors, recipient and nondonors may profoundly affect psychosocial outcomes of all involved 13, 14, and live lung donor and recipient outcomes may further complicate matters.
The two US centers with the greatest live lung donor experience, University of Southern California (USC) and Washington University (WASHU)/Barnes-Jewish Hospital, have previously published results from short-term live lung donor outcomes studies 15-19. Though neither center reported any early postoperative deaths in their donors (USC n = 253 and WASHU n = 62; total n = 315), there was a threefold difference in morbidity incidence between the two centers (20% at USC vs. 61% at WASHU) 17, 19.
Due to a paucity of accurate, reliable and comprehensive outcomes data for live lung donors, potential donors do not have robust information on which to base an informed consent decision for undergoing donor evaluation and surgery. In addition to limited and inconsistent data regarding short-term donor morbidity, the long-term risk of pulmonary dysfunction and other adverse outcomes remains unknown. Investigators have not published results from large-scale and long-term studies of the effects of live lung donation on donor pulmonary function, health-related quality of life and psychological well-being.
Limited data existed regarding comprehensive and long-term live lung donor outcomes when the Renal and Lung Living Donors Evaluation Study (RELIVE) began in 2006. Between 1993 and 2006, 12 168 deceased lung donations and 460 live lung donations occurred in the United States 20. During that period, the USC and WASHU transplant teams performed 369 live lung donation operations, accounting for 80% of live lung donations in the United States 20. This study of live lung donors describes: (1) perioperative donor characteristics; (2) the types and incidence of short-term complications following live lung donation; and (3) the incidence rates of postdonation mortality and the development of severe lung disease requiring lung transplantation.
Methods
Study design/patients and setting/study eligibility criteria
Investigators conducted a retrospective cohort study of all patients from USC (Los Angeles, CA) and WASHU (St. Louis, MO) who underwent live lung donation (i.e. lobectomy) between January 1, 1993 and December 31, 2006. Study personnel collected donor medical record data extant through January 31, 2007, death events that occurred through May 31, 2010, and lung transplant events that occurred through November 30, 2011. After review of the study protocol and approval by the RELIVE Steering Committee and the National Institute of Allergy and Infectious Diseases, each site and the University of Michigan data coordinating center (DCC) obtained investigational review board (IRB) approval at their respective sites (USC IRB approval number HS-07-00332-CR006; WASHU IRB approval number 201101865; DCC IRB approval number CR00032674 and protocol number HUM00004345).
Variables
Trained study personnel collected donor data for preoperative and intraoperative characteristics, intraoperative and postoperative complications, serious and nonserious postoperative complications during the donation hospitalization, and early rehospitalization (i.e. at the same medical center and within 30 days after donation). The protocol defined serious complications as those that required significant treatment (i.e. treatment typically requiring administration in a hospital setting), were potentially life threatening, or led to prolonged hospitalization. The protocol considered all other complications as nonserious (i.e. not treated or easily treatable, and not potentially life-threatening or leading to prolonged hospitalization). Two investigators (RDY and MLB) adjudicated all complications as serious or nonserious by consensus.
Data sources
Study personnel identified lobar donors from the medical records and databases of the USC and WASHU programs, and they collected existing patient data from paper and electronic medical records and databases. The study obtained donor death and lung transplant event information from the Social Security Death Master File and the Scientific Registry of Transplant Recipients databases, respectively. Outcomes of donors living outside the United States may not have been captured in the national database searches.
Data collection methodology and quality control
Study investigators and personnel created a manual of operations, and each site also operationalized local procedures for the study. The study used standardized definitions for terms. DCC personnel trained the site study coordinators. The coordinators abstracted data from paper and electronic medical records and entered the data into an electronic database. Key outcomes underwent central adjudication (see the Variables section). The electronic case report form data entry system had embedded quality control measures. The DCC addressed additional data concerns through queries to coordinators. Chart audits confirmed source documentation and appropriate study conduct.
