Disturbance of the rainforest has the potential to enhance egg parasitism of lepidopteran noctuid stemborers in Kisangani, DR Congo
Abstract
enLandscape context influences population dynamics of insects and impacts biological processes within communities. It was expected that anthropogenic disturbances of the rainforest landscape in DR Congo would lead to a decreased level of noctuid stemborer egg parasitism as a consequence of a decoupling between stemborers and their naturally occurring parasitoids through dispersal. To test this hypothesis, noctuid egg batches were collected in maize fields along an anthropogenic disturbance gradient to assess change in the rates of eggs parasitism and maize plant infestation with noctuid egg batches. Our results showed that, in contrast to what was initially expected, egg parasitism increased from less to highly disturbed landscape whereas maize infestation had an inverse tendency. Discovery efficiency and mean egg parasitism were 1.416 and 1.392 times higher, respectively, in the most than in the less disturbed landscape. The numbers of eggs and egg batches per 100 maize plants were 0.55 times and 0.532 times the value in the less disturbed landscape, suggesting a dilution of the stemborer population within a large habitat patch encompassing cultivated fields and the surrounding wild host plants. It was concluded that the presence of suitable host plants enhances noctuid stemborers egg parasitism in adjacent maize fields.
Résumé
frLe contexte paysager influence la dynamique des populations d'insectes et a un impact sur les processus biologiques au sein des communautés. L'on s'attendait à ce que les perturbations anthropiques du paysage de forêt pluviale en RD Congo entraînent une diminution du parasitisme des œufs de des lépidoptères foreurs de la famille des noctuidés, résultant du découplage entre ces insectes foreurs et leurs parasitoïdes dû à la dispersion. Pour tester cette hypothèse, nous avons collecté des paquets d’œufs de noctuidés dans des champs de maïs le long d'un gradient de perturbations humaines pour évaluer les changements du taux de parasitisme des œufs et l'infestation des plants de maïs par des amas d’œufs de noctuidés. Nos résultats ont montré que, contrairement à ce qui était attendu, le parasitisme des œufs augmentait des paysages moins perturbés vers les paysages très perturbés alors que l'infestation du maïs connaissait une tendance inverse. L'efficacité des découvertes et le parasitisme moyen des œufs étaient respectivement 1,416 et 1,392 fois plus élevés dans le paysage plus perturbé que dans le moins perturbé. Le nombre d’œufs et celui d'amas d’œufs par 100 plants de maïs étaient 0,55 et 0,532 fois celui dans le paysage moins perturbé, ce qui suggère une dilution des populations de foreurs dans un vaste espace d'habitats comprenant des champs cultivés et les plantes hôtes sauvages environnantes. Nous concluons que la présence de plantes hôtes propices augmente le parasitisme des œufs de noctuidés foreurs dans les champs de maïs proches.
Introduction
Landscape attributes influence population dynamics of insects and affect their biotic interactions. Landscape composition and habitat connectivity are factors explaining the difference in insect community processes (With, 2004; Heisswolf et al., 2009). For example, Heisswolf et al. (2009) found the population density of the beetle Cassida canaliculata Laich. (Coleoptera: Chrysomelidae) to be positively correlated with the density of its host plant Salvia pratensis in the landscape. Insects move between patches to meet their resource requirements such as hosts and mates (Schellhorn, Bianchi & Hsu, 2014). However, their ability to move from one patch to another is dependent on, among others, the biophysical characteristics of the surrounding matrix habitat (Dunning, Danielson & Pulliam, 1992; Taylor et al., 1993). In some instances, matrix habitat has been shown to behave as a barrier or a filter to insects dispersing through the landscape (Ricketts, 2001; Cronin, 2003). Such an effect was observed by Cronin (2003) whereby a matrix habitat consisting of mudflat was more resistant to movement of Anagrus columbi Perkins (Hymenoptera: Mymaridae), egg parasitoid of Prokelisia crocea (Van Duzee) (Hemiptera: Delphacidae), than was a matrix habitat made up of the invasive grass, Bromus inermis, or a mixture of native grass species. In agricultural landscapes, the matrix habitat was also reported to provide resources in terms of alternative hosts, high amounts of food resources (nectar and pollen), and shelter from disturbances that enhance parasitoids fitting and pest control in neighbouring agroecosystems (Thies & Tscharntke, 1999; Bianchi, Booij & Tscharntke, 2006; Zhao et al., 2013; van Rijn & Wäckers, 2015). Thies & Tscharntke (1999) reported high parasitism rates of the rape pollen beetle Meligethes aeneus (Coleoptera: Nitidulidae) along old field margins and old fallows. The authors concluded that matrix habitat type enabled parasitoid populations to build up and disperse into the cultivated field. Ultimately, the landscape context does not affect all insect species the same way (Kruess & Tscharntke, 1994; Zabel & Tscharntke, 1998; Thies, Steffan-Dewenter & Tscharntke, 2003) and this difference is likely to affect host–parasitoid interactions (Roland & Taylor, 1997; van Nouhuys, 2005). For example, comparing the abundances of the bean stinkbug Riptortus pedestris (Hemiptera: Alydidae) and its egg parasitoid Ooencyrtus nezarae (Hymenoptera: Encyrtidae) between forest edges and soya bean fields, Tabuchi et al. (2014) found the abundance of O. nezarae to be lower in soya bean fields than in the forest edges comparatively to R. pedestris. This difference was attributed to differential dispersal abilities and species response to landscape structure. Those evoked different mechanisms of landscape context effects on insect community suggest that changes of the structure and composition of an agricultural landscape coupled with insect species specific traits are likely to influence population dynamics of insect pests and affect natural biological control in agroecosystems.
