Erythropoietin prevents LPS-induced preterm birth and increases offspring survival
Jie Zhang and Xianqiong Luo contributed equally to this work.
Abstract
Problem
Preterm delivery is the leading cause of neonatal mortality and contributes to delayed physical and cognitive development in children. At present, there is no efficient therapy to prevent preterm labor. A large body of evidence suggests that infections might play a significant and potentially preventable cause of premature birth. This work assessed the effects of erythropoietin (EPO) in a murine model of inflammation-associated preterm delivery, which mimics central features of preterm infections in humans.
Method of study
BALB/c mice were injected i.p. with 20 000 IU/kg EPO or normal saline twice on gestational day (GD) 15, with a 3 hours time interval between injections. An hour after the first EPO or normal saline injection, all mice received two injections of 50 μg/kg LPS, also given 3 hours apart.
Results
EPO significantly prevented preterm labor and increased offspring survival in an LPS induced preterm delivery model. EPO prevented LPS-induced leukocyte infiltration into the placenta. Moreover, EPO inhibited the expression of pro-inflammatory cytokines, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumour necrosis factor-α (TNF-α) in maternal serum and amniotic fluid. EPO also prevented LPS-induced increase in placental prostaglandin (PG)E2 and uterine inducible nitric oxide synthase (iNOS) production, while decreasing nuclear factor kappa-B (NF-κβ) activity in the myometrium. EPO also increased the gene expression of placental programmed cell death ligand 1 (PD-L1) in LPS-treated mice.
Conclusions
Our results suggest that EPO could be a potential novel therapeutic strategy to tackle infection-related preterm labor.
1 INTRODUCTION
Preterm delivery (birth before 37 completed weeks of gestation) occurs in 5%-15% of all pregnancies and remains a significant public health concern worldwide.1 Premature birth is associated with 75% of infant mortality and 50% of long-term neurological handicaps.2, 3 Despite the advances made in obstetrics and neonatology, the rate of premature delivery has not decreased over the past 20-30 years. At present, there is no efficient therapy to prevent preterm labor and to increase offspring survival. Although the etiology is multifactorial, intrauterine infections account for 25%-40% of all premature deliveries.2 Lipopolysaccharide (LPS) stimulates nuclear factor kappa B (NF-κβ)4, 5 and cytokine responses in the chorioamnion membrane and placenta, leading to an increase in gene expression and protein production levels of proinflammatory cytokines, including tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), prostaglandins (PGs), and nitric oxide (NO),6 and these factors play a role in preterm labor.7-9 Moreover, it has been reported previously that a successful pregnancy is closely related to the maintenance of tolerance to the fetus at the feto-maternal interface (FMI) and a dysregulation in the tolerance to the fetus is associated with adverse gestational outcomes, resulting in preterm labor and fetal loss.10 Studies have shown that the PD-1/PD-L1 (programmed death-1/programmed death-ligand 1) pathway is particularly important for the maintenance of tolerance to the fetus.10 The blockade of the pathway results in a pro-inflammatory environment and preterm labor in allogeneic mouse models of pregnancy.10, 11
Erythropoietin (EPO) is a pleiotropic hormone that regulates the production of red blood cells by binding to the homodimeric EPO receptor and has been widely used to treat anemia.11, 12 It has been shown to produce protective effects on kidneys via modulation of the proinflammatory NF-κB pathway.5 EPO is also known to reduce proinflammatory cytokines and decrease the levels of serum inflammatory parameters in human brains.13, 14 EPO also decreases iNOS/NO and COX-2/PG expression in the spinal cord ventral horn.15, 16 Thus, EPO has been used to treat illnesses such as hepatic ischemia/reperfusion (I/R), preterm brain injury, hypoxic-ischemic encephalopathy (HIE), and burn-induced neuromuscular dysfunction and other related illnesses that have inflammatory etiologies.15, 17
It was previously detected that, apart from the kidney in adults, EPO is expressed in the uterus18, 20, 19 and placenta throughout the gestation period.21, 22 EPO has a protective effect in the placenta by downregulating acute inflammation induced by exposure to LPS.21, 23 It also regulates the survival of the first trimester trophoblast cells and decidual stromal cells (DSCs). Deficiency in EPO expression at the FMI could lead to unwanted pregnancy outcomes.22, 24 These results suggest that EPO could have an important role in the maintenance of gestation.
