Living in the suburbs: Space use by vervet monkeys (Chlorocebus pygerythrus) in an eco-estate, South Africa
Data Availability Statement: Data are available on request.
Abstract
enWe examined vervet monkey (Chlorocebus pygerythrus) space use using GPS/UHF telemetry data from 10 vervet monkeys across six troops over 9 months within a 420 ha suburban eco-estate. We documented a mean home range of 0.99 km2 (95% MCP) and 1.07 km2 (95% KDE) for females (n = 6), 1 km2 (95% MCP) and 1.50 km2 (95% KDE) for males (n = 4) and 0.87 km2 (95% MCP) and 1.12 km2 (95% KDE) for troops (n = 6), respectively, indicating that males and larger troops had larger home ranges. These relatively small home ranges included shared territorial boundaries and high home range overlap. Vervet monkey movements indicated higher morning activity levels, and habitat selection indicated significantly more use of golf course, urban residential and forest, thicket and woodland areas, and avoidance of wetland, grassland and shrub, and urban built-up areas. Our results suggest that modified habitat use by vervet monkeys is a consequence of behavioural facilitation to access highly available food resources, thereby facilitating their persistence in green spaces in urban areas of South Africa. Conflict management is dependent on the conservation of sufficient natural habitats and food resources, to minimise their dependence on anthropogenic supplementary food resources and consequently reduce human–monkey conflict.
Résumé
frNous avons examiné l'utilisation de l'espace chez le singe vervet (Chlorocebus pygerythrus) à`l'aide des données de télémétrie GPS/UHF de dix singes vervets répartis sur six troupes pendant neuf mois dans un parc écologique de banlieue de 420 ha. Nous avons documenté un domaine vital moyen de 0.99 km2 (95 % MCP) et 1.07 km2 (95 % KDE) pour les femelles (n = 6), 1 km2 (95 % MCP) et 1.50 km2 (95 % KDE) pour les mâles (n = 4) et 0.87 km2 (95 % MCP) et 1.12 km2 (95 % KDE) pour les troupes (n = 6), respectivement, indiquant que les mâles et les troupes plus importantes avaient un plus grand domaine vital. Ces domaines vitaux relativement petits comprenaient des limites territoriales communes et un chevauchement élevé de domaines vitaux. Les déplacements des singes Vervet indiquaient des niveaux d'activité plus élevés le matin et la sélection de l'habitat indiquait une utilisation beaucoup plus importante du terrain de golf, des zones urbaines résidentielles et forestières, des bosquets et des zones boisées, et l’évitement des zones humides, les prairies et les arbustes et les agglomérations urbaines. Nos résultats suggèrent que l'utilisation de l'habitat modifié par les singes Vervets est une conséquence de la facilitation comportementale pour accéder à des ressources alimentaires hautement disponibles, facilitant ainsi leur persistance dans les espaces verts des zones urbaines d'Afrique du Sud. La gestion des conflits dépend de la conservation d'habitats naturels et de ressources alimentaires suffisantes, afin de minimiser leur dépendance vis-à-vis des ressources alimentaires anthropiques supplémentaires et, par conséquent, de réduire les conflits entre les humains et les singes.
1 INTRODUCTION
The way animals use the space available has important bearings on their ecology, and in transformed landscapes, daily movements influence social interactions as well as human–wildlife conflicts (Inskip & Zimmerman, 2009; Fehlmann, O'Riain, Kerr-Smith, & King, 2017). The first spatial ecology study on primates took place more than eight decades ago (Carpenter, 1934) and since extensive variability in ranging patterns has been documented in primates, within and between species (Altman, 1974; Pearce, Carbone, Cowlishaw, & Isaac, 2013). Climatic variability in rainfall, temperature and day length is additional influences on ranging patterns (Higham et al., 2009; Isbell, 1983), as direct impactors on primate behaviour (Dunbar, 1993; Hill et al., 2003, 2004) and indirectly on natural resources (Bronikowski & Altmann, 1996). Primate spatial ecology is also influenced by troop size (Barton, Whiten, Strum, Byrne, & Simpson, 1992; Ganas & Robbins, 2005), intergroup competition (Isbell, Cheney, & Seyfarth, 1991; Wrangham, Gittleman, & Chapman, 1993), and social ranking and food preferences (van de Waal, van Schaik, & Whiten, 2017).