In the DR Congo, the rainforest is subjected to intense anthropogenic disturbances among which logging and conversion of natural vegetation to agricultural land through shifting cultivation (de Wasseige et al., 2009; Katembera et al., 2015). These activities have led to the fragmentation of the native forest vegetation and spread of grasses (Nshimba, 2008; Bamba, 2010). In areas dominated by the native primary forest vegetation, cultivated fields are distributed in an oceanlike vegetation encompassing thick stands of high trees. This vegetation might hamper the dispersal of agricultural pests and/or their natural enemies in the landscape. Forest logging opens corridors that enhance insects’ dispersal through the landscape and their colonization of new patches (Haddad & Baum, 1999). Haddad & Baum (1999) reported enhanced high densities of three butterfly species in patches connected by corridors made up of early-successional vegetation within a pine forest in South Carolina, U.S.A. This effect was attributed among others to higher rates of interpatch movement through the corridors, the presence of essential host plants and higher immigration rates into patches.
Maize is the most important cereal crop in sub-Saharan Africa where it is a staple food for 50% of population (M'mboyi et al., 2010). In the DR Congo, maize is cultivated throughout the country where it accounts for 12.6% of consumed calories. In the former Eastern Province encompassing this study area, maize production, which was estimated at 97 765 tons in 2002, has more than doubled in 13 years reaching 243 537 metric tons in 2014. This trend suggests a rising interest towards this cereal (INS, 2015). These figures are expected to grow in the near future in tandem with the growing human population and related food needs.
Lepidopteran stemborers are among the most economically important constraint to maize production in Africa (Bosque-Pérez & Schulthess, 1998; Seshu Reddy, 1998). Feeding by larvae on maize plants results in crop losses ranging from 10 to 100% (Kfir et al., 2002). However, the importance of these losses depends upon several factors among which the efficiency of the pool of natural enemies intervening at each developmental stage of the stemborers (Setamou & Schulthess, 1995; Ndemah et al., 2003; Cugala et al., 2006).
Management of agricultural landscapes has been identified as one of the sustainable means to control pests in agroecosystems through conservation biological control (Barbosa, 1998; Landis, Wratten & Gurr, 2000). In this regard, egg parasitoids represent an important source of mortality as they kill the pest before it damages the crop (Temerak, 1981). A positive relationship has been found between the rates of egg parasitism and maize yield at harvest (Setamou & Schulthess, 1995; Ndemah et al., 2003). In contrast to what was expected up to recently, lepidopteran stemborers, especially those belonging to the family Noctuidae, are very dispersive (Dupas et al., 2014). On the other hand, numerous studies suggest that the third-trophic level insects tend to have limited dispersal abilities (Zabel & Tscharntke, 1998; Tscharntke, Rand & Bianchi, 2005) and experience a sparser landscape than their phytophagous hosts (van Nouhuys, 2005). As such, it is expected that anthropogenic disturbances of the rainforest in the DR Congo are likely to lead to a decreased potential of natural biological control as a consequence of stemborers dispersing and escaping from their natural enemies (Visser et al., 2009). Noctuid stemborers such as Busseola fusca (Fuller 1901) and Sesamia calamistis Hampson 1910 infest maize early in the season and are responsible for loss of plants (Cardwell et al., 1997). Thus, the objective of this study was to assess egg parasitism of noctuid stemborers along a gradient in anthropogenic disturbance in the rainforest of DR Congo.
