Differences in cancer clinical trial activity and trial characteristics at metropolitan and rural trial sites in Victoria, Australia
Abstract
Objective
Cancer clinical trials (CCTs) provide access to emerging therapies and extra clinical care. We aimed to describe the volume and characteristics of CCTs available across Victoria, Australia, and identify factors associated with rural trial location.
Methods
Quantitative analysis of secondary data from Cancer Council Victoria's Clinical Trials Management Scheme dataset.
Design
A cross-sectional study design was used.
Setting
CCTs were available Victoria-wide in 2018.
Participants
There were 1669 CCTs and 5909 CCT participants.
Main Outcome Measures
Rural CCT location was assessed as a binary variable with categories of ‘yes’ (modified Monash [MM] categories 2–7) and ‘no’ (MM category 1). MM categories were determined from postcodes. The highest (‘least rural’) MM category was used for postcodes with multiple MM categories.
Results
Of 1669 CCTs, 168 (10.1%) were conducted in rural areas. Of 5909 CCT participants, 315 (5.3%) participated in rural CCTs. There were 526 CCTs (31.5%) with 1907 (32.3%) newly enrolled participants. Of 1892 newly enrolled participants with postcode data, 488 (25.8%) were rural residents. Of them, 368 (75.4%) participated in metropolitan CCTs. In a multivariable logistic regression analysis for all 1669 CCTs, odds of a rural rather than metropolitan CCT location were significantly (p-value <0.05) lower for early-phase than late-phase trials and non-solid than solid tumour trials but significantly (p-value <0.05) higher for non-industry than industry-sponsored trials.
Conclusions
In Victoria, 10% of CCTs are at rural sites. Most rural-residing CCT participants travel to metropolitan sites, where there are more late-phase, non-solid-tumour and industry-sponsored trials. Approaches to increase the volume and variety of rural CCTs should be considered.
What is already known on this subject
- Cancer clinical trials provide best-practice care.
- Access to cancer clinical trials may help improve the survival outcomes of those outside of metropolitan areas.
What this paper adds
- This is the first state-wide analysis of cancer clinical trial activity demonstrating statistically significant differences in clinical trial characteristics between rural and metropolitan trial sites.
- This paper highlights that most people residing in rural areas must travel to a metropolitan centre to participate in clinical trials.
- Approaches should be considered to increase the volume and variety of rural CCTs, particularly the availability of early-phase, solid-tumour and industry-sponsored trials at rural sites.
1 INTRODUCTION
Cancer clinical trials (CCTs) inform evidence-based cancer care.1 While clinical trials have improved treatment options and survival for cancer patients, only 5%–14% of patients from high-income countries participate in clinical trials.2 Within this small proportion of patients who do participate, there are known underrepresented populations who do not participate. Rural-residing cancer patients are one of these underrepresented populations.2, 3 Increasing rural residents' participation in CCTs provides an opportunity to bridge the survival gap between rural and metropolitan populations, particularly considering the evidence suggesting that similar outcomes can be achieved when participating in trials regardless of rurality.4
Rural residents experience barriers to CCT participation. Unger and Cook5 describe four categories of barriers related to participation, which are structural (availability of a clinical trial), clinical (trial eligibility criteria), physician-related (attitude) and patient-related (attitude). The impact of these barriers differs according to patient demographic and socioeconomic factors.5 For example, older age has an unfavourable influence on CCT participation.6 A systematic review and meta-analysis of studies conducted in the USA found that a lack of trial availability and strict eligibility criteria prevent an estimated 75% of adult cancer patients from participating in clinical trials.7 A recent scoping review highlighted barriers specific to rural-residing cancer patients, including travel and distance, absence of a trial protocol, out-of-pocket expenses and physician and patient attitudes and knowledge about CCTs.2 Some of these barriers are similar to those faced by rural-residing people accessing health care more generally, including transportation issues, the financial burden of treatment and the lack of local availability of services.8-10
The importance of improving access to clinical trials for rural-residing cancer patients is highlighted by recent North American and Australian initiatives that include tele-trials (trials that use telehealth technology to communicate between primary trial sites and satellite sites11) and the establishment of clinical trial networks, as described in a recent scoping review.2 Tele-trials can increase access to CCTs for cancer patients living in rural areas by enabling trial activity through satellite sites closer to patients' homes.12 Clinical trial networks increase rural participants' access to trials by forming partnerships among academic medical centres, community groups and rural health services.13 They may also provide financial support to establish new trial sites. Understanding trial participation and availability is essential to measure changes resulting from new initiatives.
We aimed to first describe the volume and characteristics of CCTs running in rural and metropolitan areas of the Australian state of Victoria in 2018 and, second, assess associations between trial characteristics and rural trial location.