As primate troop sizes increase, so scramble and/or contest competition generally increase, forcing larger troops to cover larger areas to obtain enough food for all troop members (Wrangham et al., 1993; Chapman, Wrangham & Chapman, 1995). Thus, increase in troop size should result in increase in day range length and home range size (Chapman & Chapman, 2000). This pattern has been widely, but not consistently, found in studies of primates (Gillespie & Chapman, 2001). Troop size correlated positively with home range size and day range length in studies of geladas (Theropithecus gelada; Iwamoto & Dunbar, 1983), red colobus (Procolobus badius; Gillespie & Chapman, 2001), Thomas's langurs (Presbytis thomasi; Steenbeck & van Schaik, 2001), northern muriquis (Brachyteles arachnoides hypoxanthus, Dias & Strier, 2003—home range size only) and mountain gorillas (Gorilla gorilla beringei; Watts, 1991, 1998; Ganas & Robbins, 2005). However, troop size was shown not to correlate with day range length in Pata's monkeys (Erythrocebus patas; Chism & Rowell, 1988), blue monkeys (Cercopithecus mitis; Butynski, 1990), black and white colobus (Colobus guereza; Fashing, 2001), red-tail monkeys (Cercopithecus ascanius; Struhsaker & Leland, 1988), western chimpanzees (Pan troglodytes verus; Lehmann & Boesch, 2003), kipunji (Rungwecebus kipunji; De Luca, Picton Phillipps, Machaga, & Davenport, 2009) and several Asian colobine species (Yeager & Kool, 2000). Takahashi (2018) examined the nutritional ecology of adult, female C. mitis in the Kakamega Forest, Kenya. Daily path length was not related to group size, with comparison to previous dietary studies showing study groups moving into new areas and habitats capitalised on the new food resources, strengthening their position as flexible feeders.
Typically, our knowledge of primate spatial ecology stems from studies of single troops (Strier, 2017). Studies with large sample sizes of troops (e.g. Bronikowski & Altmann, 1996; van de Waal, 2018) or complete populations (e.g. Hamilton, Buskirk & Buskirk, 1976; Iwamoto, 1978; Takasaki, 1981) are rare. However, within species, disjointed populations living under different ecological conditions may differ more from one another in their ranging patterns than they do from closely related species (Dunbar, 1993; Strier, 2017; van de Waal, 2018). The same may be true for troops within the same population that occupy habitats with differential availability, distribution and quality of resources (e.g. Bronikowski & Altmann, 1996, van de Waal, 2018). Thus, regardless of the intensity or duration of research, studies with small sample sizes are unable to assess the effects of local habitat differences, or take into account individual differences among troops (Isbell & Young, 1993). Consequently, they may inadequately represent the variation displayed within populations and species (Bronikowski & Altmann, 1996; Strier, 2017). Instead, studies of multiple troops within a population may be more meaningful (Isbell & Young, 1993). The current study represents such a study.
There is currently relatively little known about the urban spatial ecology of vervet monkeys. Several authors have noted variations and flexibility in the ranging behaviour of vervet monkey troops based on food availability; however, their studies refer only to troops in relatively natural areas (Isbell, Pruetz, & Young, 1998; McFarland, Barrett, Boner, Freeman, & Henzi, 2014; De Moor & Steffens, 1972; Pasternak et al., 2013; Pruetz & Isbell, 2000; Struhsaker, 1967; Teichroeb, 2015; Teichroeb & Smeltzer, 2018; Tournier et al., 2014). Research on primate behavioural flexibility in anthropogenic habitats has increased markedly since the 2000s; however, this only includes 17% of currently recognised species (McLennan, Spagnoletti, & Hockings, 2017). Therefore, while vervet monkeys are shown to persist in urban areas (Chapman & Fedigan, 1984; Horrocks & Baulu, 1994; McLennan et al., 2017; Patterson, Kalle, & Downs, 2016, 2017a,2017b; Shimada & Shotake, 1997; Thatcher, Downs, & Koyama, 2018, 2019; Wolfheim, 1983), the absence of urban spatial data has greatly curtailed the efficacy of vervet monkey management efforts in transformed landscapes like KZN. To date, most management decisions have been based on previous practices, public opinion and the suggestions of researchers both with and without relevant experience and knowledge of the local vervet monkey population (Simbithi Environmental Group, 2016 pers. comm.). It is thus likely that until urban vervet monkey habitat and land use patterns are incorporated into management plans, urban vervet monkey management and conservation efforts will remain largely reactionary and serve only to address short-term conflicts as they arise.
Our aim was to determine the home ranges and habitat use of adjacent urban vervet monkey troops, based on comparisons between spatial data collected. We predicted that urban areas would have higher densities of vervet monkeys (numbers in space) as food is abundant and predators are rare. We predicted that daily distances moved would be smaller in urban versus natural areas due to easier access to available food sources and that troops would exhibit preferential use of areas with higher productivity and avoid open, less productive areas. Spatially, primates reliant on widely dispersed, unpredictably available food sources will travel further than those primates who feed on evenly spaced, reliably available foods (Hoffman & O'Riain, 2012; Oates, 1987). We predicted that urban troops would show increased overlap in spatial use based on inherent constraints imposed on urban-living primates, including adapted foraging strategies in areas of limited resources (Barrett, Barrett, Henzi, & Brown, 2016). At a finer scale, we predicted that movement and home range sizes would increase in the drier, winter months, with food availability and distribution offering the best explanation for the variation seen in ranging patterns (Clutton-Brock & Harvey, 1977; McFarland et al., 2014; Riley, 2008). Temporally, during the drier times of the year, seasonal shifts in the distribution of available food sources may mean further movement during food scarce times compared with food abundant times of the year, as found in previous studies (Buzzard, 2006; Isbell & Young, 1993; McFarland et al., 2014).