EPO binds to two cell surface Epo receptors (EPORs), which form disulfide-linked homodimers, and selectively activates JAK2 kinase, which in turn phosphorylates Jak2 and EPOR. This process activates a variety of signaling cascades leading to STAT3 and p-STAT3 activation, which are transcription factors of the anti-apoptotic gene family consisting of Bcl-2 and Bcl-xL proteins. The JAK2/STAT3 signaling pathway is an upstream regulator of PD-L1.23, 24 PD-L1 has been shown to play an important role in the maintenance of gestation and its blockade results in preterm labor,10 but whether the effect of EPO on maintenance of gestation is related to PDL1 is unknown. With this background, the aim of this work was to study the effect of EPO on preterm labor and offspring survival in a murine model of LPS induced preterm delivery.
2 MATERIALS AND METHODS
2.1 Animals
Female BALB/c mice from the Institute of Experimental Animals at Guangzhou Medical University were housed in an approved animal room with ad libitum access to food and water. Controlled conditions of humidity (50%-60%) and temperature (21 ± 2°C) were maintained and the animals were exposed to a 12-h dark/12-h light cycle. Virgin female mice were mated with fertile males of the same strain. Pregnancy confirmation was determined by visual inspection of the vaginal plug, which was defined as day 0 of pregnancy. The average gestational length for BALB/c mice is typically 19-20 days. Animals were killed in a CO2 chamber and all efforts were made to minimize their suffering. All the experimental protocols were approved by Guangzhou Medical University Animal Care and Use Committee and were performed following the guidelines of the National Institutes of Health on the care and ethical treatment of animals.
2.2 Treatments
On gestational day (GD) 15 (day 0 = vaginal plug observed), pregnant mice were assigned to one of four groups: (i) CRL mice (n = 10) were intraperitoneally injected with 0.2ml normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of normal saline, also given 3 hours apart. (ii) LPS mice (n = 14) were intraperitoneally injected with 0.2 mL normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of 50 μg/kg LPS (Escherichia coli 0111:B4; Biochemist), also given 3 hours apart. (iii) EPO/LPS mice (n = 11) were intraperitoneally injected with 20 000 IU/kg recombinant erythropoietin (EPO, NeoRecormon®; Boehringer-La Roche) twice on GD15, with a 3 hours time interval between injections. One hour after the first rhEPO injection, mice then received two injections of 50 μg/kg LPS, also given 3 hours apart. (iv) EPO mice (n = 10) were intraperitoneally injected with 0.2 mL normal saline twice on GD15, with a 3 hours time interval between injections. One hour after the first normal saline injection, mice then received two injections of 20 000 IU/kg EPO, also given 3 hours apart. Animals were observed every hour after the first LPS or vehicle solution injection for any signs of morbidity (decreased movement and/or diarrhea), vaginal bleeding, and preterm delivery. The beginning of preterm delivery was defined by the delivery of the first pup. Viability of the pup was assessed by tactile stimulation.
A second experiment was performed in which the pregnant mice were treated as the first experiment. The CRL, LPS, EPO/LPS, and EPO groups of pregnant mice were deeply anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours post-injection of the first vehicle or LPS, (ie, before the onset of preterm labor), and the uterus, placenta, amniotic fluid, and blood samples were collected. To investigate the effects of FA on high-dose.
2.3 Enzyme-linked immunosorbent assay
The concentrations of IL-1β, IL-6, and TNF-α in mouse maternal serum and amniotic fluid were determined by enzyme-linked immunosorbent assay kit (eBioscience) according to the manufacturer's instructions.