This study explored the following research questions: 1. How many patients participated in CCTs in Victoria, Australia, during 2018, both overall and by trial location (metropolitan and rural)? 2. What are the characteristics of CCTs available in Victoria, Australia? 3. Which trial characteristics are associated with rural trial location?
2 METHODS
2.1 Study design
A cross-sectional study was undertaken from 1 January 2018 to 31 December 2018.
2.2 Data source
Cancer Council Victoria (CCV) is a not-for-profit cancer charity that collects data on CCT activity from trial units within Victoria, Australia. Victoria is Australia's second most populous state or territory, with a resident population of 664310014 people living predominately (77%) in metropolitan areas.15 In 2018, there were 35 203 new cancers diagnosed in Victoria.15 Clinical trial data have been collected by CCV as part of the Clinical Trials Management Scheme (CTMS) since 1988 to inform funding for clinical trial activity. The CTMS data set only includes data on those clinical trials that provide therapeutic treatment and meet the World Health Organization (WHO) definition of a clinical trial: ‘any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes’.16 This data set, therefore, excludes supportive care, palliative care, survivorship and preventative cancer trials. De-identified data are collected annually from 43 participating sites. The CTMS data are reported at the clinical trial level and include two unique clinical trial identifiers: scientific title and trial registration number. Clinical trials are registered on public web-based registries such as the Australia New Zealand Clinical Trials Registry (ANZCTR) or United States National Library of Medicine Clinical Trials Registry (Clinicaltrials.gov) to ensure public accessibility and accountability.17 Data items collected include tumour type, phase, sponsorship, site, number of new participants and number of ongoing participants. Year of birth and postcode data are only available for newly enrolled participants, not ongoing participants. The absence of patient identifiers may result in non-unique participants enrolled in multiple trials.
Where possible, missing trial data were obtained from alternative data sources: the ANZCTR18 or ClinicalTrials.gov.19 ANZCTR and ClinicalTrials.gov are examples of online clinical trial registries that provide information about clinical trials to the public.
2.3 Ethical considerations
CCV sought permission from each of the 43 Victorian CCT units to release the de-identified data for the study period. Once permission was received, CCV provided the Clinical Trial Management Scheme (CTMS) data set for each site to the researchers. Monash University Human Research Ethics Committee (Project ID 22593) and Monash Health Human Research Ethics Committee (HREC/62727/MonH-2020) provided ethical approval for this study.
2.4 Characteristics of participating in trial sites
2.4.1 Geographical location
The address of trial sites (publicly available information) was used to categorise the location of trial sites according to the modified Monash (MM) model.20 The MM model categorises areas according to geographical remoteness and population size and is informed by the Australian Statistical Geography Standard – Remoteness Areas framework.20 The MM model uses the following categories to classify remoteness and population size: MM1 (major cities), MM2 (inner and outer regional centres), MM3 (large rural towns), MM4 (medium rural towns), MM5 (small rural towns), MM6 (remote communities) and MM7 (very remote communities).20 Trial sites located in MM2-7 were categorised as rural and will be referred to as such hereafter. Concerning assessing associations between other trial characteristics and trial location, the outcome variable of interest was the binary variable ‘rural trial location’ (yes or no).
2.4.2 Trial phase
The trial phases provided in the CTMS data set were Phase I, Phase I/II, Phase II, Phase II/III, Phase III, Phase III/IV and Phase IV trials, as well as no phase (i.e. non-drug) trials. Trials that included more than one phase were regrouped into one of five phases (I, II, III, IV or no phase). The phase that was the trial's primary outcome was selected: I/II trials were regrouped as Phase I trials, and Phase II/III and Phase III/IV trials were regrouped as Phase III trials. Regroupings were determined by a co-author (SJH) with expertise in medical oncology and clinical trials. To obtain adequate cell sizes for assessing trial characteristics associated with rural trial locations, Phase I and II trials were reclassified as early-phase trials and Phase III and IV trials as late-phase trials.
2.4.3 Tumour type
The CTMS data categorised trials based on the location of individual solid tumours (e.g. lung, breast and head and neck) and grouped all haematological tumours. To provide more detailed information, haematological tumours were separated into four types: lymphoma, leukaemia, myeloma and other haematological tumours. A new binary variable was also created, with solid and non-solid tumour categories.
2.4.4 Trial intervention
Trial intervention was categorised as drug, radiotherapy, surgery, combination (i.e. chemo-radiotherapy), screening (ctDNA testing) or other (theranostics, imaging, Cart T-cell therapy or nutrition). The intervention was determined from the information available in the ANZCTR18 or ClinicalTrials.gov.19 To obtain adequate cell sizes for assessing the statistical significance of any variation in interventions between trial sites, trial interventions were also dichotomised into a new binary variable with the following categories: drug and non-drug interventions.