2 METHODS
2.1 Study site
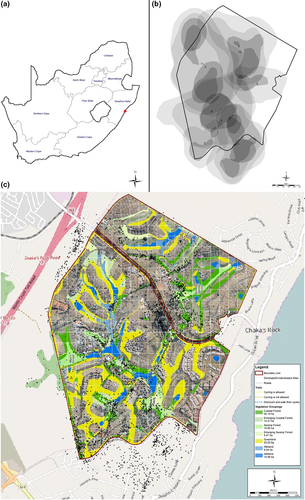
This study was conducted in suburbia in Simbithi Eco-Estate in the suburb of Ballito, north of Durban city centre (420 ha, alt. range: 30–80 m a.s.l., 31°13”11.42’ E; 29°30”48.99’S; Figure 1, Alexander, Ehlers Smith, Ehlers Smith, & Downs 2019a; Alexander, Ehlers Smith, Ehlers Smith, & Downs 2019b), KZN, South Africa, between February and November 2016. Highland sourveld grassland with Themeda triandra and patches of indigenous forest with bush clumps are the dominant natural vegetation (Mucina & Rutherford, 2006). The study area, rehabilitated from mainly sugarcane Saccharum officinarum plantations and alien vegetation, has many naturally occurring streams and wildlife species within natural habitats inside the estate, including coastal forest, swamp forest, grassland and wetland, with some sections extensively converted into residential land (Alexander et al., 2019a, 2019b; Simbithi Environmental Group, pers. comm.). The mean annual ambient temperature ranges from 18.7 to 25.1°C, and the mean monthly rainfall is 91.6 mm (D. Lilienfeld's Weather Station, Simbithi Eco-Estate, pers. comm.). Six known troops of vervet monkeys share resources within this estate and troop sizes and movements were previously monitored on an ad hoc basis from 2014 to 2016 (Simbithi Environmental Group, pers. comm.).
2.2 Trapping and monitoring procedures
Fourteen telemetry units were fitted to adult and sub-adult vervet monkeys (five sub-adult males and nine adult females) from February to June 2016. Adult females were targeted based on knowledge of the females’ influence over troop dynamics (Young, McFarland, Barrett, & Henzi, 2017), social security (Henzi et al., 2017; Josephs, Bonnell, Dostie, Barrett, & Henzi, 2016) and avoidance of conflict (Arseneau-Robar, Taucher, Schnider, van Schaik, & Willems, 2017). Sub-adult males were chosen as they were more likely to remain with or close to the troop than sexually mature males who migrate into and out of troops with the accessibility of adult females (Henzi & Lucas, 1980).
A remote-controlled steel cage trap baited with raw nuts and bananas was used to trap vervet monkeys along their known travelling routes within the estate (Grobler & Turner, 2010). With the assistance of an experienced veterinarian, captured monkeys were anaesthetised using 0.10 mg/kg ketamine injected intramuscularly, and WW1500AS-TERRESTRIAL GPS/UHF tracking collars (www.wireless-wildlife.org) were fitted. Morphological measurements, faecal, blood and hair samples, and photographs for identification were taken. The approximate ages of individuals were determined by morphological characteristics, including weight, sexual development and an assessment of teeth size and wear. No drugs were required for recovery, and anaesthetised individuals were closely monitored following release. All capture efforts undertaken followed the procedures outlined by the ethical clearance from the University of KwaZulu-Natal Animal Research Ethics Committee (Downs 020/15/animal), adhered to the legal requirements of South Africa and adhered to the American Society of Primatologists' Principles for the Ethical Treatment of Primates.
Collars were 60 g and weighed <2% of the body mass of individuals. A duty cycle of 1 location (accuracy 5–30 m) per 4 h, from 06:00 to 18:00 daily, was employed, resulting in four fixes per day. This duty cycle predicted a lifespan of 1,356 locations (339 days). The 4 hourly duty cycle was chosen to minimise serial auto-correlation, allowing the use of minimum convex polygon (MCP) and fixed kernel density estimation (KDE) methods (Worton, 1989). Data were downloaded from the collars by a solar power-supported UHF receiver base station positioned at a selected vantage point within the estate and a car-mounted UHF base station moved to vantage points within core areas when signal communication was lost from our static base station. Audio signals verified a successful download on the base stations and were validated on the website server (www.wireless-wildlife.co.za) 6–24 hr later. Data downloads from telemetry units occurred every 4 weeks and more frequently towards the end of the unit's battery life. These data were stored on an online server. The complete dataset of telemetry locations was verified by a technical supplier and then obtained from the server in February 2017.