Materials and methods
Description of the surveyed areas
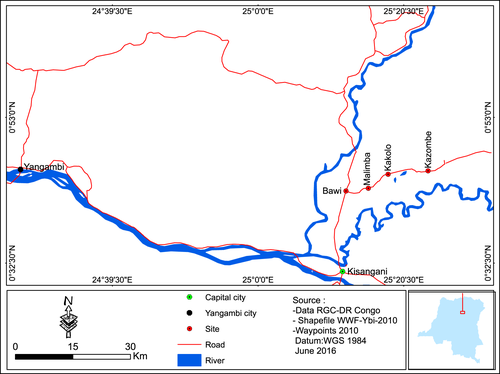
The study was carried out in the northern outskirt of Kisangani (0°31′N, 25°11′E, 376 masl) from March 2013 to December 2014. The region is situated in the moist rainforest in the Congo basin. The rainfall regime is of a bimodal type with precipitations distributed between March to June (referred to as the first cropping season) and September to December (referred to as the second cropping season). However, rains can be observed in periods normally considered as dry. The annual mean rainfall is 1875 mm. The monthly mean temperature vary around 25° C with slight amplitudes during the year (Van Wambeke & Evrard, 1954).
Four sites (more or less 4 Km2 each) representing a human disturbance gradient were chosen for this study (Fig. 1). Landscape structure and composition parameters were generated through the analysis of a 2010 satellite image using the software ArcGis 9.3 and interpreted following Mcgarigal & Marks (1994). Three land-use types were identified in the survey area, that is the native forest dominantly constituted of the mixed moist semi-evergreen Guineo-Congolian rainforest sensu White (1983), the secondary Guineo-Congolian rainforests at differing stages of regeneration (Lebrun & Gilbert, 1954) and a mixture of fields and fallows with differing abundance of grasses. A floristic inventory provided the abundance and diversity of grasses in each site following the procedure designed by Otieno et al. (2006). Wild host plants of noctuid stemborers were listed in each site based on previous studies in sub-Saharan Africa and our own data (Le Ru et al., 2006a,b; Ndemah et al., 2007; Matama-Kauma et al., 2008; Ong'amo et al., 2006, 2013, 2014; Moolman et al., 2014; Mubenga, in prep.). This classification resulted in four landscapes with characteristics as shown in Table 1.
| Site | FP (%) | FS (%) | TF (%) | FF (%) | GR | GA | GD | GE | PD (Patch/100 ha) |
|---|---|---|---|---|---|---|---|---|---|
| Bawi | 5.64 | 3.56 | 9.2 | 90.8 | 11 | 682 | 0.8401 | 0.8417 | 14.8 |
| Malimba | 72.11 | 27.03 | 99.14 | 0.86 | 12 | 564 | 0.496 | 0.4396 | 14.7 |
| Kakolo 2 | 74.03 | 23.05 | 97.08 | 2.92 | 5 | 541 | 0.3057 | 0.3184 | 13.8 |
| Kazombo | 99.8 | 0.1 | 99.9 | 0.1 | 5 | 166 | 0.4308 | 0.4968 | 0.46 |
- FP: proportion of ‘primary forest’ land-use type; FS: proportion of ‘secondary forest’ land-use type; TF: proportion of total forested area (sum FP and FS); FF: proportion of ‘field and fallows’ land-use type; GR: grasses species richness; GA: abundance of grasses (individuals); GD: diversity of grasses (Simpson index); GE: equitability of distribution of grasses in the landscape; PD: patch density index: number vegetation patches per 100 ha.
Bawi: The patch density index was 14.8 patches/100 ha. The land-use type ‘fields and fallows’ dominated the landscape covering 90.8% of the land and the total forested area covered 9.2%. Wild host plants of stemborers were represented by 11 plant species with a Simpson's diversity index of 0.8401. Their relative abundance in the landscape was estimated to 682 individuals/quadrat. Wild hosts plants of noctuid stemborers were distributed as Panicum maximum Jacq. (25%), Paspalum paniculatum L. (22%), Pennisetum purpureum Schumach. (15%), Hyparrhenia diplandra (Hack.) Stapf (10%), Pennisetum polystachyon (L.) Schult. (9%), Sorghum arundinaceum (Desv.) Stapf (4%) and Setaria megaphylla (Steud.) T. Durand & Schinz (1%). An equitability value of 0.8417 suggests a fair distribution of grasses in the landscape. Chromolaena odorata (L.) King and Robinson, an invasive weed, was also present showing an advanced degradation status of the native forest vegetation.
Malimba: The patch density index amounted to 14.7 patches per 100 ha. The ‘primary forest’ and ‘secondary forest’ land-use types occupied 72.11% and 27.03%, respectively, with a total forested area amounting to 99.14% of the landscape. The relative abundance of grasses in the landscape was estimated to 564 individuals/quadrat representing 12 species with a Simpson's diversity index of 0,496. Wild host species of stemborers found in this landscape were P. paniculatum (69%), P. maximum (11%), P. polystachyon (3%), P. purpureum (2%), S. megaphylla (1%), H. diplandra (1%) and S. arundinaceum (1%). The ‘fields and fallows’ land-use type covered 0.86%. An equitability value of 0.4396 suggests an uneven distribution of grass species on the landscape.