2.4.5 Trial sponsorship
Trial sponsorship refers to how the trial was financed. Sponsorship categories included industry sponsored, collaboratively sponsored and other sponsorship (investigator-initiated trials and trials with more than one sponsor listed). A binary variable of industry compared with non-industry sponsored trials was created for the regression analysis.
2.4.6 Characteristics of new participants
In the CTMS data set, variables for the year of birth and residential postcode were only available for participants newly enrolled in 2018. These variables were used to create two binary variables for new participants: estimated age (≥65 years or <65 years) and residential area (rural/metropolitan), as detailed below.
2.4.7 Estimated age
In the subset of trials that recruited new participants, age in years at trial enrolment was estimated at the participant level by subtracting year of birth from year of enrolment. These estimated ages were then used to calculate the mean age of participants for each separate trial. The continuous variable for mean age was collapsed into a binary variable indicating whether or not each separate trial's participant cohort was, on average, estimated to be older (aged ≥65 years).
2.4.8 Residential area
In the subset of trials that recruited new participants, each postcode was cross-referenced with the MM model20 to create a binary variable with ‘metropolitan’ and ‘rural’ categories as well as a polytomous variable with the following MM categories: ‘MM1 (metropolitan)’, ‘MM2 (regional centres)’, ‘MM3 (large rural towns)’, ‘MM4 (medium rural towns)’, ‘MM5 (small rural towns)’ and ‘MM6-7 (remote or very remote communities)’. When a postcode had more than one MM model category, the highest (i.e. ‘least rural’) category was used for categorisation. Another geographical variable was the state of residence for newly recruited participants. The percentage of Victorian CCT participants residing inside and outside Victoria was calculated.
The percentage of rural-residing patients was calculated for each trial, providing a non-normally distributed continuous variable. To understand the geographic distribution of trial sites with only rural-residing residents and to obtain adequate cell sizes for a cross-tabulation of residential areas with the trial area, trials with new participants were categorised in terms of whether or not they had 100% rural-residing patients in their particular participant cohorts.
2.5 Statistical analysis
Descriptive analyses were performed on all study variables. Calculations included frequencies, percentages and ratios for categorical variables as well as the mean (standard deviation [SD] and five-number summary (minimum, quartile 1 (Q1), median, quartile 3 (Q3) and maximum)) for continuous variables, including the mean of mean ages per trial. The age range of all new participants across all trials was also calculated for metropolitan and rural trial sites. Trial characteristics expressed as categorical variables (trial site, trial phase, tumour type, intervention and sponsorship) were compared between metropolitan and rural trial sites using Pearson's chi-squared test. The assumptions for the chi-squared test were assessed.21
In the entire sample of CCTs, univariable and multivariable binary logistic regression models were used to assess associations between each of four trial characteristics (solid tumours, early-phase trials, industry-sponsored trials and drug intervention trials) and the binary outcome variable: rural CCT location.
A subgroup analysis was conducted for those trials that had new participants. For this subgroup, univariable and multivariable binary logistic regression models were used to assess associations between two binary demographic variables (mean age ≥ 65 years and less than 100% rural-residing participants) and the binary outcome variable: rural CCT location. This subgroup analysis utilised a complete-case approach to manage missing demographic data.
All analyses were conducted using SPSS Version 26 (IBM Corp., Armonk, NY). P-values <0.05 were considered statistically significant.
3 RESULTS
3.1 Trial sites
In 2018, 1669 CCTs were conducted across 43 clinical trial sites in Victoria, Australia. Of these, 67 (4.0%) trials were paediatric trials conducted at two metropolitan specialist paediatric services. Overall, 1501 clinical trials (89.9%) were located in metropolitan Victoria (MM1), and 168 (10.1%) were located in rural areas (MM2&3) (Table 1). Recruitment of new participants to clinical trials at each site ranged from 1 to 42 participants per trial, while mean recruitment was 3.6 participants (SD = 3.7, median = 2.0, Q1 = 1.0, Q3 = 4.0). There were 529 (31.7%) trials with new participants enrolled in 2018 and 1140 (68.3%) trials with nil new participants enrolled in 2018. There were 783 (46.9%) trials with participants in follow-up. The number of participants per trial at each site ranged from 1 to 189, with a mean of 5.11 participants (SD = 9.5, median = 3.0, Q1 = 1.0, Q3 = 6.0).
