Effects of roads on small mammal diversity and abundance in the northern Serengeti, Tanzania
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abstract
enThe high biodiversity of small mammal species in the Serengeti ecosystem provides this ecosystem with important conservation value. However, whether the extensive development of roads has negative impacts on the small mammal population has not been tested. Small mammal population diversity and abundance were examined in this study using live trapping at sites close to (experimental) and away (control) from the main gravel road during the short rainy seasons in November and December 2011 and 2012. A total of 138 individuals from three orders representing six families and fourteen species were collected over 4,860 trap nights. There were no significant differences in the species richness, diversity or abundance of small mammals between the control and experimental sites (p > 0.05), suggesting that the current gravel road does not have a significant impact on the small mammal population. These findings were ascribed to the availability of favourable habitats at both distances as a result of little road usage due to poor conditions. Should the road be improved, the control of anthropogenic activities in the area should be given high priority. Continuous monitoring of the small mammal populations in the area is recommended.
Resume
frLa grande biodiversité des espèces de petits mammifères de l'écosystème du Serengeti confère à cet écosystème une valeur de conservation importante. Cependant, on ne sait pas si le développement extensif des routes a des effets négatifs sur la population de petits mammifères. La diversité et l'abondance de la population de petits mammifères ont été examinées dans cette étude en utilisant le piégeage vivant dans des sites proches (expérimentaux) et éloignés (témoins) de la route de gravier principale pendant les courtes saisons des pluies de novembre et décembre 2011 et 2012. Un total de 138 individus Sur trois ordres représentant six familles et quatorze espèces, 4860 nuits-pièges ont été recueillies. Il n'y avait pas de différences significatives dans la richesse en espèces, la diversité ou l'abondance des petits mammifères entre les sites témoins et expérimentaux (p> 0,05), ce qui suggère que le chemin de gravier actuel n'a pas d'impact significatif sur la population de petits mammifères. Ces résultats ont été attribués à la disponibilité d’habitats favorables aux deux distances en raison de la faible utilisation de la route en raison de mauvaises conditions. Si la route devait être améliorée, le contrôle des activités anthropiques dans la région devrait être hautement prioritaire. Une surveillance continue des populations de petits mammifères dans la région est recommandée.Mots clés: Abondance, Diversité, Route du Serengeti, Petits mammifères.
1 INTRODUCTION
Roads are important infrastructural tools for economic development in rural areas (Chomitz & Gray, 1996). However, road construction destroys habitats, creates edge effects, promotes the introduction of exotic plant species (Kija, Kittle, Mneney, & Mwita, 2011; Runyoro, Nkya & Nyahongo, 2011; Elisante, Mokiti, & Ndakidemi, 2013), reduces the free movement of wildlife across the landscape (Garland & Bradley, 1984; McGregor, Bender, & Fahrig, 2008; Jaeger, 2012) and may lead to high wildlife mortalities through collision with vehicles (Bellis & Graves, 1971; Drews, 1995). Several studies have indicated that animal populations, including those of large mammals, birds and amphibians, tend to have lower densities along edges created by roads in comparison with the corresponding interior habitats due to the increase in disturbances, such as noise, surface barriers and dust deposition, caused by the movement of vehicles on the roads (Forman & Deblinger, 2000; Ndibalema, Mduma, Stokke, & Røskaft, 2007; Newmark, Boshe, Sariko, & Makumbule, 1996). On the other hand, roads can positively affect some animals by facilitating their access to food or by increasing the food supply (Adams & Geis, 1983; Ascensão, Clevenger, & GLIRO, 2012; Bissonette & Rosa, 2009; Garland & Bradley, 1984).