2.3 Home range analyses
Downloaded data were provided with location (WGS 1984), date, time and velocity. Our data were first assembled onto a time series, and NULL locations were counted. We estimated the home range size of individuals and troops using both MCP and KDE methods in the adehabitatHR (Calenge, 2006) package so that methods were comparable with previous studies. Generally, KDE estimates provide the best estimate of home range with the advantage of being able to provide estimates when there are limited data points (Wartmann, Purves, & van Schaik, 2010; Worton, 1989). We calculated home range estimates using 100% MCP, 95% MCP and 95% KDE, and core areas were defined by the 50% KDE isopleth (Campioni et al., 2013; Seaman & Powell, 1996). We followed an ad hoc bandwidth selection procedure which allowed for the reference bandwidth to be reduced until the smallest home range with a contiguous polygon was determined. Consequently, it avoided over-smoothing and unnecessary fragmentation of home ranges (Ramesh, Kalle, & Downs, 2016a; Ramesh, Kalle, & Downs, 2016b).
Further, we investigated the relationship between habitat variables and use from the telemetry data of the ten units. Eight of the units covered four troops with data from a collared sub-adult male and adult female, and two troops with data from a collared adult female. For each troop, individual telemetry data were used as surrogates for the movement of the whole troop. The habitat area available to each troop was determined by the area used within the MCP of each collared individual and subsequently subdivided into 100 × 100 m sub-units (grids) within an area coverage of 10.32 km2, representing a range of 40–106 sampling units for individual coverage and 52–135 sampling units for troop coverage. We superimposed 100 100 m grids over the eco-estate polygon area using GIS software. Earlier studies (Isbell et al., 1998) recorded 82.6 m as the distance travelled by large vervet groups in 30 min; hence, we decided to set our grid cell size to 100 × 100 m as it will allow for the independence of site use every 4 hr. Hence, this grid cell size is the adequate scale for measuring habitat use as the number of GPS fixes in relation to the available habitat in the grid. A total of 625 grid cells were included inside the polygon area of the eco-estate. Grid cells allow for accurate calculation of the proportion of habitat and number of GPS fixes per grid cell as habitat use of vervet troops. We characterised habitat use by calculating the number of GPS locations within a sample grid unit, resulting in vervet use densities. A total of 415 sampling sub-units, encompassing movement of all individuals, resulted from this procedure. We considered independence of neighbouring sampling units because the study landscape was highly mosaic in nature due to a focus on habitat management of plants and wildlife within the eco-estate. Prior to this extraction, we made use of the 2014 land cover map for KZN (Ezemvelo KZN Wildlife, 2014) which classified the study area into six land use classes, including golf course, grassland and shrub, forest, thicket and woodland, urban, wetland and cultivation. In each sampling unit, we calculated the available area of land use from the classified 2014 land cover map for KZN (Ezemvelo KZN Wildlife, 2014). We assessed the habitat use based on the number of GPS fixes within each land use class. Statistical analyses were performed in the open-source software R, version 3.0 (R Development Core Team, 2014).
2.4 Habitat selection analyses
We used a generalised linear mixed model (GLMM; Breslow & Clayton, 1993) to investigate the relationship between predictors and habitat use. All the land use classes were chosen as fixed effects, and troop names were included as random effects. The number of fixes per troop was used as a proxy for habitat use. Models were run assuming a Poisson distribution. We ran all possible combinations of the independent variables as predictors of habitat use. Based on the Akaike's information criterion (AIC) and Akaike weights (wi), the best-fit models explaining troop habitat use were those with ΔAIC ≤ 2. The relative importance of each predictor was calculated using the relative ΔAIC weight of predictors, which varied from 0 (no support) to 1 (complete support) relative to the overall models (Burnham & Anderson, 2002). Statistical analyses were performed in the software R, version 3.0 (R Development Core Team, 2014). We conducted all statistical analyses using packages lme4 (Bates, Maechler, Bolker, & Walker, 2015), MASS (Venables & Ripley, 2002), effects (Fox, 2003), rJava (Urbanek, 2010), glmulti (Calcagno & de Mazancourt, 2010) and MuMIn (Bartoń, 2013).