| Characteristic | n (column %) | Ratio | ||
|---|---|---|---|---|
| Total | Metro | Rural | Metro:Rural | |
| Number of individual trials | 1669 | 1501 | 168 | 9:1 |
| Phase | ||||
| Phase I* | 329 (19.7) | 316 (21.1) | 13 (7.7) | 24:1 |
| Phase II | 370 (22.2) | 337 (22.5) | 33 (19.6) | 10:1 |
| Phase III* | 921 (55.2) | 805 (53.6) | 116 (69.0) | 7:1 |
| Phase IV | 16 (1.0) | 14 (0.9) | 2 (1.2) | 7:1 |
| Phase N/A | 33 (2.0) | 29 (1.9) | 4 (2.4) | 7:1 |
| Tumour type | ||||
| Breast | 238 (14.3) | 208 (13.9) | 30 (17.9) | 7:1 |
| GU | 184 (11.0) | 160 (10.7) | 24 (14.3) | 7:1 |
| Lymphoma* | 162 (9.7) | 137 (9.1) | 25 (14.9) | 6:1 |
| Leukaemia* | 162 (9.7) | 149 (9.9) | 7 (4.2) | 31:1 |
| Lung* | 156 (9.3) | 132 (8.8) | 24 (14.3) | 6:1 |
| Multiple Tumours* | 140 (8.4) | 136 (9.1) | 4 (2.4) | 34:1 |
| Myeloma* | 116 (7.0) | 112 (7.5) | 4 (2.4) | 29:1 |
| Haematological other* | 114 (6.8) | 111 (7.4) | 3 (2.6) | 37:1 |
| UGI* | 94 (5.6) | 77 (5.1) | 17 (10.1) | 5:1 |
| LGI* | 91 (5.5) | 71 (4.7) | 20 (11.9) | 4:1 |
| Skin | 76 (4.6) | 73 (4.9) | 3 (1.8) | 24:1 |
| Gynaecological | 55 (3.3) | 48 (3.2) | 7 (4.2) | 7:1 |
| Brain and CNS | 50 (3.0) | 50 (3.3) | 0 | – |
| Head and neck | 24 (1.4) | 24 (1.6) | 0 | – |
| Sarcoma | 13 (0.8) | 13 (0.9) | 0 | – |
| Tumour type | ||||
| Solid Tumour | 1121 (67.2) | 992 (66.1) | 129 (76.8) | 8:1 |
| Non-Solid Tumour* | 548 (32.8) | 509 (33.9) | 39 (23.2) | 13:1 |
| Intervention | ||||
| Drug | 1515 (90.8) | 1361 (90.7) | 154 (91.7) | 8:1 |
| Radiation | 66 (4.0) | 58 (3.9) | 8 (4.8) | 10:1 |
| Other | 17 (1.0) | 17 (1.1) | 0 | – |
| Combined treatments | 38 (2.3) | 38 (2.5) | 0 | – |
| Screening | 25 (1.5) | 19 (1.3) | 6 (3.6) | 3:1 |
| Surgical | 8 (0.5) | 8 (0.5) | 0 | – |
| Intervention | ||||
| Drug | 1518 (90.8) | 1361 (90.7) | 154 (91.7) | 9:1 |
| Non-Drug | 154 (9.2) | 140 (9.3) | 14 (8.3) | 10:1 |
| Sponsorship | ||||
| Industry* | 1114 (66.7) | 1019 (67.9) | 95 (56.5) | 11:1 |
| Collaborative* | 323 (19.4) | 270 (18.0) | 53 (31.5) | 5:1 |
| Other Non-Industry | 172 (10.3) | 159 (10.6) | 13 (7.7) | 12:1 |
| Combined | 60 (3.6) | 53 (3.5) | 7 (4.2) | 8:1 |
- Abbreviations: CNS, Central nervous system (brain and spinal cord); − No trials available; GU, Genitourinary (prostate, bladder and renal); upper gastrointestinal (oesophageal, stomach); lower gastrointestinal (colorectal); Metro, metropolitan.
- * p-value <0.05 (based on Pearson's chi-square test for which all assumptions were met).
3.2 Trial phase
For eight trials with unknown trial phases in the CTMS data set, missing data were obtained from ANZCTR18 or ClinicalTrials.gov.19
Phase III was the most frequently reported trial phase, accounting for 55.2% of all trials. The least frequently reported trial phase was IV, accounting for 1% of all trials. This was consistent for metropolitan and rural trial sites considered separately (Table 1).
There was a statistically significant variation in the numbers of Phase I and III trials conducted at metropolitan and rural sites. At rural sites, there were significantly fewer Phase I and significantly more Phase III trials (Table 1).