The effects of roads on animals in wildlife parks were first described by Pienaar (1968); since then, many researchers have examined animal mortality along highways (Bellis & Graves, 1971; Drews, 1995; Evenden, 1971; Newmark et al., 1996). However, only a few animal taxa, such as amphibians, birds and large mammals (Trombulak & Frissell, 2000; Forman & Deblinger, 2000; Ndibalema et al., 2007; Mahulu, Senzota, & Magige 2015), have received much attention. In the Serengeti ecosystem, most small mammal studies have focused on ecological and socio-ecological issues (Magige, 2012, 2013; Magige & Senzota, 2006; Senzota, 2012; Timbuka & Kabigumila, 2006), and no studies have addressed the effects of roads on small mammals.
The Serengeti ecosystem is one of the largest mosaics of open grassland and woodland located in northern Tanzania and supports large and varied wildlife populations (Sinclair, 1995). The northern part of this ecosystem consists of a network of gravel roads that cut across the Serengeti National Park (SNP). Like many protected areas in Africa, this ecosystem is faced with increasing pressure from development associated with road construction and tourism-related facilities (Elisante et al., 2013; Fyumagwa et al., 2013; Jaeger, 2012; Kija et al., 2011). The construction and improvement of road networks improve economic access, which in turn supports community development (Fyumagwa et al., 2013). However, upgrading gravel roads to tarmac standards may have negative effects on the ecosystem (Drews, 1995; Newmark et al., 1996; Forman & Alexander, 1998; Ndibalema et al., 2007). For instance, in Mikumi National Park, the improvement of a highway that cuts across the park has resulted in the death of thousands of wildlife since 1992 (Drew, 1995). To monitor and mitigate the impacts associated with road development, we need to characterise the current baseline data in the area before and after road construction (Fyumagwa et al., 2013; Røskaft et al., 2012).
The present study aims to determine the effects of roads on the species richness, species diversity and abundance of small mammals along the main gravel road in the northern part of the Serengeti ecosystem. A preliminary survey in the area recorded 133 species of birds and 11 species of small mammals, with most of them identified as rodents (Røskaft et al., 2012). Because road edges represent areas of high disturbance (Adams & Geis, 1983; Ascensão, 2012), it was hypothesised that survey sites away from the main road would have a higher species richness, diversity and abundance of small mammals than sites adjacent to the main road.
2 MATERIALS AND METHODS
2.1 Study area description
The Serengeti ecosystem is located in northern Tanzania and extends to south-western Kenya between the latitudes of 1° and 3°S and the longitudes of 34° and 36°E (Figure 1). The study area included some of the open areas (areas without protection) in the eastern and western portions of the SNP, a section of the SNP (total protection) and the Loliondo Game Controlled Area (LGCA) in Loliondo District (partial protection). The ecosystem is part of the high interior plateau of East Africa and slopes from the highest point (1850 m) on the eastern plains towards the Gulf of Speke (920 m) in Lake Victoria (Sinclair, 1995). The climate of the area is typically semi-arid, with a mean maximum temperature of 28°C. The average minimum temperatures vary between 13°C in the wet seasons and 15°C in the dry seasons. The rainfall follows a bimodal pattern, in which "short rains" fall in November to December and "long rains" fall in March through May. The average annual rainfall increases from the south-east (500 mm) to the west and northern section of the ecosystem (1,200 mm). The soil in the northern section of the ecosystem is mainly composed of sandy and loamy soils from granite rocks (Northon-Griffiths, Helocker, & Pennycuick., 1975; Sharam, Sinclaire, Turkington, & Jacob, 2009; Sinclair, 1995).