3 RESULTS
3.1 Body mass, telemetry deployment and data acquisition
The mean body mass of collared vervet monkeys was 4.6 ± 0.3 kg (n = 14). Adult males had a mean body mass of 5.2 kg (±1.16 SD, N = 4) and females 3.6 kg (±0.63 SD, N = 6). Age of study animals ranged from approximately 2–7 years. For our study, we obtained a maximum of 46–214 days of data from each telemetry unit used for the analysis, which yielded 79–607 GPS fixes. We did not use one unit's data as it came off after 26 days, yielding only 66 locations. After filtering the data, a total of 3,588 GPS fixes were obtained (unit range: 79–606 GPS fixes) with a sampling duration range of 40–248 days (Table 1).
| Individual ID | Sex | Start date | End date | No. of days | No. of GPS fixes | Body mass (kg) |
|---|---|---|---|---|---|---|
| V1 | F | 15/02/2016 | 03/09/2016 | 199 | 495 | 2.9 |
| V2 | M | 01/03/2016 | 13/08/2016 | 166 | 387 | 4.2 |
| V3 | F | 15/02/2016 | 02/06/2016 | 108 | 311 | 3.0 |
| V4 | M | 15/02/2016 | 27/07/2016 | 164 | 407 | 6.4 |
| V5 | M | 15/02/2016 | 23/07/2016 | 160 | 255 | 6.0 |
| V6 | F | 15/02/2016 | 30/06/2016 | 137 | 345 | 3.5 |
| V7 | M | 29/02/2016 | 02/10/2016 | 217 | 606 | 4.2 |
| V8 | F | 23/05/2016 | 13/08/2016 | 82 | 243 | 3.5 |
| V9 | F | 26/05/2016 | 07/11/2016 | 248 | 460 | 4.4 |
| V10 | F | 06/06/2016 | 16/07/2016 | 40 | 79 | 4.3 |
3.2 Population structure
We obtained repeated, reliable counts from five vervet monkey troops, with a mean troop size of 31.6 (±8.84 SD) (Table 2). All troops had access to permanent water sources; however, two troops had relatively more access to non-natural foods than others (Thatcher, Downs, & Koyama, 2019). These two troops had significantly larger troop sizes than troops more reliant on natural food sources.
| Individual | Sex | 100% MCP (km2) | 95% MCP (km2) | 95% KDE (km2) | 50% KDE (km2) | Troop | Troop size | 100% MCP (km2) | 95% MCP (km2) | 95% KDE (km2) | 50% KDE (km2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | F | 2.48 | 1.36 | 1.82 | 0.32 | HE | 40 | 3.57 | 1.48 | 1.79 | 0.32 |
| V2 | M | 3.27 | 1.21 | 1.67 | 0.33 | ||||||
| V3 | F | 1.46 | 1.26 | 1.78 | 0.49 | SA | 30 | 1.5 | 1.27 | 1.73 | 0.45 |
| V4 | M | 1.21 | 1.11 | 1.82 | 0.44 | ||||||
| V5 | M | 1.58 | 0.89 | 1.4 | 0.37 | IW | NR | 1.7 | 0.92 | 1.06 | 0.23 |
| V6 | F | 0.89 | 0.73 | 0.82 | 0.17 | ||||||
| V7 | M | 1.1 | 0.82 | 1.09 | 0.33 | GO | 39 | 1.1 | 0.82 | 1.06 | 0.32 |
| V8 | F | 0.55 | 0.5 | 0.96 | 0.25 | ||||||
| V9 | F | 0.93 | 0.37 | 0.49 | 0.12 | BG | 18 | 0.93 | 0.37 | 0.49 | 0.12 |
| V10 | F | 1.36 | 0.38 | 0.57 | 0.12 | FY | 31 | 1.36 | 0.38 | 0.57 | 0.12 |
- NR: not recorded.
3.3 Home range and daily distance moved
The 100% MCP estimates ranged from 55 to 327 ha for individuals and from 93 to 357 ha for troops (Table 2). As expected, the MCP estimates were greater than the 95% KDE estimates (Table 2). Regardless of estimation methods, home range sizes varied markedly, with one male exhibiting a home range more than double the size of the overall mean. Mean home range sizes of 95% MCP and 95% KDE were 99 and 107 ha for females (n = 6), 100 and 150 ha for males (n = 4) and 87 and 112 ha for troops (n = 6), respectively, indicating that males and larger troops had generally larger home ranges (Table 2).