Statistical analyses
Data analysis and reporting utilized descriptive statistics, chi-square statistics to compare categorical data among groups, and two-tailed t-tests and Wilcoxon tests to compare continuous data between groups. To assess donor selection and care learning effects at each site on complication rates, we compared complication rates within sites based on early and late subgroup (i.e. first 40 donors and last 40 donors at each site, and first and last 81 donors at USC); we also compared complication rates among era of donation for the combined (USC and WASHU) cohort. We log-transformed hospital length of stay for inferential analyses. We used logistic regression to identify significant predictors of complications, and linear regression for predictors of log-transformed hospital length of stay (see frequencies and descriptive statistics in Tables S1, S2 and S3). Historical and predonation variables tested as predictors in the models included donor age, race, ethnicity, sex, height, weight, BMI (tested independently of height and weight), history of alcohol/tobacco/illicit drug use, bronchodilator use, reported lung disease (e.g. asthma), chronic pain, psychiatric conditions and predonation pulmonary function data (i.e. percentage of predicted pre- and postbronchodilator forced expiratory volume in one second and forced vital capacity, total lung capacity, residual volume and diffusing capacity for carbon monoxide). Intraoperative variables tested as predictors included incision type, location of donated lobe, right middle sacrifice, rib removal, stump treatment, use of a double lumen endotracheal tube, use of a bronchoplastic procedure and medical center. We assessed model assumptions and performed regression diagnostics. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). The authors used the Strengthening the Reporting of Observational Studies in Epidemiology Statement 21 to guide the reporting of results. The sample size for this study was fixed and the study utilized data from all lung donors during the study period at the two sites.
Reporting
We published a limited subset of data from this study in abstract form and presented it at the 2011 American Transplant Congress 22. An older subset of these data was reported in previous publications 17, 19. However, compared to those studies, the RELIVE study included more patients from each center, used more rigorous and standardized study methodology, had a longer follow-up period to assess for donor death or lung transplantation, and used national databases to assess outcomes.
Results
Donor characteristics
The study cohort consisted of 369 adult live lung donors (287 USC, 82 WASHU) (Table 1). Donations were made to 186 recipients (145 USC, 41 WASHU). Donors were predominantly white, non-Hispanic and male. Most had a biological relationship to their lung transplant recipient. About one-third of donors (n = 109) were parents of the recipient. Both parents served as donors for 25 recipients (17 at USC, 8 at WASHU). Many of the recipients were children (age less than 18 years). USC had 48 (33%) child recipients (age of 1 recipient was unknown) and WASHU had 33 (80%) child recipients.
| Variable | USC (N = 287) | WASHU (N = 82) | All (N = 369) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Male | 186 | 65 | 46 | 56 | 232 | 63 |
| Non-Hispanic | 256 | 89 | 76 | 93 | 332 | 90 |
| Caucasian | 268 | 93 | 79 | 96 | 347 | 94 |
| Relation to recipient | ||||||
| Biological | 194 | 68 | 70 | 85 | 264 | 72 |
| Parent | 72 | 25 | 37 | 45 | 109 | 30 |
| Sibling | 52 | 18 | 9 | 11 | 61 | 17 |
| Aunt/uncle | 33 | 11 | 17 | 21 | 50 | 14 |
| Cousin | 29 | 10 | 6 | 7 | 35 | 9 |
| Other | 8 | 3 | 1 | 1 | 9 | 2 |
| Nonbiological | 86 | 30 | 12 | 15 | 98 | 27 |
| Friend | 59 | 21 | 5 | 6 | 64 | 17 |
| Spouse | 8 | 3 | 0 | 0 | 8 | 2 |
| Other | 19 | 7 | 7 | 9 | 26 | 7 |
| Unknown | 7 | 2 | 0 | 0 | 7 | 2 |
| Age (range 18.2–58.5) | Mean | SD | Mean | SD | Mean | SD |
|---|---|---|---|---|---|---|
| 37.1 | 9.8 | 39.5 | 9.8 | 37.6 | 9.8 |
- USC, University of Southern California; WASHU, Washington University (St. Louis).
Live lung donation surgery
Based on lung size and anatomical issues, lung donation typically consisted of removal of a single lower lobe. The paired lung donations usually consisted of a right lower lobe from one donor and a left lower lobe from another donor. All but three of the recipients received lobes from two donors to achieve a bilateral lung transplant.