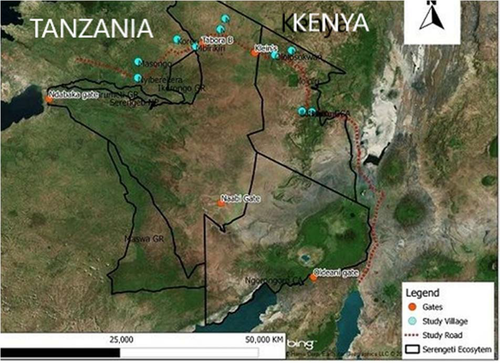
The vegetation characterising the study area varies from patches of grass plains in the south, woodlands in the centre and wooded grasslands in the north. The western corridor is dominated by savannah woodlands (Belsky, 1985). The present study was conducted along the main gravel road (171.9 km) in the northern part of the Serengeti ecosystem. The road is a mixture of unpaved gravel and dirt lane sections that mainly traverse through rural settings and a number of villages, and a portion of it (54.9 km) passes through the SNP and LGCA (Figure 1). The section within the SNP becomes impassable during the rainy seasons due to the loss of shape and presence of longitudinal gullies (TANROADS, 2010). However, road segments outside the SNP are in better condition due to periodic maintenance by local authorities, and the road is occasionally used to carry people and goods between villages. Normal economic activities (such as livestock husbandry and farming) are carried out via the use of the road outside the national park, but there are restrictions within the park, and only authorised vehicles, particularly patrol and research vehicles, are allowed on the main gravel road.
For the purpose of this study, the road was divided into five segments: two in the eastern part of the ecosystem (segments I and II), two in the western part (segments IV and V) and one (segment III) within the SNP. Within each segment, one study site was established adjacent to the main road (experimental site), while another was established approximately 5 km away and perpendicular to the main road (control site), to create a total of 10 study sites. The selected experimental sites were named Maaloni, Ololosokwan, Tabora B, Mbirikiri and Nyiberekera, while the control sites were Loosoito, Endulele, Tembo, Koreri and Wageti. The global positioning system (GPS) coordinates of each site were recorded using a GPS receiver (GPS 12XL, Garmin). The study sites were mainly composed of four types of habitats: grassland, woodland, shrubland and cultivated or fallow land. Highly dense grasses were widely scattered at the study sites, and the most dominant trees and shrubs were Terminalia, Commiphora and Acacia, with Acacia seyal and Acacia robusta being frequently encountered within the SNP. Datura stramonium, Lantana camara and Opuntia spp. were the most common alien species observed along this road. With the exception of segment V, where a permanent river flows, the riparian habitats in the remaining study sites included seasonal rivers and streams. Considering that the effects of roads are normally confounded with those of other site variables, such as slope and vegetation type (Brehme, Tracey, McClenaghan, & Fisher, 2013; Rotholiz & Mandelik, 2013), efforts were made to account for these variables by setting traps in similar habitat types, with shrubland habitat selected for all sites (Simonetti, 1989).
2.2 Animal sampling
Two intensive small mammal trapping sessions were conducted in November and December during the short rainy seasons of 2011 and 2012. Because of the broad range of body sizes among mammal species and their differential responses to traps, the use of traps of different sizes and types was required to sample the widest variety of species occurring at the study sites (Bissonette & Rosa, 2009; Torre, Guixé, & Sort, 2010). Therefore, small mammals were sampled using a combination of four live traps: Sherman (80 mm × 90 mm × 230 mm), Tomahawk (230 mm × 230 mm × 790 mm), wire mesh (200 mm length, 100 mm diameter) and pitfall trap (200 ml). The wire mesh traps were oval-shaped, locally made traps consisting of a frame of interweaved steel wires with a small opening that prevents an animal from escaping once captured. No animal sampling was conducted during the dry season due to limitations in terms of time in the field.
Measures of vegetation composition were evaluated within quadrats at each study site along the main gravel road. The measures included habitat type, dominant woody plants/shrubs, and grass height, thickness and colour along a scale of 1–6. For example, deep green grasses were assigned a score of six, while burned and dry/brown grasses were scored one and two, respectively. A more detailed description of the vegetation sampling techniques used in this study is provided in Røskaft et al. (2012).
2.3 Sampling protocol
Trap lines: At each study site, six trap lines spaced 10 m apart were established on the ground. Each line consisted of 10 Sherman traps, and the distance between traps was 10 m. To maximise captures, Tomahawk and wire mesh traps were strategically set along the trap lines at locations seemed likely to be visited frequently by small mammals. All traps were baited with roasted pieces of coconut and sardines (coated in peanut butter) and were monitored for six consecutive days.