Mean 50% KDE core areas of female vervet monkeys were smaller than the males and smaller troops (females 25 ha; males 40 ha; Table 2), and the 50% KDE core areas of smaller troops were smaller than the larger troops (smallest BG: n = 18, 50% KDE core area: 12 ha; largest HE: n = 40, 50% KDE core area: 32 ha). Troop movements (distance (m) and step length (m)) per 4 hr decreased during the afternoon compared with the morning (Table 3). During the morning, the maximum distances moved ranged from 487.3 to 2145.9 m and mean step lengths ranged from 286.7 to 416 m, while in the afternoon, the maximum distances movement ranged from 301.8 to 1318.8 m and mean step lengths ranged from 144.5 to 325.2 m (Table 3).
| Troop | Time | Min (m) | Max (m) | Mean (m) | Sum (m) |
|---|---|---|---|---|---|
| HE | 06h00–10h00 | 10.0 | 2145.9 | 371.1 | 34138.6 |
| 14h00–18h00 | 5.2 | 301.8 | 128.2 | 1667.1 | |
| SA | 06h00–10h00 | 36.4 | 846.6 | 375.4 | 18019.4 |
| 14h00–18h00 | 27.6 | 1318.8 | 325.2 | 25042.1 | |
| IW | 06h00–10h00 | 105.7 | 691.1 | 374.7 | 20610.2 |
| 14h00–18h00 | 10.5 | 745.9 | 282.8 | 34789.9 | |
| GO | 06h00–10h00 | 55.5 | 842.0 | 416.0 | 50341.7 |
| 14h00–18h00 | 13.2 | 628.6 | 251.6 | 5283.5 | |
| BG | 06h00–10h00 | 51.9 | 487.3 | 286.7 | 15484.4 |
| 14h00–18h00 | 63.7 | 348.6 | 176.2 | 3172.4 | |
| FY | 06h00–10h00 | 18.9 | 668.3 | 320.8 | 21491.7 |
| 14h00–18h00 | 36.9 | 319.2 | 144.5 | 1589.8 |
3.4 Habitat selection
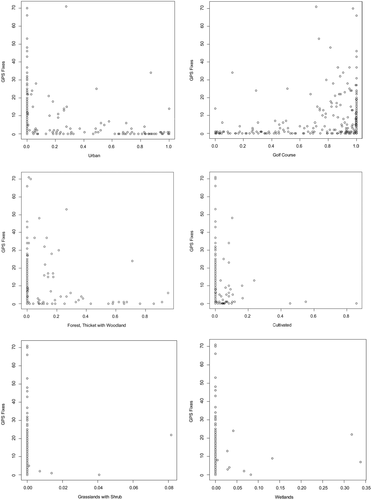
When we modelled the space use of vervet monkeys with the habitat variables, the top models (ΔAIC ≤ 2) identified included urban, golf course and forest, thicket with woodlands, cultivated lands, grassland with shrubs and wetlands, which were substantially associated with vervet monkey resource use as important predictors and provided better fit to the model (Table 4; Figure 2). Among these six variables, vervet monkey use increased with area availability of the urban and golf course habitat types and was influenced by the forest, thicket with woodland habitat type, while vervet monkey use decreased with area availability of the cultivated, grassland with shrub and wetland habitat types. Vervet monkey space use indicated that vervet monkey use was mostly dependent on urban, golf course and forest, thicket with woodland. Overall, our model showed that urban and golf course had high relative importance (Figure 2).
| Selected models | df | logLik | AIC | deltaAIC | Weight |
|---|---|---|---|---|---|
| Urban + golf course + forest, thicket with woodlands | 4 | −270.90 | 5415.8 | 0.00 | 0.35 |
| Urban + golf course + forest, thicket with woodlands + cultivated lands | 5 | −270.85 | 5415.9 | 0.04 | 0.34 |
| Urban + golf course + cultivated lands + grassland with shrubs + wetlands | 6 | −270.91 | 5416.0 | 0.20 | 0.31 |
| Urban + golf course | 3 | −272.90 | 5439.9 | 24.05 | 0 |
| Urban + forest, thicket with woodlands + cultivated lands + grassland with shrubs + wetlands | 6 | −271.70 | 5441.6 | 25.78 | 0 |
| Golf course | 2 | −272.17 | 5446.4 | 30.54 | 0 |
| Urban + forest, thicket, woodlands + grassland with shrubs | 4 | −273.40 | 5462.9 | 47.09 | 0 |
| Urban + forest, thicket with woodlands | 3 | −273.58 | 5469.2 | 53.40 | 0 |
| Urban + grassland with shrubs | 3 | −273.70 | 5469.4 | 53.55 | 0 |
| Urban | 2 | −274.18 | 5474.4 | 58.57 | 0 |
| Cultivated lands | 2 | −282.30 | 5642.5 | 226.72 | 0 |
| Wetlands | 2 | −282.50 | 5643.0 | 227.20 | 0 |
| Grassland with shrubs | 2 | −282.51 | 5645.1 | 229.24 | 0 |
| Forest, thicket with woodlands | 2 | −282.20 | 5650.4 | 234.60 | 0 |
3.5 Seasonal movement characteristics
All vervet monkeys collared within the Simbithi Eco-Estate urban mosaic stayed in the area for the entire study period, except for V7 and V8 (Goodies Troop), who moved between the estate and urban surrounds. Mean monthly distance moved by individuals differed significantly (range: 160.6–585.3 m; Table S1). The overall mean monthly distance was greatest in the month of May (463.6 ± 49.1 m; Table S1). Individuals covered slightly longer distances in the months of May to and shorter distances during the months of March and April (Table S1). For instance, V7 covered a mean monthly distance of 258.4 m in the month of March and 385.2 m in the month of July (Table S1). Overall, individuals covered longer distances in autumn (mean = 338.8 ± 29.7 m) and shorter distances in spring (mean = 322.2 ± 26.1 m) (Table S1).