Complications
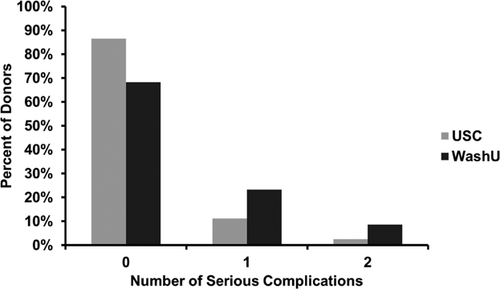
One hundred fifty-six in-hospital (intraoperative or postoperative) serious or nonserious (e.g. nausea, pain, fever) complications occurred in 29% (107/369) of donors. Seventy-nine serious complications occurred in 17.6% (65/369) of donors (Table 2 and Fig. 1); 13.8% (51/369) had only one serious complication and 3.8% (14/369) had two serious complications. The most common serious complication was postoperative cardiac arrhythmia that led to treatment. Pericarditis occurred in 3.3% of donors, and this only occurred in left side donors (p = 0.0003). Additional/unexpected chest tube drainage was required in 3.3% of recipients; two-thirds of these events were associated with pneumothorax and one-third with pleural effusion and/or bleeding. Reoperation occurred in 2.2% of donors. No donors had tracheostomy, stroke, myocardial infarction, sepsis or venous thromboembolism. No deaths occurred during the donation hospitalization. The two centers had significantly different incidences of serious complications (p < 0.001) (Fig. 1). We did not find evidence of learning curve effects on the incidence of serious complications either within sites or overall.
| Complication | Number of complications | Percent of donors with each complication |
|---|---|---|
| Treated arrhythmia | 13 | 3.5 |
| Pericarditis1 | 12 | 3.3 |
| Tube thoracostomy—total | 12 | 3.3 |
| Pneumothorax | 8 | 2.2 |
| Pleural effusion/bleeding | 4 | 1.1 |
| Reoperation | 8 | 2.2 |
| Pneumonia | 8 | 2.2 |
| Air leak | 6 | 1.6 |
| Intra-operative right middle lobe sacrifice | 5 | 1.4 |
| Ileus | 5 | 1.4 |
| Blood transfusion | 4 | 1.1 |
| Empyema | 2 | 0.5 |
| Pulmonary artery thrombosis (not pulmonary embolism) | 2 | 0.5 |
| Bronchial stricture | 1 | 0.3 |
| Cardiac arrest | 1 | 0.3 |
- USC, University of Southern California; WASHU, Washington University (St. Louis).
- N = 369 [287 USC, 82 WASHU].
- Seventy-nine serious in-hospital complications occurred in 65 of the 369 donors; 17.6% (65/369) had ≥1 serious complication; 13.8% (51/369) had only 1 serious complication and 3.8% (14/369) had 2 serious complications.
- 1 Pericarditis only occurred in left-sided donors (p = 0.0003).
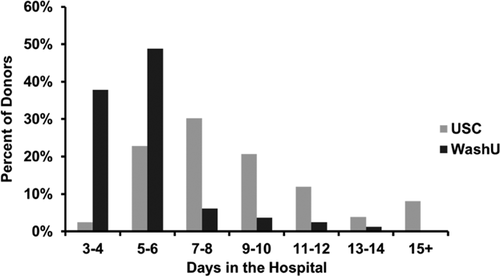
Hospital length of stay and rehospitalization
The hospital length of stay averaged 8.3 ± 4.2 days (median 7 days; range 3–32 days) (Fig. 2). Early rehospitalization was documented in 24 (6.5%) donors. However, the study lacked complete rehospitalization reporting because some donors did not undergo follow-up with the donor site team after discharge from the hospital. Most documented early rehospitalizations occurred in association with ongoing or recurrent pericarditis, arrhythmia, pleural effusion or airway/chest bleeding (Table 3).