2.4 Western blots
For Western blot analysis, five animals were randomly selected from each group. Mice were anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours after the first vehicle or LPS. Their uteruses were removed and frozen at −80°C until use. The samples were homogenized on ice in 15 mole/L Eris buffer containing a cocktail of proteinase inhibitors and phosphatase inhibitors. The protein samples were separated via gel electroplate (SDS-PAGE) and transferred onto a polyvinylidene fluoride (PVDF) membrane. The membranes were placed in the blocking buffer for 1 hour at room temperature (RT) and incubated with a primary rabbit antiserum against iNOS (1:1000; CST) or phosphorylated-NF-κB-65 (1:1000; CST) overnight at 4°C. Then, the membranes were incubated in horseradish peroxidase-conjugated IgG. An enhanced luminescence (ECL) solution (Pierce) was used to detect the immune complexes. Each band was quantified using a computer-assisted imaging analysis system (NIH ImageJ).
2.5 Immunohistochemistry
Five animals were randomly selected from each group. Mice were deeply anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours after the first vehicle or LPS treatment. The placentae were removed and fixed in 4% paraformaldehyde solution and embedded in paraffin. Paraffin sections (5 μm) were cut, dehydrated, and microwaved in citrate-phosphate buffer (pH 6.0) to retrieve antigens. Then, the sections were treated with 3% hydrogen peroxide (H2O2), followed by 10% normal goat serum blocking at RT for 30 minutes. This was followed by incubation with PD-L1 (1:200; Abcam) or PGE2 antibody (1:200; Abcam) diluted in PBS containing 1% BSA for 24 hours at 4°C. The sections were incubated with mouse anti-rabbit IgG for 30 minutes at RT after washing with PBS (3 minutes × 3 times). The signals were detected by using a biotin-streptomycin-hydroxide system using diamorphine (Charismatic) as the chromosome. Negative controls were performed with primary antibody replaced by PBS.
2.6 Hematoxylin and Eosin (H&E staining)
Five animals were randomly selected from each group. Mice were deeply anesthetized using sodium pentobarbital (50 mg/kg, i.p.) on GD15 at 12 hours after the first vehicle or LPS injection. The placentae were removed and immediately fixed in 10% formalin saline for 24 hours. The placentae were transferred to 70% ethanol and then processed to paraffin-embedded blocks to generate 5-µm-thick sections for hematoxylin and eosin (H&E) staining. The slides were observed under a light microscope for cellular changes of inflammation and photographs of different magnifications were taken digitally with FSX100 (Olympus).
3 DATA PRESENTATION AND STATISTICAL ANALYSIS
Kaplan-Meier survival curves for preterm labor experiments were compared between different treatment groups using the log-rank test. Latency intervals (mean ± SD) were analyzed between different experimental groups using a one-way analysis of variance (ANOVA). Comparisons were made with post hoc Tukey's test. IL-β, IL-6, and TNF-α levels in the mother's serum or amniotic fluid and phosphorylated-NF-κB-65 and iNOS levels in uterine were analyzed between different experimental groups using an ANOVA test. Comparisons were made with post hoc Tukey's test. Statistical analyses were performed using GraphPad Prism 7 (GraphPad) software. The level of significance was set at P < .05 for all analyses.
4 RESULTS
4.1 Effect of EPO on LPS-induced preterm delivery
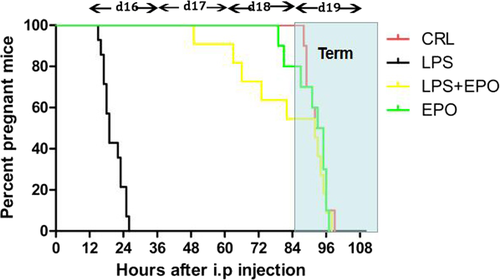
Figure 1 illustrates the cumulative percentage of pregnant animals at each time point. In CRL and EPO groups, no pregnant mouse delivered before GD19. LPS resulted in 100% pregnant mice delivering before GD19. Preterm delivery rate dropped to 45.5% when LPS-treated mice were pretreated with EPO. EPO significantly reduced the preterm delivery rates.