4 DISCUSSION
4.1 Body mass and condition
Turner et al.’s (2018) study on the morphological variation within the Chlorocebus genus presented body mass sample sizes from Ethiopia, Kenya, South Africa, and St. Kitts and Nevis Islands. Their mean adult weight for free-living South African males was 5.69 kg (0.73 SD) and for females 4.09 kg (0.66 SD). Over all the current study male body mass ranged from 4.02 to 5.69 kg and female body mass from 2.41 to 4.09 kg. In our study, mean body mass of vervet monkey's trapped compared with Turner et al.’s (2018) South African means for males and females, had lighter body masses for both sexes, but with both sexes means falling within the body mass ranges. This indicated a similarity in body mass between general wild and urban individuals from the current study, unlike other studies where urban-living monkeys introduced to non-natural foods have generally shown marked increases in body mass (up to 50%) (Altmann, Schoeller, Altmann, Muruthi, & Sapolsky, 1993). This has sometimes resulted in serious negative impacts on body condition, including lower activity levels, high cholesterol, obesity, diabetes, malnourishment and reduced lifespans (Aggimarangsee, 1992;Saj, Sicotte, & Paterson, 1999; Kemnitz et al., 2002; Chatpiyaphat & Bonratana, 2013; van Velden, 2013). Lack of marked variation in body mass between wild individuals and this study's individuals is indicative of the fact that, while nonhuman food sources were accessible, the eco-estate still provided these troops with the necessary resources for relatively healthy living.
4.2 Home range and territory structure
To our knowledge, we present the first telemetry results of home range and habitat use of vervet monkeys in an urban environment in South Africa. In order to understand the habitat use by each of the collared vervet monkeys, we first determined the total area used by each individual using the 100% MCP. Although home range sizes can be overestimated by including infrequently used areas (Burgman & Fox, 2003), the MCP method is the simplest home range estimation technique that gives an approximation of the total area used by an animal while making no assumptions regarding the statistical independence of radio-fixes (De Solla, Bonduriansky, & Brooks, 1999). Our results showed that the total area covered by each individual and troop varied seasonally (from 0.55 to 3.27 km2) with troops travelling significantly longer distances in colder, drier months compared with warmer months, as per previous studies (Barrett, Brown, Barrett, & Henzi, 2010; Dasilva, 1992; McFarland et al., 2014; Nakagawa, 2000).
Typically, we found that larger troops had larger home ranges than smaller ones (Table 3). In general, larger groups demand larger movement to obtain food resources (Clutton-Brock & Harvey, 1977; Borries, Larney, Lu, Ossi & Koenig, 2008; Pasternak et al., 2013). However, Takahashi's (2018) study showed group size to be unrelated to movement, with the avoidance of within-group scramble competition over food being a more important impact on daily foraging movement. Additionally, troop movements correlated with adjustments in diet in response to seasonal food availability within the environment, with regular use of non-natural, high-carbohydrate foods found in human-modified habitats. Troops with ready access to these foods derived up to one-third of their daily calories from non-natural foods, without having to move far to reach their daily consumption needs. Smaller troops in our study had smaller home ranges, reflecting highly resourceful areas with adequate food resources and suitable sleeping sites as in some other studies (Teichroeb & Aguado, 2016). The smaller home ranges of vervet monkeys in developed areas are likely related to the higher density of food resources within smaller areas compared with wild home ranges (ca. 1.76 km2) in the reserve areas in South Africa (Pasternak et al., 2013). As shown in Isbell's , Cheney, and Seyfarth (1990) study, vervet monkeys shifted into the home ranges of neighbouring groups during periods of low abundance of fever trees Vachellia xanthophloea. Similarly, our study shows support for the persistence of vervet monkeys in urban landscapes being dependent on their ability to use a variety of habitat matrix; therefore, less suitable habitats may be used when necessary (Barrett et al., 2016; Isbell et al., 1990).
4.3 Habitat selection
Our results showed preferential habitat use of the golf course and urban areas within the eco-estate, and these two land classes appear to be key predictors for vervet monkey resource use in this urban-green space mosaic. Horrocks and Baulu's (1994) study also showed preferences for modified habitats and non-natural foods. Similarly, Takahashi's (2018) study showed clear preferential use by two troops for human-modified habitat and the non-natural food resources it provided. One study troop's daily intake of natural foods outweighed that of the other troops, with habitat differences between the home ranges playing an important factor. Those troops exposed to more availability of human-modified areas took advantage of this.