| 1st rehospitalization (N = 24 donors) | 2nd rehospitalization (N = 3 donors) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Primary discharge diagnosis | ||||
| Pneumothorax | 5 | 20.8 | 1 | 33.3 |
| Pain (n = 1 atypical chest pain; n = 2 incisional) | 3 | 12.5 | 0 | 0.0 |
| Pneumonia | 3 | 12.5 | 0 | 0.0 |
| Pericarditis | 2 | 8.3 | 0 | 0.0 |
| Postoperative pleural effusion | 2 | 8.3 | 0 | 0.0 |
| Arrhythmia | 1 | 4.2 | 0 | 0.0 |
| Dehydration due to vomiting due to drug s/e | 1 | 4.2 | 0 | 0.0 |
| Empyema | 1 | 4.2 | 1 | 33.3 |
| Hemorrhage | 1 | 4.2 | 0 | 0.0 |
| Hydropneumothorax | 1 | 4.2 | 0 | 0.0 |
| Infection (unknown site) | 1 | 4.2 | 0 | 0.0 |
| Left lower lobe atelectasis | 1 | 4.2 | 0 | 0.0 |
| Nausea/vomiting/abdominal pain | 1 | 4.2 | 0 | 0.0 |
| Severe shortness of breath | 1 | 4.2 | 0 | 0.0 |
| Bronchopleural fistula | 0 | 0.0 | 1 | 33.3 |
| Secondary discharge diagnosis | ||||
| Postoperative pleural effusion | 2 | 8.3 | 0 | 0.0 |
| Bronchopleural fistula | 1 | 4.2 | 0 | 0.0 |
| Elevated aPTT | 1 | 4.2 | 0 | 0.0 |
| Hemoptysis | 1 | 4.2 | 0 | 0.0 |
| Left-sided chest discomfort | 1 | 4.2 | 0 | 0.0 |
| Pneumonia | 1 | 4.2 | 0 | 0.0 |
| Pleural cavity abscess | 0 | 0.0 | 1 | 33.3 |
| Pneumothorax | 0 | 0.0 | 1 | 33.3 |
| Right pleural effusion | 0 | 0.0 | 1 | 33.3 |
| Unknown/missing | 17 | 70.8 | 0 | 0.0 |
| Tertiary discharge diagnosis | ||||
| Bronchial inflammation | 1 | 4.2 | 0 | 0.0 |
| Pneumonia | 1 | 4.2 | 0 | 0.0 |
| Unknown/missing | 22 | 91.7 | 3 | 100 |
- s/e, side effects; aPTT, activated partial thromboplastin time; SC, University of Southern California; WASHU, Washington University (St. Louis).
- Total cohort N = 369 (287 USC, 82 WASHU).
Lung transplantation and death outcomes
No donors died or underwent lung transplantation during follow-up that ranged from 4 to 17 years for mortality and 5 to 19 years for transplant (4267 person-years at risk for mortality; 4820 person-years at risk for lung transplantation). Eleven donors (3.0% of 369) were not living in the United States at the time of donation; nine (3.1% of 287) donated at USC and two (2.4% of 82) donated at WASHU. For four donors (1.1% of 369), all from USC (1.4%; 4/287), we do not know whether they were living in the United States or not.
Predictive models
A predictive logistic regression model for in-hospital serious complications identified the center as an independent predictor (p < 0.001; Table 4). A predictive linear regression model for log-transformed hospital length of stay (Table 5) identified medical center (Figure 2), bronchodilator use (ever), anterolateral incision approach and right middle lobe sacrifice (not planned as part of the operation) as independent predictors of longer length of stay (model p < 0.001). Among the many assessed variables (see Tables S1, S2 and S3), the models did not identify age, sex or weight as significant independent predictors.
| Variable | OR | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| Intercept | 0.46 | 0.29 | 0.74 | 0.0012 |
| Medical center | 0.34 | 0.19 | 0.60 | 0.0002 |
- USC, University of Southern California.
- WASHU, Washington University (St. Louis).
- N = 369 (287 USC, 82 WASHU); C = 0.608; model chi-square 14.43.
| Variable | Beta | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| Intercept | 1.57 | 1.49 | 1.65 | <0.0001 |
| Medical center | 0.54 | 0.45 | 0.63 | <0.0001 |
| Bronchodilator use: ever (reference: never, unknown) | 0.50 | 0.17 | 0.82 | 0.0031 |
| Incision: anterolateral (reference: all other incision types) | 0.13 | 0.00 | 0.25 | 0.0486 |
| Right middle lobe sacrifice | 0.39 | 0.06 | 0.72 | 0.0190 |
- USC, University of Southern California; WASHU, Washington University (St. Louis).
- N = 367 (285 USC, 82 WASHU).
- Adjusted R2 0.28; F-test 37.41.
- The model excluded two patients from USC with missing hospitalization length of stay.
- Duration of hospital length of stay (days) was log-transformed.