Pitfall lines: One pitfall line of 11 buckets was established along the ground at each study site. The pitfall traps consisted of plastic buckets spaced five metres apart and buried in the ground with their rims flush with the ground surface. The pitfall line was then bisected by a drift fence (approximately 50 cm high) composed of a black polythene sheet and supported by strong wood pegs. The fence acted as a barrier by preventing animals from bypassing the trap. To prevent overflowing and the loss of captured animals when rainfall occurred, all pitfall buckets had holes at the bottom to allow the drainage of rainwater. The pitfall traps were also monitored for 6 consecutive days. All captured animals were identified to the species level using Kingdon field guides (Kingdon, 1974, 1997), and the samples were thereafter preserved at the Tanzania Wildlife Research Institute (TAWIRI) veterinary laboratory for virological studies.
2.4 Data processing and analysis
The collected data were analysed to compare the abundance, diversity and richness of species between the experimental and control sites along the main gravel road. Due to the small sample size, data from different sites were pooled together based on their spatial distance from the main road, and they were analysed mainly using nonparametric tests.
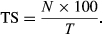
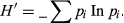
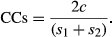
3 RESULTS
A total of 138 small mammals were trapped during 4,860 trap nights corresponding to three orders, six families and 14 species (Table 1). Rodents accounted for 84.1% of all animals captured, followed by soricomorphs (14.5%), and the least abundant group was elephant shrews (1.4%). The multimammate rat, Mastomys natalensis, was the most widely distributed species across all study sites and was captured at seven of the ten study sites.
| Species Captured | Experimental Sites | Control Sites | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAL | OLO | TBR | MBR | NYB | LOO | END | TEM | KOR | WAG | |
| Rodents | ||||||||||
| Aethomys kaiseri | X | X | — | — | — | X | — | — | X | — |
| Arvicanthis niloticus | X | — | — | — | — | X | X | — | X | — |
| Zelotomys hildegardeae | — | — | — | X | — | — | — | — | — | — |
| Dasymys sp. | — | — | — | X | — | — | — | — | X | — |
| Dendromus sp. | — | — | — | — | — | — | X | X | — | X |
| Thallomys paedulcus | — | — | — | — | — | X | — | — | — | — |
| Gerbilliscus sp. | — | — | — | — | X | X | — | — | X | X |
| Graphiurus murinus | — | — | X | — | — | X | X | X | X | — |
| Mastomys natalensis | X | — | — | X | X | X | X | X | X | — |
| Mus minutoides | — | X | — | X | X | — | X | X | X | X |
| Praomys sp. | X | — | — | — | — | — | — | — | — | — |
| Steatomy sparvus | — | — | X | — | — | — | — | — | — | — |
| Soricomorpha | ||||||||||
| Crocidura sp. | — | — | X | X | — | — | — | X | X | X |
| Macroscelidea | ||||||||||
| Elephantulus sp. | X | — | — | — | X | — | — | — | — | — |
- Abbreviations: END: Endulele; KOR: Koreri; LOO: Loosoito; MAL: Maalon; MBR: Mbirikiri; NYB: Nyiberekera; OLO: Ololosokwan; TBR: Tabora B; TEM: Tembo; WAG: Wageti and X: species present; —: species absent.