3.3 Tumour type
The most common tumour types represented were breast cancer (14.3%), genitourinary cancer (11.0%) and lymphoma (9.7%) (Table 1). There was statistically significant variation in trial numbers among the tumour types of trials conducted at metropolitan and rural sites. In rural areas, there were significantly more trials for the following tumour types: lymphoma, lung cancer, UGI and LGI cancer. Conversely, there were significantly fewer trials in rural areas for leukaemia, multiple solid tumours, myeloma and other haematological tumours.
The most common tumour types for metropolitan trial sites were breast cancer (13.9%), genitourinary cancer (10.7%) and leukaemia (9.9%). The most common tumour types for rural trial sites were breast (17.9%), lymphoma (14.9%), genitourinary (14.3%) and lung (14.3%) cancers. In contrast to metropolitan sites, no trials were available at rural sites for head and neck cancers, sarcomas or brain and CNS cancers.
When comparing solid and non-solid tumours, there was a statistically significant difference between metropolitan and rural trial sites (Table 1). Rural sites had significantly fewer trials for non-solid tumours.
3.4 Intervention
In most trials (90.8%), the interventions were pharmacological (i.e. drug trials). This was consistent across metropolitan (90.7%) and rural trial sites (91.7%). In contrast to metropolitan trial sites, there were no surgical trials, trials that combined more than one modality or other trials at rural sites. The variation in interventions between metropolitan and rural sites was not statistically significant (Table 1).
3.5 Sponsorship
One trial had no sponsorship listed, and this lack of available information was verified using data from ANZCTR.18 Two-thirds of trials were sponsored by an industry sponsor (Table 1). Rural sites had a significantly lower proportion of industry sponsorship than metropolitan sites but a significantly higher proportion of collaborative sponsorship.
3.6 Characteristics of new CCT participants and locations
A total of 5909 participants were enrolled on a CCT in 2018. There were 1907 (32.3%) new participants and 4002 (67.7%) existing participants. Ninety-five per cent of participants attended a metropolitan trial site (Table 2). For every 18 cancer patients enrolled at a metropolitan trial site, there was 1 cancer patient enrolled at a rural trial site. Year of birth was available for 1848 (96.9%) new participants. The age range of new participants was 1–89 years for metropolitan trial sites and 28–89 years for rural trial sites. The mean age of new participants for each trial was 61.42 years (SD = 14.54, median 64.0 years, Q1 = 56.50, Q3 = 70.11), and the mean trial ages ranged from 1.0 to 86.0 years. Most trials had new CCT participants with a mean age ≥ 18 years (n = 1648, 98.7%). The mean age of new participants for trials at metropolitan trial sites was 61.04 years (SD 14.84, median 64.00 years, Q1 = 56.50, Q3 = 70.00), and for rural trial sites was 65.65 years (SD 9.79, median 65.80 years, Q1 = 61.33, Q3 = 73.00).
| Participanta type | n (column %) | N (row %) | Ratio | |
|---|---|---|---|---|
| Total | Metro | Rural | Metro:Rural | |
| New | 1907 (32.3) | 1778 (93.2) | 129 (6.8) | 14:1 |
| Existing | 4002 (67.7) | 3816 (95.4) | 186 (4.6) | 21:1 |
| Total | 5909 (100) | 5594 (94.7) | 315 (5.3) | 18:1 |
- Abbreviations: n, frequency; metro, metropolitan.
- a The term ‘participant’ refers to a unique combination of an individual patient and an individual trial.
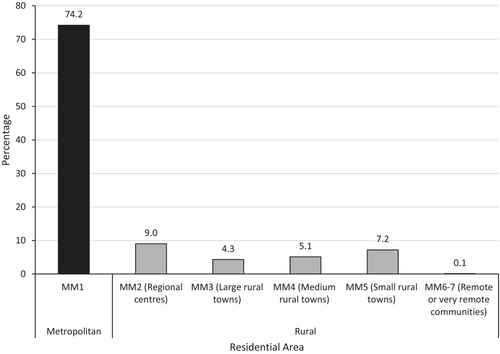
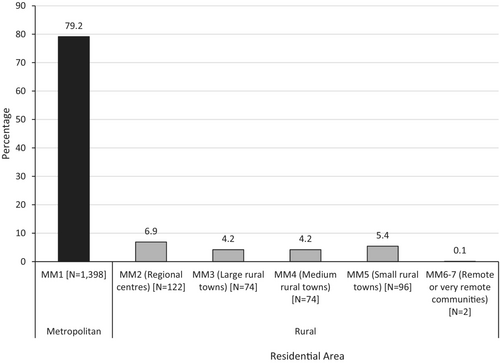
Of the 1907 new participants enrolled on a clinical trial, 1892 (99.2%) had postcodes with corresponding MM values available to determine geographic location. One hundred and three participants in Victorian CCTs (5.4%) resided outside Victoria. Of the 1892 participants, 1404 (74.2%) resided in a metropolitan area (MM1) and 488 (25.8%) resided in a rural area (MM2-7) (Figure 1). The most common rural residential areas were MM2 (regional centres) and MM5 (small rural towns), accounting for 9.0% and 7.2% of the 1892 newly enrolled participants respectively (Figure 1). Of the 488 rural participants newly enrolled on a clinical trial, 368 (75.4%) were recruited to a metropolitan site. The rates of participation in CCTs at metropolitan (as opposed to rural) sites were 6.9% for residents of MM2 (regional centres), 4.2% for residents of MM3 (large rural towns), 4.2% for residents of MM4 (medium rural towns), 5.4% for residents of MM5 (small rural towns) and 0.1% for MM 6–7 (remote or very remote communities) (Figure 2).