4.2 Effect of EPO on fetal survivals after LPS injection
Table 1 illustrates EPO reduced fetal mortality. 100% of fetuses were dead in LPS-treated mice. EPO reduced fetal mortality by 46%, significantly alleviating LPS-induced fetal death (Table. 1).
| Viable pup % number/total pup number | Viable pup number/mother | |
|---|---|---|
| CLR | 100 | 8 ± 2 |
| EPO | 100 | 7 ± 2 |
| LPS | 0**** | 0 |
| EPO + LPS | 46**** | 5 ± 1 |
- **** P < 0.0001 vs LPS;
- **** P < 0.0001 vs CRL.
4.3 Effect of EPO on LPS-induced upregulation of PGE2 in placenta
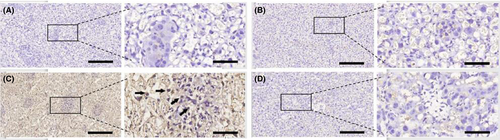
LPS markedly increased the levels of placental PGE2 which expressed in placental trophoblast cells (Figure 2C),when compared to the negative controls (Figure 2A). EPO itself had no effect on PGE2 production (Figure 2B). EPO pretreatment attenuated LPS-induced upregulation of PGE2 in placentae (Figure 2D).
4.4 Effect of EPO on LPS-induced leukocyte infiltration in placenta
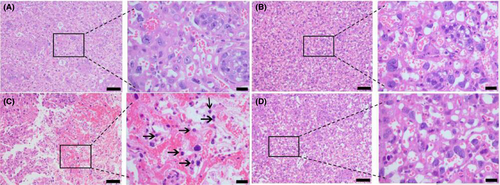
Lipopolysaccharide caused an increased leukocyte infiltration in the placenta (Figure 3C), when compared to the negative controls (Figure 3A). EPO itself had no effect on leukocyte infiltration in the placenta (Figure 3B). EPO prevented LPS-induced leukocyte infiltration in the placenta (Figure 3D).
4.5 Effect of EPO on LPS-induced release of inflammatory cytokines in maternal serum
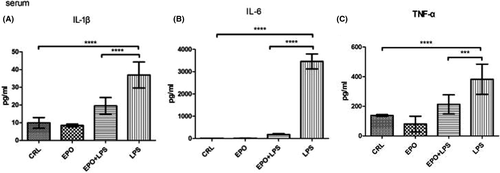
As shown in Figure 4, maternal serum IL-1β, IL-6, and TNF-α levels significantly increased in LPS-treated animals, whereas EPO significantly reduced the effect of LPS on this parameter.
4.6 Effects of EPO on LPS-induced release of inflammatory cytokines in amniotic fluid
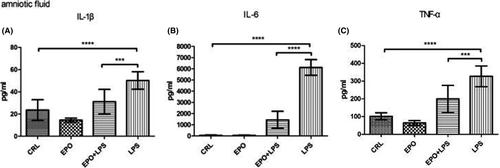
As shown in Figure 5, amniotic fluid IL-1β, IL-6, and TNF-α levels significantly increased in LPS-treated animals, whereas EPO significantly reduced the effect of LPS on this parameter.
4.7 Effect of EPO on LPS-induced NF-κB activation and iNOS protein level in uterus
Uterine phosphorylated-NF-κB-65 and iNOS levels were assessed in control and LPS-injected mice both in the presence and absence of EPO. As shown in Figure 6, the levels of phosphorylated-NF-κB-65 and iNOS protein were significantly increased in uteruses of mice injected with LPS. EPO significantly prevented increases in phosphorylated-NF-κB-65 and iNOS protein levels induced by LPS (Figure 6A,B, respectively).

4.8 Effects of EPO on the expression of placental PD-L1 after LPS injection
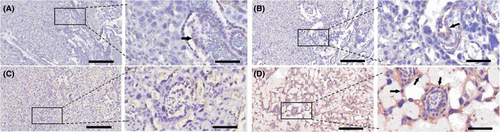
LPS decreased the expression of placental PD-L1 (Figure 7C), when compared to the mice receiving saline injection (Figure 7A). EPO, which on its own had no effect on PD-L1 expression (Figure 7B), increased the expression of placental PD-L1 after LPS injection (Figure 7D).