Kakole 2: The patch density index amounted to 13.8 patches per 100 ha. The ‘primary forest’ and ‘secondary forest’ land-use types occupied 74.03% and 23.05%, respectively, with a total forested area estimated as 97.08% of the landscape. Grasses abundance was 541 individuals/quadrat representing five species with a Simpson diversity index of 0.3057. Wild host species of stemborers were distributed as P. paniculatum (62%), P. maximum (16%), P. purpureum (2%), Setaria megaphylla (3%) and Cyperus distans (2%). The ‘fields and fallows’ land-use type covered 2.92%, suggesting a low level of agricultural activities. An equitability value of 0.3184 suggests an uneven distribution of grass species on the landscape.
Kazombo: It is the least fragmented landscape with a patch density index of 0.46 patches/100 ha of landscape. The total forested area occupies 99.9% in the site, the field and fallow land-use type representing only 0.1%. Grasses were represented by five species with a relative abundance estimated as 166 individuals/quadrat and a Simpson's diversity index of 0.4308. However, it is worth noting that the grasses diversity in this site is overestimated as grasses were mostly concentrated in the area close to the roadside. P. paniculatum (72%), P. maximum (4%), S. megaphylla (2%) and S. arundunaceum (1%) were the dominant wild hosts of noctuid stemborers found in the landscape. An equitability value of 0.4968 suggests an uneven distribution of grass species on the landscape.
The patch density index measures the level of fragmentation and heterogeneity in a landscape whereby the most fragmented landscape is the most disturbed as well, providing a range of resources for widely distributed species. The results of the landscape characterization showed that Bawi, Malimba and Kakolo 2 had similar patch density indices, suggesting a similar degree of fragmentation in all of them. Nevertheless, a proportion of ‘fields and fallows’ as higher as 90.8% associated with a higher abundance of grasses suggested that Bawi was the most disturbed site. In contrast, Kazombo was the less disturbed site with the lowest patch density index. It also had the lowest species richness of grasses. Kakolo 2 and Malimba were roughly similar with regard to the proportions of the primary forest, secondary forest, total forested area in the landscape and abundance of grasses. The two sites were only different in terms of grasses species richness and diversity that were higher in Malimba than Kakolo 2. While the fields in Bawi and Malimba belonged to smallholder farmers, fields in Kakolo 2 and Malimba were part of large concessions with limited access.
P. paniculatum and P. maximum were the most common wild host grasses found in all four landscapes. P. paniculatum was more abundant in landscapes with high total forested area, that is Kazombo, Kakolo and Malimba. In contrast, P. maximum was most abundant in Bawi where grasses dominated and its abundance decreased towards Kazombo, the less disturbed landscape. Its deep, dense and fibrous root system allows this grass to survive water stress conditions that happen in degraded landscapes such as Bawi (Aganga & Tshwenyane, 2004).
Sampling design
In Kisangani, maize is cropped almost the all year round. For ease of data analysis, the period spanning from January to June was referred to as the ‘first cropping season’ while the period spanning from July to December was referred to as the ‘second cropping season’ (Van Wambeke & Evrard, 1954). Egg batches were collected in fields where maize was the only cereal cropped and planted since less than 4 weeks. Every field visited was divided into four sections, and a 25-m2 quadrat was installed in each section. In the quadrat, lower leaf sheaths of 25 maize plants taken randomly were opened to search for egg batches. After a visual egg count, egg batches were individually transferred to glass vials (diameter x height: 15 × 70 mm) plugged with cotton wool and kept in the laboratory for 3 weeks until emergence of parasitoids, stemborer larvae or both. Emerging larvae were reared on maize cuttings in plastic tubes (diameter x length: 25 × 210 mm) and kept at laboratory temperature until pupation. Pupae were individually placed in plastic tubes and provided with small maize stem cuttings to maintain a good level of humidity and thus avoiding their desiccation until emergence of moths or parasitoids. All containers were plugged with cotton wool. Emerging moths were identified by dissection of the genitalia after immersion in boiling NaOH 10% following the procedure by Maes (1998) whereas parasitoid were kept in 70% ethanol, pre-identified at Centre de Surveillance de la Biodiversité (CSB) and finally identified at icipe, Nairobi, Kenya, and at Institut de Recherche pour le Développement, Gif-sur-Yvette Cedex, France, through gene sequencing. Voucher specimens of adult stemborers and parasitoids are kept at CSB and IRD.
The rate of maize infestation was estimated as the ratio between the number of plants bearing egg batches and the total number of plants observed in a field multiplied by 100. Two types of egg parasitism were defined: (a) mean egg parasitism per field calculated as percentage of eggs parasitized within individual batches, averaged over all egg batches found in the field which provide information about parasitoid fecundity; (b) percentage of egg batches with parasitoids per field – referred to as ‘discovery’ efficiency – which provides information about the searching ability of a parasitoid (Bin & Vinson, 1991).