3.7 Associations between trial characteristics and rural trial location among all CCTs
Among all 1669 CCTs, the multivariable binary logistic regression analysis of trial characteristics (Table 3) found that the adjusted odds of a rural trial site are 51% lower for early-phase trials than late-phase trials, 63% higher for non-industry sponsored trials than industry-sponsored trials and 36% lower for non-solid tumour trials than solid tumour trials.
| Factor | Metro N (row %) | Rural N (row %) | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| Phase | ||||
| Late (III or IV) | 819 (87.4) | 118 (12.6) | 1.0 | 1.0 |
| Early (I or II) | 653 (93.4) | 46 (6.6) | 0.49 (0.34–0.70)* | 0.49 (0.35–0.71)* |
| No phase (non-drug) | 29 (87.9) | 4 (12.1) | 0.96 (0.33–2.77) | 0.66 (0.23–1.96) |
| Tumour | ||||
| Solid | 992 (88.5) | 129 (11.5) | 1.0 | 1.0 |
| Non-solid | 509 (92.9) | 39 (7.1) | 0.59 (0.41–0.86)* | 0.64 (0.44–0.93)* |
| Sponsorship | ||||
| Industry | 1019 (91.5) | 95 (8.5) | 1.0 | 1.0 |
| Non-industry | 482 (86.8) | 73 (13.2) | 1.63 (1.18–2.25)* | 1.63 (1.17–2.27)* |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; n, Frequency/numerator; N, total number of trials/denominator; OR, odds ratio.
- *p-value <0.05.
3.8 Associations between trial demographics and trial location among CCTs recruiting new participants
Of the 529 trials with newly recruited participants, 526 trials had postcode and year of birth data available for complete-case subgroup analysis. Seventy (13.3%) of the 526 trials only recruited rural-residing people with cancer. Of these 70 trials, 37 (52.9%) were located at rural trial sites. In the multivariable binary logistic regression analysis, having less than 100% rural-residing residents was associated with 99% lower odds of a rural trial location while having a mean participant age ≥ 65 years was unrelated to rural trial location (Table 4).
| Factor | n (row %) | OR (95% CI) | aOR (95% CI) | |
|---|---|---|---|---|
| Metro | Rural | |||
| Mean trial age | ||||
| <65 years (N = 279) | 260 (93.19) | 19 (6.81) | 1.0 | 1.0 |
| ≥65 years (N = 247) | 224 (90.69) | 23 (9.31) | 1.41 (0.75, 2.65) | 0.84 (0.36, 1.96) |
| 100% rural residentsa | ||||
| Yes (N = 70) | 33 (47.14) | 37 (52.86) | 1.0 | 1.0 |
| No (N = 456) | 451 (98.90) | 5 (1.10) | 0.01 (<0.01, 0.03)* | 0.01 (<0.01, 0.03)* |
- Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; n, Frequency/numerator; N, total number of trials/denominator; OR, odds ratio.
- a Those trials for which 100% of participants reside in MM 2–7.
- * p-value <0.05.
4 DISCUSSION
This cross-sectional study set in Victoria, Australia, in 2018, found 1669 CCTs, of which 10% were conducted at rural trial sites. A total of 5909 participants were enrolled in CCTs, and of them, 5% attended a rural trial site. The proportion of newly enrolled participants living in rural areas (26%) exceeds the proportion of Victorian residents living in rural areas (23%).15 However, approximately three-quarters of these rural residents, attended a metropolitan trial site, which highlights the additional burden of travel that rural residents face.
There was statistically significant variation between metropolitan and rural sites in relation to three trial characteristics: phase, tumour type and sponsorship, which has implications for rural residents accessing CCT.