In our study, the relatively high use of the golf course by vervet monkeys indicated that open areas provide suitable protection and foraging opportunities. Therefore, the positive influences of open areas in the space use of vervet monkeys may bridge the gap between sustainable indigenous vegetation management practices and the ecological requirements of generalist feeders. The intensive use of modified habitats by generalist primates has been observed in many studies in agricultural and developed landscapes (Fehlmann et al., 2017; Hoffman & O'Riain, 2012). This has a major influence on wide-ranging species because of their high energy requirements when resources are distributed patchily as a result of habitat fragmentation (Lindstedt, Miller, & Buskirk, 1986). Species like vervet monkeys are likely to use developed areas leading to human–monkey conflict, particularly when the main land use includes housing and entertainment with supplementary anthropogenic food opportunities (Patterson, Kalle, & Downs, 2017b). Overall, the eco-estate and its sports and leisure developments influenced the habitat use of vervet monkeys within the home ranges. Vervet monkeys spent more time in modified habitats than the other habitats, thus allowing them to exploit the easily available resources. The highly fragmented patches of forest, thicket and woodland had less of an influence over vervet monkey use.
4.4 Seasonal movement characteristics
Seasonal variability has been shown to play a pivotal role in the behavioural flexibility of primates to respond to environmental change (McFarland et al., 2014). Thus, examining differences in the time spent resting and foraging by the study troops can help us understand the seasonal behavioural differences and similarities between wild and urban troops. Mean monthly distance moved by our study troops’ individuals differed significantly with the overall mean monthly distances greatest in the month of May. Individuals covered longer distances in the months of May to July (autumn–winter) and shorter distances during the months of March and April (summer–autumn). These results showed higher temperatures associated with an increase in time spent resting and colder temperatures associated with an increase in time spent foraging. Similar results were found in McFarland et al.’s (2014) study of wild troops, where data indicated that climate had a direct effect on animal activity. In both studies, urban and wild troops were shown to be behaviourally flexible enough to tolerate current environmental variability. However, they simultaneously predict that the time individuals have available for critical behaviours to their survival will be limited by temperature variability in the future.
5 CONCLUSIONS AND RECOMMENDATIONS
Our study demonstrated the habitat use of urban vervet monkeys in modified habitats in terms of habitat area requirements in highly fragmented landscapes containing a patchy distribution of natural habitat. This shows that eco-estates provide alternative habitats for vervet monkeys. Since the major portions of previous agricultural land have been replaced with living spaces on the north coast of KZN, the management of these conflict-prone generalists is dependent on the conservation of sufficient natural habitats to decrease its dependence on anthropogenic food resources for long-term persistence of the species. Therefore, there are several factors to be considered in land use planning in developed mosaics of KZN. The viable long-term management options could preserve sufficient natural habitats such as forest, thicket, woodland, grassland and shrub areas to enhance the natural resource availability through ecological restoration. Otherwise, these species are attracted to easy food resources (anthropogenic) in human residential areas leading to retaliatory killing of the species and thus may have an impact on the ecosystem balance, particularly on small mammals.
In South Africa, vervet monkeys are often persecuted by farmers and homeowners (Wimberger & Downs, 2010; Wimberger, Downs, & Boyes, 2010); however, vervet monkeys could be important ecosystem engineers, which may prove beneficial to conservation concerns (Foord, Aarde, & Ferreira, 1994). During our study, some of the noncollared vervet monkeys were lost to intergroup fighting and vehicle collisions (Patterson, unpublished data). Hence, vervet monkey management must be prioritised within the urbanised landscape by considering behavioural changes of small mammals as structural changes in the habitat will affect foraging and movement behaviour of species in this landscape. Studies on these human–monkey conflicts are highly valuable in urban landscapes and we suggest that future studies focus on population and fecundity rates of vervet monkeys in the urban landscape with varying degrees of vegetation management/reintroduction under changing land use scenarios to help in the mitigation of human–monkey conflicts.
ACKNOWLEDGEMENTS
We thank the University of KwaZulu-Natal (UKZN) for providing financial support to the first author under the Postgraduate Research Programme. In addition, we thank the National Research Foundation (ZA) for funding. We would like to thank P. Coulon, M. Riley and the Environmental Management Department of Simbithi Eco-Estate for research permission. D. Lillienfeld (Veterinarian) is thanked for enabling capturing and collaring of vervet monkeys. Graduate students who helped in the field are highly appreciated. A special thanks to B. Gijsbertsen, UKZN, for ArcGIS support and P. Peiser for R-program statistical support.
Open Research
DATA AVAILABILITY
Data are available on request.