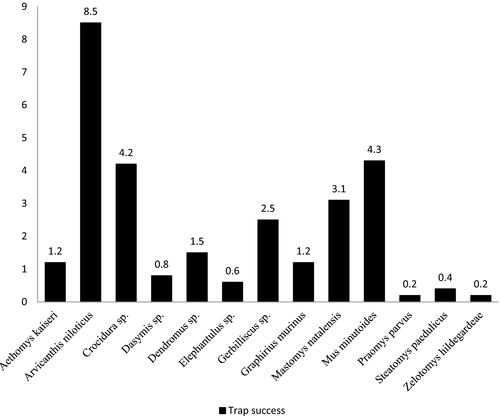
Overall, the diversity and TS of the study area were found to be 2.11 and 28.3%, respectively. Furthermore, the small mammal communities did not significantly vary between the control and experimental sites with respect to species richness (χ2 = 0.18, df = 1, p = 0.67), diversity (t = 0.85, df = 103, p = 0.39) or abundance (Mann–Whitney: U = 38.5; n1 = 10, n2 = 12, p = 0.08) (Table 2). The relative abundances of small mammal at both the experimental and control sites were mostly influenced by the African grass rat, Arvicanthis niloticus, which exhibited an overall TS of 8.5% (equivalent to 30% of the total captures), followed by the African pygmy mouse, Mus minutoides (4.5%), and the least abundant species were Thallomys paedulicus and Zelotomys hildegardeae, which were captured at only one site each (Figure 2). Although we did not calculate the capture efficiency of each trap type, shrews were exclusively captured in pitfall traps, and Tomahawk traps appeared to perform well in trapping large-bodied species, such as Gerbilliscus sp. and Aethomys kaiseri.
| Experimental sites | Control sites | Experimental versus Control sites | P value | |
|---|---|---|---|---|
| Species richness | 12 | 10 | χ2 = 0.18, df = 1 | 0.67 |
| Species diversity | 2.05 | 1.96 | t = 0.85, df = 103 | 0.39 |
| Trap success (%) | 12.76 | 15.64 | Mann–Whitney: U = 38.5, n1 = 10, n2 = 12 | 0.08 |
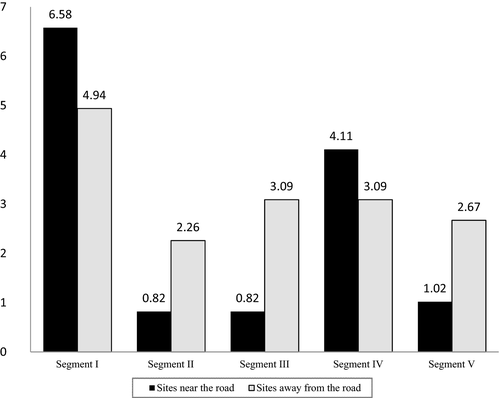
Similarly, paired experimental and control sites were compared in terms of their species richness and diversity. There were no significant differences in the species richness of experimental and control sites in any segment (Table 3). However, the difference in species diversity between the paired experimental and control sites in segments II and IV were statistically significant (Ololosokwan vs. Endulele: t = 3.28, df = 7, p = 0.02; Mbirikiri vs. Koreri: t = 5.00, df = 23, p < 0.001). Although the number of species trapped at corresponding experimental and control study sites did not significantly differ, the largest number of animals was trapped in segment I, while the least animals were trapped in segment II (Figure 3).
| Segments | Experimental versus control sites | Species richness | Species diversity | |||
|---|---|---|---|---|---|---|
| χ 2 | P | t | df | P | ||
| I | Maalon/Loosoito | 0.09 | 0.30 | 0.65 | 46 | 0.52 |
| II | Ololosokwan/Endulele | 1.29 | 0.26 | 3.28 | 7 | 0.02 |
| III | Tabora B/Tembo | 0.50 | 0.50 | 1.8 | 9 | 0.11 |
| IV | Mbirikiri/Koreri | 0.69 | 0.41 | 5.00 | 23 | 0.001 |
| V | Nyiberekera/Wageti | 0.00 | 1.00 | 0.60 | 14 | 0.56 |
Note
- for chi-square analyses, df = 1.