4.1 Phase
Reduced rural availability of early-phase trials means potentially less access to emerging anti-cancer therapies. Phase I trials are designed to determine the safety and tolerability of a new treatment or combination of treatments and establish the recommended dosing for Phase II trials.22 Phase I trials have been seen as a last resort for those patients who have exhausted standard-of-care options.23 However, advances in cancer care have seen an improvement in the therapeutic benefit of Phase I trials. Emerging evidence indicates that Phase I trials have improved survival rates without an increase in treatment-related death.23, 24 The potential benefits of Phase I trials are largely limited to metropolitan clinical trial units, meaning that rural residents must travel to participate.
4.2 Tumour type
There were no available trials in rural areas for head and neck cancers, sarcomas and brain and CNS cancers. These tumour streams had the least number of trials available of all the tumour streams. There have been fewer treatment developments for sarcoma25 and brain cancer26 than for other tumour types, resulting from reduced clinical trial activity. There are known difficulties in conducting clinical trials for brain cancer related to trial design and eligibility criteria.26 Rural-residing head and neck cancer patients are more likely to travel to receive treatment, which means there are fewer patients receiving care locally.27 The complete absence of clinical trials for some tumour streams outside of metropolitan clinical trial units again highlights the requirement to travel for those living outside of metropolitan areas.
4.3 Trial sponsorship
In our study, rural trial sites had fewer industry-sponsored CCTs available than metropolitan trial sites. With reduced access to sponsored trials, rural participants have reduced access to travel reimbursement. Industry-sponsored trials can reimburse travel, accommodation and food expenses to relieve some of the financial burden. Compared to usual care, clinical trial participation leads to more visits to a cancer care provider, placing additional financial burden on participants.28 This burden is further compounded for those rural residents who must travel to metropolitan centres to receive care. Financial toxicity is worse for those clinical trial participants living greater than 100 miles (166 km) away from their clinical trial unit and those with lower socioeconomic status.29
4.4 Travel
Among participants newly enrolled in CCTs, the most common rural residential area was MM2 (regional centres). Furthermore, residents of MM2 had a higher rate of metropolitan CCT participation than those residing in more rural residential areas. This likely reflects that people residing in regional centres tend to be closer to metropolitan Victoria, where more CCTs are available, as well as the fact that there are more CCT units in Victoria's regional centres than in more rural and remote parts of Victoria.30 The lack of clinical trial opportunities rurally requires most clinical trial participants to travel to a metropolitan trial unit. In our study, 368 rural residents travelled to a metropolitan trial unit. A third of these participants resided in MM2 (regional centre), which may reflect the closer proximity to metropolitan clinical trial units.
The cost and time required for travel for clinical trial participation are significant. The out-of-pocket expenses calculated for rural-residing cancer patients participating in early-phase trials are at least US $600 per month for travel, accommodation, parking and food and US $200 per month for direct medical costs.29 This represents a large outlay of money for participation that may or may not be covered by the commercial sponsor of the trial. Travel is a barrier to clinical trial participation for rural patients. Increased travel time is associated with a reduced likelihood of clinical trial participation.31 In addition to being patient-related barriers, travel and distance are also perceived barriers for physicians and may prevent physicians from referring patients to clinical trials in the first instance.32 In a US study that was not specific to cancer,33 it was found that rural residents have 77% lower odds of being invited to clinical trials compared to metropolitan residents, which the authors concluded was due to limited trial availability locally.
4.5 Tele-trials and clinical trial networks facilitate local access to clinical trials
Our findings suggest that clinical trials can be conducted exclusively with rural participants at rural trial sites. Seventy trials recruited only participants from rural areas, of which 37 (53%) were completed at a rural trial site. Local access to clinical trials removes the barrier of travel; however, increased opportunities for clinical trials to be conducted from rural trial sites are needed to improve equity of access. Initiatives such as clinical trial networks13, 30 and tele-trials34 have demonstrated increased participation of rural cancer patients by providing clinical trial opportunities closer to home.
To improve access to clinical trials in rural Victoria, a clinical trials network – namely the Regional Trials Network (RTN) – was established in 2017 with funding from CCV.30 Preliminary data from the RTN show that, in rural Victoria, there are increased numbers of available CCTs and patients recruited to CCTs compared to this study.30 However, the RTN used a broader CCT definition than used for the CTMS data and included data from a site that would be classed as a metropolitan (MM1) trial site under the definitions used in our study.
Tele-trials are yet to be captured in the CTMS data (personal communication, 2022). The targeted thromboprophylaxis in patients receiving anticancer therapies (TARGET-TP), tele-trial did not meet the CCV eligibility criteria for inclusion in the 2018 CTMS data.35 There has been increasing activity in tele-trials in recent years (post-2018).12, 36, 37 This activity has been accelerated by the need to increase the number of rural participants in CCTs and provide cancer care remotely during the COVID-19 pandemic.36
In line with the importance of local access to clinical trials is the specific need for early-phase trials in rural areas. In the literature, a US example of a rurally based, community oncology Phase I clinical trial unit is described by Powell and Bleeker.38 Over an 8-year period, 21 early-phase clinical trials were conducted, and 218 participants enrolled, with over half of participants from rural areas at this centre.