Statistical analysis
Collected data were analysed using the generalized linear model under the software SPSS 20.0 (IBM Statistics, 2011). Percentages data (infestation and egg parasitism) were analysed using a binomial error distribution with a logit-link function while count data (parasitoid species abundance and number of eggs and egg batches per 100 maize plants) were analysed using a Poisson error distribution with a log link function (Mccullagh & Nelder, 1989). As egg parasitism and maize infestation were expected to depend upon abundance of wild host plants of borers (Schulthess, Chabi-Olaye & Goergen, 2001), we added this variable as a covariate. Models were fitted using the Fisher's criteria method and scaled with the Pearson's chi-square. Estimated mean differences were computed to compare infestation and parasitism parameters in Bawi, Malimba and Kakolo 2 to Kazombo. Kazombo was considered the reference site as it had the lowest disturbance level (Table 1). Least significant difference was adjusted for multiple comparisons. Estimated marginal means were estimated based on the original scale. Bivariate correlation analysis was further computed to explore relationships between landscape parameters, maize infestation and egg parasitism. Significance level was set at P ≤ 0.05.
Results
Parasitoids and stemborer species diversity
A total of 61 fields were surveyed, 19 in Bawi, 27 in Malimba, 8 in Kakolo 2 and 7 in Kazombo. In total, 685 egg batches containing 10272 eggs were collected. Three parasitoid species emerged from stemborer eggs, Trichogramma sp (Hymenoptera: Trichogrammatidae), Telenomus sp (Hymenoptera: Scelionidae) and Gonatocerus sp (Hymenoptera: Mymaridae). Trichogramma sp was the most abundant species accounting for 56.4% of the parasitoid community, followed by Telenomus sp and Gonatocerus sp representing 43.3% and 0.3%, respectively. About 59.4% or 407 of the collected egg batches yielded parasitoids. Two stemborer species, B. fusca and S. calamistis, were detected with each species constituting 35.1% and 64.9%, respectively.
Effects of the disturbance gradient on maize infestation and egg parasitism
Across sites, maize infestation with egg batches decreased along the disturbance gradient from less to highly disturbed site except in Malimba where it was higher than in the reference site Kazombo. Comparatively to Kazombo, the mean differences in infestation rates were -1.0 ± 0.1% in Kakolo 2, 5.0 ± 1.2% in Malimba and -3.0 ± 1.2% in Bawi (Table 2). The difference between Kazombo and Kakolo 2 was not significant. The probability of infestation was 1.769 times higher in Malimba (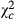 =19.771; P = 0.009), whereas it was 0.576 times lower in Bawi (
=19.771; P = 0.009), whereas it was 0.576 times lower in Bawi (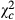 = 8.36; P = 0.004) comparatively to Kazombo. The same trend was observed in terms with the mean number of egg batches per 100 plants and the mean number of eggs per 100 plants. Hence, the mean differences in egg batches per 100 plants were −1.4 ± 1.2 in Kakolo 2, 7.3 ± 1.9 in Malimba and −6.1 ± 1.8 in Bawi with later being 0.532 times lower (
= 8.36; P = 0.004) comparatively to Kazombo. The same trend was observed in terms with the mean number of egg batches per 100 plants and the mean number of eggs per 100 plants. Hence, the mean differences in egg batches per 100 plants were −1.4 ± 1.2 in Kakolo 2, 7.3 ± 1.9 in Malimba and −6.1 ± 1.8 in Bawi with later being 0.532 times lower ( = 11.926; P = 0.001) comparatively to the reference site Kazombo while the mean number of eggs per 100 plants ranged from 178.7 ± 6.0 in Kazombo 184.8 ± 5.9 in Kakolo 2, 308.7 ± 3.7 in Malimba and 98.2 ± 2.7 in Bawi.
= 11.926; P = 0.001) comparatively to the reference site Kazombo while the mean number of eggs per 100 plants ranged from 178.7 ± 6.0 in Kazombo 184.8 ± 5.9 in Kakolo 2, 308.7 ± 3.7 in Malimba and 98.2 ± 2.7 in Bawi.