Considering that the Serengeti road traverses through a National Park, the characteristics of the small mammal populations within and outside the park were also compared. The data from this comparison indicated that the population of small mammals did not significantly vary in terms of species richness on either side of the park among the adjacent segments (north-eastern segment, segment II: χ2 = 0.07, df = 1, p = 0.80; north-western segment, segment IV: χ2 = 1.26, df = 1, p = 0.26), although there was a significant increase in diversity outside the park in the north-western segment (Table 4). Similarly, the species richness did not vary on either side of the park as the distance away from the park borders increased (>20 km), while the species diversity significantly varied (Table 4). Of the four most dominant small mammal species at the study sites (Arvicanthis niloticus, Mus minutoides, Crocidura sp. and Mastomys natalensis; Table 1, Figure 2), only one species, Arvicanthis niloticus, was captured inside the park, while the tiny fat mouse, Steatomys parvus, was trapped exclusively inside the park, albeit in low numbers. The total number of trapped animals also decreased over the sampling years. More than half (59%) of the small mammals were trapped in 2011, while 41% were trapped in 2012. Despite this reduction in catch rate, three new species (Dasymys sp., Zelotomys sp. and Steatomys parvus) were recorded in 2012.
| Segments | Approximate distance from the NP (km) | Number of captured animals | Species richness | Species diversity | Individual segment versus segment III | ||||
|---|---|---|---|---|---|---|---|---|---|
| Species richness | Species diversity | ||||||||
| χ 2 | P | t | df | P | |||||
| I | 70 | 34 | 11 | 1.10 | 0.47 | 0.49 | 2.22 | 73 | 0.02 |
| II | 20 | 15 | 7 | 1.43 | 0.07 | 0.80 | 3.14 | 32 | 0.002 |
| III | 0 | 18 | 8 | 1.65 | - | - | - | - | - |
| IV | 20 | 53 | 13 | 1.84 | 1.26 | 0.26 | 2.03 | 39 | 0.03 |
| V | 70 | 18 | 8 | 1.48 | 0.00 | 1.00 | 3.26 | 37 | 0.001 |
Note
- A segment comprises two corresponding study sites (an experimental and control site); for chi-square analyses, df = 1.
The Sørensen similarity index (CCs) was used to determine the similarity in the small mammal communities between the adjacent and distant sites surveyed in the current study. Overall, the CCs value was high (73%), indicating that the control and experimental sites resembled each other in terms of species composition. Similarly, a pairwise comparison indicated high species similarity between corresponding sites, with the exception of segment II (Ololosokwan vs. Endulele), where the similarity was <50%. Because sampling was conducted during the rainy periods, grassland habitats were common in the study area and scattered across almost every study site (Table 5).
| Segments |
Study sites Experimental/Control |
Habitat/vegetation | Dominant tree/shrub species |
|---|---|---|---|
| I | Maalon/Loosoito | 80% woodland, 10% grassland, 5% cultivated land and 5% shrubland |
Acacia Commiphora |
| II | Ololosokwan/Endulele | Shrubland/grassland | Acacia |
| III | Tabora B/Tembo (Serengeti National Park) | 45% shrubland, 35% woodland and 20% grassland |
Terminalia Acacia A. robusta A. senegal |
| IV | Mbirikiri/Koreri | 50% cultivated (farmland, fallow land),25% shrubland, 5% woodland and 20% grassland |
Terminalia Acacia A. seyal A. tortilis |
| V | Nyiberekera, Wageti |
50% cultivated, 25% shrubland, 5% woodland and 20% grassland |
Terminalia (90%) Acacia A. seyal |
4 DISCUSSION
The results of this study suggest that the main gravel road in the Serengeti ecosystem does not have significant effects on the species richness, diversity or abundance of small mammals in the northern Serengeti region. Such observations could be attributed to the absence of road-associated disturbances due to little road usage because of its poor condition. The road networks in the northern Serengeti region are poorly developed (TANROADS, 2010), with most transportation activities concentrated on the south-western side, where the main gravel roads are continuously maintained (Fyumagwa et al., 2013; Kija et al., 2011; Ndibalema et al., 2007). Furthermore, the northern road is much farther from the central tourist hub radiating from the Seronera region. Observations in the field indicated that although some road sections (e.g. the Natta–Mugumu section, segment V) surveyed in this study do undergo periodic maintenance, a large portion of the road remains underutilised throughout the year, especially during the rainy seasons due to floods.