5 DISCUSSION
The current results indicate that EPO prevented preterm labor and increased offspring survival in LPS-induced preterm delivery. These data indicate that EPO is a potential therapeutic agent for infectious preterm labor.
The administration of LPS to pregnant BALB/c mice on GD15 induced premature parturition, cervical bleeding, and opening. The two doses of 50 μg/kg LPS with 3 hours time difference between injections, resulted in reliable preterm delivery with the least variation in time to delivery between mice and the minimal maternal mortality. In addition, it was considered that prolonged exposure to bacterial products at sub-clinical doses is a more effective trigger for the onset of preterm labor and delivery.25
Non-steroid anti-inflammatory drugs (NSAIDs) are non-specific COX inhibitors. At present, NSAIDs are considered the most effective tocolytics. Indomethacin, a nonspecific COX inhibitor, was reported to be an efficient tocolytic drug, significantly delaying preterm delivery.26, 27 However, its use should be restricted in duration and limited to pregnancies below 32 weeks because of fetal ductus arteriosus closure risk and decreased urine production, responsible for oligohydramnios.28-31 However, the ideal tocolytic agent should be easy to administer, inexpensive, effective in preventing preterm birth and improving neonatal outcomes, with few maternal, fetal, and neonatal side effects, and without long-term adverse effects.32 It has been demonstrated that EPO provides a reliable protective strategy with no significant adverse effects in a high preterm and extremely low birth weight infants.33, 34 We also demonstrated that EPO prevented preterm labor in LPS-treated mice and increased the survival of offsprings.
EPO is a broad-spectrum antioxidant35, 36 and a noteworthy anti-apoptotic agent.37 Free radical damage38 and apoptosis22 are common place during pregnancy and have negative effects on the mother, placenta, and fetus. Thus, the antioxidant and antiapoptotic actions of EPO could also account for the protective effect of EPO on preterm delivery and offspring survival. Although preterm prevalence has decreased in the last decade, there are still high rates of morbidity, even under a tocolytic treatment. Prenatal exposure to inflammation is believed to be an important causal factor in adverse outcomes for children born preterm. It was shown that EPO treatment ameliorates damage to fetal brain and liver, optic nerve injury, and placenta induced by exposure to LPS through anti-inflammatory, anti-apoptotic effects and proliferative responses.21, 39 It was suggested that due to its multiple organ protection functions and various protection mechanisms, EPO not only behaves as a tocolytic agent, but also might improve fetal development by protecting the fetus. Ongoing studies are in progress to confirm the preventive effects.
Augmentation of PGE2 derived from COX-2 is a key signal in both the ripening of the cervix and stimulation of uterine contractions.40 A previous report indicated that LPS increased the levels of PGE2.41 It was also shown that EPO decreased COX-2 expression after burn injury.15 As shown herein, EPO decreased PGE2 levels after LPS injection. EPO has been reported to be an effective inhibitor of iNOS activity.42 It was shown that EPO attenuates iNOS induction during pentylenetetrazole-induced generalized seizures.46 We also demonstrated that EPO prevented the increased production of iNOS protein levels following LPS injection.
Pregnancies affected by LPS are characterized by elevated levels of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β in the amniotic fluid, myometrium, fetal membranes, and maternal serum.43, 44 EPO or EPO analogs prevented the effects of LPS on TNF-α, IL-6,,25 and IL-1β45 levels, as previously described in adipose tissues, pancreas, and brain.25, 42, 45 As shown herein, EPO significantly inhibited the production of TNF-α, IL-6, and IL-1β in amniotic fluid and maternal serum.