Discussion
RELIVE documented and classified morbidities associated with live lobar lung donation in the largest cohort reported to date that represents most of the United States and much of the international experience 5, 7. No donors underwent lung transplantation and no donor deaths occurred during the 4000+ person-years of follow-up.
Though a minority of donors had complications, about 1 out of 5 donors experienced a serious complication, and about 1 out of 25 experienced multiple serious complications. About 1 out of 50 donors required reoperation. At least 1 out of 16 donors required rehospitalization within 30 days of donation. Serious complications and hospital length of stay showed variation between the medical centers. A preoperative medical condition (i.e. bronchodilator use, ever) and some operative issues (i.e. anterolateral incision approach and right middle lobe sacrifice [not planned as part of operation]) were independent predictors of longer length of stay.
Consistent with prior reports 17, 19, 23, the current study showed significant center-based differences in the occurrence of short-term postlive lung donation complications. These differences may have resulted from differences in donor selection, management and monitoring approaches, medical record documentation and definitions and terminology used by clinicians. Differences between centers in the length of donation hospitalization may have resulted from different policies or practices at each center.
The live lung donation operation differs from and is more extensive than standard lobectomy (e.g. performed for malignancy) because it requires removal of an adequate cuff of bronchus, pulmonary artery and vein with the lung lobe for successful donor lobe implantation into the recipient. In addition, the donor's remaining tissue must allow for closure of these structures without compromise. Differences in right and left side surgical approaches appeared to affect outcomes in RELIVE. Compared to right lower lobe donors, left lower lobe donors had a higher rate of pericarditis that we attributed to a higher rate of pericardial opening.
Live donor lung transplantation has rarely occurred in the United States since implementation of the current donor lung allocation score (LAS) system in 2005. However, even in the LAS era, live lung donation may still have relevance for candidates at high projected risk of waiting list mortality and a low likelihood of obtaining deceased donor lungs (e.g. high degree of sensitization). The recent controversial issue of limited donor access for young pediatric lung transplant candidates in the United States 24 further increases the relevance of the option of live lung donation in certain settings. Live lung donation may also have greater relevance in lung transplant allocation systems that give priority based on candidate waiting list time. In addition, systems that have severe limitations in deceased donor availability due to cultural (e.g. Japan) or other issues may depend heavily on the ability to increase access to donor lungs through live lung donation. Thus, for all donor lung allocation systems, RELIVE has relevance and generates a better understanding of the risks of live lung donation. Such information will improve the informed consent process 12 and it will hopefully improve donor selection and subsequently decrease donor risks.
The limitations of RELIVE include the retrospective study design, the variability of data completeness and the complication documentation issues outlined above. The study protocol attempted to minimize errors in complication ascertainment by using standardized definitions, clear data abstraction rules and uniform study coordinator training. In addition, the investigators reviewed and categorized all complications through an adjudication process. The study lacked complete follow-up after hospital discharge that included assessment of rehospitalization and long-term outcomes. In addition, ascertainment of mortality and lung transplantation via the US national databases would not capture events in donors that resided outside of the United States. The predictive models did not undergo validation in other patients and in other settings. The next phase of RELIVE will utilize a cross-sectional design and incorporate contacting the donors from this retrospective cohort study to assess long-term donor lung function and psychosocial outcomes and to confirm mortality and lung transplant event rates from this study.
In conclusion, live lung donation for recipients provides an acceptable option when use of deceased donor lungs lacks feasibility. However, the use of two live donors for each recipient and the risk of morbidity associated with live lung donation do not justify this approach when deceased lung donors are available. Center effects and long-term live donor outcomes require further evaluation.
Acknowledgments
This research was performed as a project of the Renal and Lung Living Donors Evaluation Study (RELIVE), a collaborative clinical research project sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), Health Resources and Services Administration (HRSA) and National Heart, Lung, and Blood Institute (NHLBI). The Minneapolis Medical Research Foundation (MMRF), as the contractor for the US Scientific Registry of Transplant Recipients (SRTR), provided SRTR and/or Social Security Death Master File (SSDMF) data under a data use agreement. This study used data from the SRTR. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. HRSA, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The data reported here have been supplied by MMRF as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. This study was sponsored by the National Institutes of Health (NIH), NIAID, grants U01AI069545, U01A169550 and U01AI69491 and the HRSA.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