Similar studies indicate that heavy-use roads tend to cause more environmental disturbances than unpaved and narrow roads, which may become impassable due to poor surfacing (Brehme et al., 2013; Laurance, Goosem, & Laurance S.G.W, 2009; McGregor et al., 2008; Orłowiski & Nowak, 2006; Oxley, Fenton, & Carmody, 1974; 2007 & Kindlmann &Sedláček., 22007). In addition, the magnitudes of such impacts tend to increase with increasing vehicular traffic density (Caro, 2015; Jaeger, 2012; Kija et al., 2011; Orłowiski & Nowak, 2006) and road width (e.g. wider vs. narrow; Rich, Dobkin, & Niles, 1994; Laurance et al., 2009) and depending on the type of road surface under study (Brehme et al., 2013; McGregor et al., 2008). For instance, Orłowski and Nowak (2006) recorded high mortality among small mammal species across agricultural fields in eastern Poland due to increased traffic density in the study area. In the current study area, however, motorised vehicles are relatively rare, and the low level of maintenance experienced by this dirt road might have discouraged its high usage by humans (who are associated with vehicles and other secondary disturbances). This low level of usage has likely contributed to reduced habitat modification and hence increased the likelihood of small mammals utilising both the control and experimental sites.
The availability of favourable habitat is of great importance to the survival of small mammals and is considered to be a limiting factor in terms of food and nesting resource accessibility and protection from predators (Ascensão et al., 2012; Bissonette & Rosa, 2009; Garland & Bradley, 1984; Mulungu et al., 2008). With the exception of macroscelids, individual species of all dominant families of small mammals surveyed in the current study were similarly represented in the control and experimental sites. This result is an indication that the presence of homogenous habitat in the area might have limited the migration of species among sites. This conclusion regarding a reduced movement pattern was also supported by the high similarity index values observed and suggests the importance of maintaining habitat connectivity in the future. Over 65% of the road segments surveyed during the study cut across the protected area network of the Serengeti ecosystem (Røskaft et al., 2012; TANROADS, 2010), which confers a certain level of habitat conservation. It is possible that this conservation status, coupled with high rainfall in the northern part of the ecosystem (Belsky, 1985; Sharam et al., 2009), promotes the continuous growth of vegetation throughout the study area. In addition, habitat data from the field revealed that over 80% of the study sites in both the natural and fallow lands were covered with grasses. A similar trend was observed by Mulungu et al. (2008) on Mount Kilimanjaro in northern Tanzania, where similarity in species composition was closely related to that in vegetation type. Only a few species of small mammals could not be detected at both distances from the road, which may have occurred due to microhabitat preferences exhibited at these sites (Bissonette & Rosa, 2009; Brehme et al., 2013; Peters, Molina-Vacas, Rodriguez, & Grilo, 2013). Small mammals use some microhabitats more often than others likely because they differ in quality (Brehme et al., 2013; Simonette, 1989), although additional trapping efforts coupled with well-designed sampling techniques could be used to draw a definite conclusion.
The relatively high TS of three species (Arvicanthis niloticus, Mus minutoides and Mastomys natalensis) at most study sites was expected. These species belong to the order Rodentia, a generalist taxon that is widely distributed in Tanzania (Kingdon, 1997; Mulungu et al., 2008; Mulungu, Makundi, Massawe, & Machang'U, 2005). Rodents have also been previously reported to be the major component of the small mammal populations in many studies conducted in the Serengeti region (Magige, 2012, 2013; Magige & Senzota, 2006; Timbuka & Kabigumila, 2006) and in other parts of East Africa (Kasangaki, Kityo, & Julian, 2003; Webala, Carugati, & Fasola, 2010). The relatively slight increase in species richness and diversity outside the SNP in north-western segments could be ascribed to the presence of cultivated lands, where cereal crops attract rodents (Magige, 2012; Roskaft et al., 2012), although additional sampling efforts are necessary to confirm this relationship.