| Infestation (%) | # egg batches/100 plants | # eggs/100 plants | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cont |
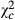
|
Pc | Coef | Cont |

|
Pc | Coef | Cont |
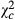
|
Pc | Coef | |
| Site | ||||||||||||
| Bawi | −3.0 ± 1.2 | 8.36 | 0.004 | 0.576 | −6.1 ± 1.8 | 11.926 | 0.001 | 0.532 | −80.4 ± 6.6 | 148.96 | <0.0001 | 0.55 |
| Malimba | 5.0 ± 1.2 | 19.771 | 0.009 | 1.769 | 7.3 ± 1.9 | 15.883 | <0.0001 | 1.563 | 130.0 ± 7.1 | 335.89 | <0.0001 | 1.728 |
| Kakolo 2 | −1.0 ± 0.1 | 0.316 | 0.574 | 0.897 | −1.4 ± 1.2 | 0.386 | 0.534 | 0.893 | 6.1 ± 8.4 | 0.534 | 0.465 | 1.034 |
| Season | ||||||||||||
| 1 | −18.0 ± 1.3 | 184.154 | <0.0001 | 0.206 | −18.7 ± 1.6 | 136.598 | <0.0001 | 0.242 | −317.5 ± 6.5 | 2363.897 | <0.0001 | 0.201 |
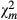
|
299.007 | 338.766 | 2363.987 | |||||||||
| df | 1 | 1 | 1 | |||||||||
| <0.0001 | <0.0001 | <0.0001 | ||||||||||
| Discovery efficiency (%) | Mean egg parasitism (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cont |
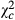
|
Pc | Coef | Cont |
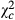
|
Pc | Coef | |
| Site | ||||||||
| Bawi | 8.0 ± 2.1 | 13.312 | <0.0001 | 1.416 | 8.0 ± 2.6 | 9.868 | 0.002 | 1.392 |
| Malimba | 0.0 ± 0.0 | 0.017 | 0.895 | 0.988 | −4.0 ± 2.5 | 3.075 | 0.079 | 0.836 |
| Kakolo 2 | −2.0 ± 2.5 | 0.99 | 0.32 | 0.901 | −8.0 ± 2.9 | 6.681 | 0.01 | 0.738 |
| Season | ||||||||
| 1 | −12.0 ± 1.9 | 38.38 | <0.0001 | 0.597 | −16.0 ± 2.1 | 63.731 | <0.0001 | 0.514 |
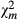
|
38.38 | 63.731 1 | ||||||
| df | 1 | 1 | ||||||
| Pm | <0.0001 | <0.0001 | ||||||
-
Cont.: difference;
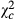 : chi-squared value for the difference; Pc: P-value for the difference estimate; Coef: estimated exponential regression coefficient;
: chi-squared value for the difference; Pc: P-value for the difference estimate; Coef: estimated exponential regression coefficient; 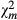 : chi-squared value for the model effects; df: degree of freedom associated with calculation of model effects; Pm: P-value of the estimation of the model effects.
: chi-squared value for the model effects; df: degree of freedom associated with calculation of model effects; Pm: P-value of the estimation of the model effects.
On the contrary to infestation rates, egg parasitism increased from Kazombo towards Bawi. Difference in discovery efficiency ranged from −2.0 ± 2.5% in Kakolo 2 to 8.0 ± 2.1% in Bawi, and there was no significant difference between Malimba and Kazombo. Only the difference between Bawi and Kazombo was significant with the probability of discovery efficiency 1.416 times higher ( = 13.312; P < 0.0001) in Bawi. Mean egg parasitism followed the same trend increasing by 1.392 times in Bawi (
= 13.312; P < 0.0001) in Bawi. Mean egg parasitism followed the same trend increasing by 1.392 times in Bawi ( = 9.868; P = 0.002). For all of the five observed parameters, estimated values were higher in the second season comparatively to the first. While infestation was five times higher (
= 9.868; P = 0.002). For all of the five observed parameters, estimated values were higher in the second season comparatively to the first. While infestation was five times higher (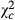 = 184.154; P < 0.0001), the increase was four times higher (
= 184.154; P < 0.0001), the increase was four times higher (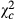 = 136.598; P < 0.0001) and five times higher (
= 136.598; P < 0.0001) and five times higher (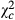 = 2363.897; P < 0.0001) for eggs and egg batches numbers, respectively.
= 2363.897; P < 0.0001) for eggs and egg batches numbers, respectively.
Effects of disturbance gradient on parasitoids abundance and proportion of egg batches parasitized per parasitoid species
Overall, parasitoids abundance showed variable trends according to the species (Table 3). Abundance of Telenomus sp decreased from Kazombo to Bawi and dropped to 0.616 times ( = 31.704; P < 0.0001) and 0.318 times (
= 31.704; P < 0.0001) and 0.318 times (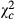 = 118.644; P < 0.0001) in Kakolo 2 and Bawi, respectively. In contrast, abundance of Trichogramma sp increased in the disturbed sites by 5.064 times in Kakolo 2 (
= 118.644; P < 0.0001) in Kakolo 2 and Bawi, respectively. In contrast, abundance of Trichogramma sp increased in the disturbed sites by 5.064 times in Kakolo 2 (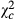 = 172.204; P < 0.0001), 10.123 times in Malimba (
= 172.204; P < 0.0001), 10.123 times in Malimba (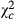 = 1151.958; P < 0.0001) and 1.823 times (
= 1151.958; P < 0.0001) and 1.823 times (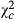 = 25.279; P < 0.0001). The proportion of egg batches parasitized by Telenomus sp followed a trend similar to that observed for the parasitoid abundance. Proportion of egg batches parasitized by Telenomus sp was 0.205 times (
= 25.279; P < 0.0001). The proportion of egg batches parasitized by Telenomus sp followed a trend similar to that observed for the parasitoid abundance. Proportion of egg batches parasitized by Telenomus sp was 0.205 times (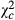 = 194.408; P < 0.0001), 0.119 times (
= 194.408; P < 0.0001), 0.119 times (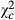 = 486.031; P < 0.0001) and 0.147 times (
= 486.031; P < 0.0001) and 0.147 times ( = 381.66; P < 0.0001) in Kakolo 2, Malimba and Bawi, respectively. The proportion of egg batches parasitized by Trichogramma sp, however, increased along the disturbance gradient and had more than doubled in Bawi (coef: 2.257;
= 381.66; P < 0.0001) in Kakolo 2, Malimba and Bawi, respectively. The proportion of egg batches parasitized by Trichogramma sp, however, increased along the disturbance gradient and had more than doubled in Bawi (coef: 2.257; 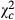 : 24.857; P < 0.0001) (Table 3).