The release of proinflammatory cytokines plays a critical role in the pathogenesis of inflammation-associated premature delivery.46, 47 NF-κB is an important factor in the regulation of inflammatory proteins,48, 49 such as IL-6, IL-1β, iNOS, and TNF-α, among others. It was reported that N,N-dimethylacetamide (DMA) significantly suppressed the activation of NF-κB signaling pathways from LPS-induced mice50 and prevents preterm birth.51 EPO also acts as a potent anti-inflammatory immune modulator by specifically targeting NF-κB.14, 52 Besides, it was shown that EPO exerts a protective effect in renal IRI via the MAPK/NF-κB pathway.5 In accord with the above studies, we also demonstrated that EPO suppressed the action of uterine NF-κB induced by LPS.
A successful pregnancy is the result of maintenance of tolerance to the fetus by the maternal immune system, as well as, protection from other harmful infections. A dysregulated maternal immunity is related to complications in pregnancy resulting in pathological preterm labor.57 The maternal immune cells present in the uterus during the period of gestation are tightly regulated, and a very fine balance exists between immunity and tolerance at the FMI. PD1/PD-L1 pathway plays an important role in fetal protection and the maintenance of tolerance to the fetus.57 Blockade of this pathway, by anti-PDL1 or anti-PD1, abrogates the fine balance of tolerance and immunity at the FMI.53 Meanwhile, blockade of the PD1/PDL1 pathway results in tolerance to the fetus at the FMI not being maintained resulting in fetal loss in allogeneic mouse model of pregnancy.54 It has been shown that the JAK2/STAT3 signaling pathway is an upstream regulator of PD-L1.23, 24 EPO activate JAK2/STAT3 signaling pathway, and p-STAT3 are transcription factors of the anti-apoptotic gene family consisting of Bcl-2 and Bcl-xL proteins. Our study first indicates that EPO increased the expression of placental PD-L1 in preventing LPS-induced preterm labor, suggesting that EPO might activate the JAK2/STAT3 signaling pathway and promote the expression of PDL1 protein to prevent preterm labor.
However, the potential therapeutic benefits of EPO therapy are outweighed by its primary erythropoietic activity with a subsequent increase in the risk for thromboembolic complications.14, 55 EPO analogs or derivatives, such as EPO-derived Helix B-surface peptide (pHBSP), carbamylated EPO (CEPO), asialo-EPO similar peptides,13, 25which have no erythropoietic activity while retaining the tissue-protective properties of EPO, might broaden the scope of clinical application.
EPO in human and sheep plasma cannot pass through the placental barrier from mother to fetus.56, 57 EPO protected the placenta and fetal liver from damages induced by LPS.21 Our study has indicated that maternally administered EPO prevented preterm labor induced by LPS and increased offspring survival rates. These findings led us to consider that the placental barrier exposed to inflammation might become more permeable, which meant that EPO could travel across the placenta to the fetus and play a protective role.
Several studies support the inference that increased NO, PG, and cytokine production play significant roles in preterm delivery.8, 58, 59 The administration of indomethacin (a COX-2 inhibitor),41 aminoguanidine (an iNOS inhibitor),41 or etanercept (a competitive inhibitor of TNF-α)8 prevents inflammation-induced preterm delivery. In addition, cytokine suppressive anti-inflammatory drugs (CSAIDs),60 which work by specifically targeting the NF-κB and p38 MAPK inflammatory signaling pathways, could be an available therapeutic option to prevent preterm labor. These and previous studies support the notion that the impairment of PG/COX-2 system,61 decrease in NO levels,41 diminishing cytokine production,62 inhibiting cytokine signaling pathways,60 or preferably the combination of these treatments, maybe a therapeutic strategy to prevent preterm delivery. Therefore, EPO could be a promising resource in the management of preterm delivery, as it alone decreased pro-inflammatory cytokine levels in maternal serum and amniotic fluid, PGE2 contents in placenta, and p-NF-κB-p65 and iNOS levels in mouse uteri from animals treated with LPS. Moreover, EPO promotes expression of placental PD-L1 in preventing preterm labor induced by LPS. Thus, EPO might be considered as a novel tocolytic agent for infection-related preterm labor.
ACKNOWLEDGMENTS
There is no funding to report for this submission. We would like to thank Professor RuiPing Pang for technical support.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.