4.6 Financial reimbursement
Patient reimbursement for trial-related costs is recommended by US government agencies39 and professional groups40, 41 as a measure to remove the financial barriers to CCT participation. Financial reimbursement for costs incurred as part of clinical trial participation can assist cancer patients in participating in clinical trials42, 43 and reduce the inequity in access between rural and metropolitan cancer patients. A cancer care equity program from the USA that provided financial assistance to meet the costs of accommodation and travel during CCT participation has been associated with increased CT enrolment.43 The program may have encouraged oncologists to discuss clinical trial participation by removing the financial barriers to participation.43
Corrigan and Fu44 recommend including financial toxicity measures as part of patient-reported outcomes collected during clinical trials. These data could accurately define the out-of-pocket costs that new treatments create, which is particularly relevant to rural residents who face increased costs.
4.7 Strengths and limitations
A strength of this study was the availability and inclusion of data from 100% of the trial sites. There were no missing data for any trial characteristics other than demographics, for which there were only small proportions of missingness. Limited CCT data are available in Australia: only three of Australia's eight states/territories collect annual data, and each state completes this process independently.45-47 The data available for analysis in the present study were for Victoria only, limiting the generalisability of results to other Australian states/territories and internationally. This does raise the question of whether more clinical trial participation data could be reported as part of clinical trial registration. Understanding whether target recruitment is being met and the participants' demographics would further enhance the transparency of clinical trial conduct and inform quality improvement initiatives.
CTMS data only include WHO-defined clinical trial intervention trials,16 which may lead to CCT underreporting due to the lack of non-interventional trials in the data set. Data on CCTs for prevention, supportive care and palliative care initiatives are also lacking. CCTs conducted outside a clinical trial site may be missing from the CTMS data set. Another limitation is the imperfect correspondence between participant residential postcode data and the MM model. As some postcodes are represented by more than one MM category, the absence of data on residential suburbs means that the accuracy of the MM category cannot be ensured.
A unique patient ID in the CTMS data would allow for patient-specific analysis. The CTMS data currently lack unique patient identifiers, making it impossible to determine participant occurrence. Also, patient-level data are lacking, making it difficult to assess social determinants of health on enrolment beyond age and residential location. The new Australian National Clinical Trial Governance Framework requires clinical trial sites for the first time to report on the Indigenous status and cultural and linguistically diverse status of clinical trial participants.48 This will assist with understanding the representation of these populations in clinical trials more broadly for Australia.
5 CONCLUSION
In 2018, most rural CCT participants in Victoria had to travel to a metropolitan site to participate in a trial due to structural barriers, such as the absence of trials in certain tumour types and the limited availability of early-phase and industry-sponsored trials. However, rural trial sites were more likely to recruit new participants exclusively from rural areas, indicating the feasibility of conducting CCTs at rural sites. New initiatives and investment in CCTs across rural Victoria may increase trial opportunities, which is much needed to improve equity and access for rural-residing cancer patients. Future evaluation of these initiatives and monitoring of rural-residing cancer patients' participation in CCTs is important for rural people diagnosed with cancer in Australia and internationally.
AUTHOR CONTRIBUTIONS
McPhee J. Narelle: Conceptualization; writing – original draft; methodology; writing – review and editing; formal analysis. Michael Leach: Conceptualization; writing – original draft; formal analysis; supervision; methodology; writing – review and editing. Claire E. Nightingale: Supervision; formal analysis; writing – review and editing; writing – original draft; conceptualization; methodology. Samuel J. Harris: Conceptualization; writing – original draft; writing – review and editing; formal analysis; supervision; methodology. Eva Segelov: Conceptualization; writing – original draft; writing – review and editing; formal analysis; supervision; methodology. Eli Ristevski: Supervision; conceptualization; writing – original draft; writing – review and editing; methodology; formal analysis.
ACKNOWLEDGEMENTS
The project team acknowledges the use of Clinical Trials Management Scheme data collected from 43 participating trial sites by Cancer Council Victoria, with support from the Victorian Government through the Victorian Cancer Agency. Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
The authors received no specific funding for this work.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest to disclose about this research. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
ETHICS APPROVAL
Monash University Human Research Ethics Committee (Project ID 22593) and Monash Health Human Research Ethics Committee (HREC/62727/MonH-2020) provided ethical approval for this study.