: 24.857; P < 0.0001) (Table 3).
| Ab.Teln | Ab. Trich | Propteln (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cont |
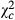
|
P c | Coef | Cont |
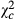
|
P c | Coef | Cont |
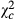
|
P c | Coef | |
| Site | ||||||||||||
| Bawi | −43.3 ± 3.9 | 118.644 | <0.0001 | 0.318 | 9.5 ± 1.9 | 25.279 | <0.0001 | 1.823 | −45.0 ± 2.3 | 381.66 | <0.0001 | 0.147 |
| Malimba | 12.9 ± 4.4 | 8.805 | 0.003 | 1.188 | 105.4 ± 3.1 | 1151.958 | <0.0001 | 10.128 | −49.0 ± 2.2 | 486.031 | <0.0001 | 0.119 |
| Kakolo 2 | −26.4 ± 4.7 | 31.704 | <0.0001 | 0.616 | 46.9 ± 3.6 | 172.204 | <0.0001 | 5.064 | −38.0 ± 2.7 | 194.408 | <0.0001 | 0.205 |

|
518.972 | 1300.881 | 499.969 | |||||||||
| df | 3 | 3 | 3 | |||||||||
| Pm | <0.0001 | <0.0001 | <0.0001 | |||||||||
| Season | ||||||||||||
| 1 | −92.6 ± 3.8 | 598.883 | <0.0001 | 0.188 | −120.6 ± 4.9 | 590.684 | <0.0001 | 0.076 | 35.0 ± 2.1 | 287.87 | <0.0001 | 4.748 |
|
598.883 | 590.684 | 287.87 | |||||||||
| df | 1 | 1 | 1 | |||||||||
| Pm | <0.0001 | <0.0001 | <0.0001 | |||||||||
| Proptrich (%) | Proptelntrich (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cont |

|
P c | Coef | Cont |

|
P c | Coef | |
| Site | ||||||||
| Bawi | 5.0 ± 1.0 | 24.857 | <0.0001 | 2.257 | 2.0 ± 0.5 | 243.989 | <0.0001 | 5.21E+12 |
| Malimba | −1.0 ± 0.7 | 1.816 | 0.178 | 0.752 | 6.0 ± 0.6 | 103.48 | <0.0001 | 1.26E+12 |
| Kakolo 2 | 3.0 ± 1.2 | 4.912 | 0.027 | 1.088 | 21.0 ± 3.2 | 44.828 | <0.0001 | 5.58E+12 |
|
51.473 | 197.324 | ||||||
| df | 3 | 3 | ||||||
| Pm | <0.0001 | <0.0001 | ||||||
| Season | ||||||||
| 1 | −4.0 ± 0.8 | 28.168 | <0.0001 | 2.332 | 0.0 ± 0.0 | 30.236 | <0.0001 | 0.104 |
|
38.38 | 30.236 | ||||||
| df | 1 | 1 | ||||||
| Pm | <0.0001 | <0.0001 | ||||||
-
Cont.: difference;
 : chi-squared value for the difference; Pc: P-value for the difference estimate; Coef: regression exponential estimated coefficient;
: chi-squared value for the difference; Pc: P-value for the difference estimate; Coef: regression exponential estimated coefficient; 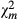 : chi-squared value for the model effects; df: degree of freedom associated with calculation of model effects; Pm: P-value of the estimation of the model effects.
: chi-squared value for the model effects; df: degree of freedom associated with calculation of model effects; Pm: P-value of the estimation of the model effects.