The poor representation of some small mammal species (e.g. Zelotomys sp., Elephantulus sp.) could be explained by their low densities or because the trapping methods were not effective in capturing such species. For instance, most small mammal studies involving live trapping methods underestimate the abundance of elephant shrews (Timbuka & Kabigumila, 2006; Webala et al., 2010), although Mulungu et al. (2008) and Kihaule and Stanley (2016) obtained different results on the Kilimanjaro and Meru mountains, respectively. Alternatively, sampling was conducted only during the wet seasons, when rainfall was plentiful, which may have reduced animal movement, causing fewer species to be caught in the standard traps, for example due to the weather or availability of abundant food and nesting resources (Venance, 2009). In addition, due to time constraints in the field, it was not possible to sample small mammals during the dry season to compare the two seasons. To obtain a wider picture of the population trends at the study site, we suggest future monitoring programmes to consider sampling during both wet and dry seasons because the activities of some small mammal species tend to vary with season (Hieronimo et al., 2014; Rico, Kindlmann, & Sedláček., 2007; Webala et al., 2010). In addition to seasonal variations, the density of small mammals also tends to vary with available predators in the area because they form a prey base for many predators in the ecosystem. Therefore, their relationship with predators in the study area must be evaluated for monitoring purposes. At present, data on the predator–prey relationships of small mammals in the Serengeti plains are scarce (Senzota, 1990, 2012).
While these data provide baseline information, they highlight the importance of maintaining vegetative structures in the environment if the conservation of small mammal populations is of great concern. The data also reveal a linear relationship between the intensity of road usage and the impacts of roads on the environment. This observation is of practical importance to conservation managers when focusing on activities aimed at minimising habitat changes in the area, especially during the construction phase. Likewise, should the road be improved, other factors, such as invasive plant species, climate change, anthropogenic activities and socio-economic aspects that may affect habitat quality and animal populations in general, need to be studied and monitored, as suggested by several authors (Fyumagwa et al., 2013; Hopcraft, Bigurube, Lembeli, & Borner, 2015; Røskaft et al., 2012). For instance, there is a close relationship between an increasing human population and fire frequency, which in turn may affect habitat quality (Strauch & Eby, 2012), animal abundance (Namukonde, Kueblar, & Ganzhorn, 2017) and feeding relationships in grassland plains (Nkwabi, Sinclair, Metzger, & Mduma, 2010). The increase in tourism activities, for example the western side of the Serengeti ecosystem, has been linked to widespread alien species invasions due to the increased movement of people, vehicles and livestock in the area (Elisante et al., 2013; Kija et al., 2011). The occurrence of some invasive alien species (e.g. Datura stramonium and Opuntia sp.) was also noted along this road and in preliminary surveys by Røskaft et al. (2012), although the understanding of their distribution and magnitude of invasion would require further study.
5 CONCLUSION
In this small snapshot study, the unpaved gravel road does not seem to impact the small mammal populations. Should there be a need to convert the northern Serengeti road to tarmac road, mitigation measures that will minimise the effect on small mammals coupled with continued monitoring activities should be given high priority. One possible approach would be to minimise road-associated disturbances, such as the movement of invasive plant species into natural areas, through monitoring programmes (Underhill & Angold, 2000). Conducting preconstruction inventory studies and documenting the diversity and quality of the vegetation along this road are two such approaches.
ACKNOWLEDGEMENTS
Funding for this work was provided by the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) through the Norwegian Directorate for Nature Management. We thank R. Fyumagwa, Director of the Serengeti Wildlife Research Centre (SWRC), for logistical support and assistance with the traps. We also thank A. Nkwabi, J. Mcheto, E. Maulid and A. Mahulu for their assistance with field data collection and the late R. Senzota, who provided many helpful suggestions regarding the manuscript.
Open Research
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.