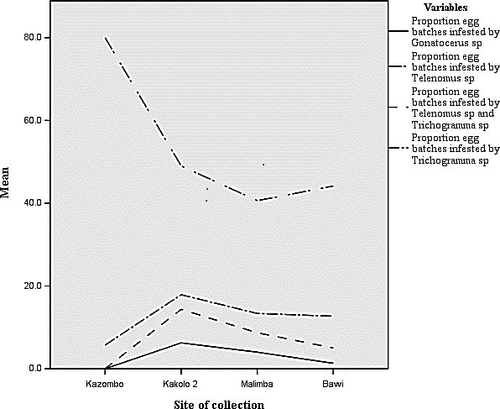
Relative importance of egg parasitoid species along the disturbance gradient
Telenomus sp was found to be the most influential parasitoid in the surveyed sites. Across sites and seasons, it parasitized more egg batches than any other parasitoid species along the disturbance gradient. It was followed by Trichogramma sp and Gonatocerus sp, respectively, the last being the least influential species also showing a marked sensibility to landscape disturbances (Fig. 2). A total of 19.4% of collected egg batches were parasitized by Telenomus sp and Trichogramma sp together. Multiparasitism was positively correlated with maize infestation (r = 0.453; P < 0.01), number of egg per 100 maize plants (r = 0.468; P < 0.01), and mostly with abundance of Telenomus sp (r = 0.604; P < 0.01) than that of Trichogramma sp (r = 0.477; P < 0.01) (Table 4).
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | (k) | (l) | (m) | (n) | (o) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) | 1 | ||||||||||||||
| (b) | 0.094** | 1 | |||||||||||||
| (c) | 0.674** | 0.651** | 1 | ||||||||||||
| (d) | 0.148 | 0.469** | 0.309* | 1 | |||||||||||
| (e) | −0.279* | −0.590** | −0.235 | −0.967** | 1 | ||||||||||
| (f) | 0.048 | −0.069 | 0.151 | −0.277* | 0.310* | 1 | |||||||||
| (g) | 0.027 | 0.057 | 0.049 | 0.100 | −0.095 | 0.186 | 1 | ||||||||
| (h) | 0.064 | 0.119 | 0.093 | 0.187 | −0.182 | 0.164 | 0.894** | 1 | |||||||
| (i) | −0.061 | −0.122 | 0.029 | −0.170 | 0.200 | 0.710** | 0.275* | 0.371** | 1 | ||||||
| (j) | 0.106 | 0.034 | 0.126 | −0.152 | 0.157 | 0.791** | 0.164 | 0.210 | 0.653** | 1 | |||||
| (k) | −0.301* | −0.273* | −0.237 | −0.027 | 0.056 | −0.082 | 0.503** | 0.354** | 0.124 | −0.144 | 1 | ||||
| (l) | 0.125 | 0.104 | 0.021 | −0.038 | 0.004 | 0.449** | 0.250 | 0.238 | 0.282* | 0.546** | −0.110 | 1 | |||
| (m) | 0.178 | 0.111 | 0.002 | −0.164 | 0.108 | 0.453** | 0.255* | 0.358** | 0.604** | 0.477** | −0.093 | 0.374** | 1 | ||
| (n) | 0.055 | −0.062 | 0.165 | −0.271* | 0.306* | 0.983** | 0.172 | 0.177 | 0.765** | 0.861** | −0.093 | 0.456** | 0.481** | 1 | |
| (o) | 0.068 | −0.042 | 0.156 | −0.251 | 0.278* | 0.949** | 0.145 | 0.170 | 0.749** | 0.913** | −0.125 | 0.475** | 0.468** | 0.985** | 1 |
- (a): number patches per 100 ha; (b): abundance grass; (c): grasses richness; (d): grasses equitability; (e): total forested area; (f): maize infestation; (g): discovery efficiency; (h): mean egg parasitism; (i): abundance Telenomus sp; (j): abundance Trichogramma sp; (k): proportion of egg batches parasitized by Telenomus sp; (l): proportion of egg batches parasitized by Telenomus sp and Trichogramma sp; (m): proportion of egg batches parasitized by Trichogramma sp; (n): number egg batches per 100 plants; (o): number eggs per 100 plants.
- *Correlation is significant at the 0.05 level (2-tailed)
- **correlation is significant at the 0.01 level (2-tailed).
Discussion
Wasps in the families Scelionidae and Trichogrammatidae are widespread in sub-Saharan Africa as egg parasitoids of stemborer (Bosque-Perez, Ubeku & Polaszek, 1994; Schulthess, Chabi-Olaye & Goergen, 2001; Ndemah et al., 2003; Chabi-Olaye et al., 2006; Bruce et al., 2009). The genus Telenomus alone is represented by ten species (Polaszek, 1997). Gonatocerus sp, however, has not been collected as an egg parasitoid of maize stemborer in this region. Our collection of Gonatocerus sp on stemborer eggs appears to be a first record in the rainforest.
Our results showed that egg parasitism (discovery efficiency and mean egg parasitism) significantly increased from less to highly disturbed sites (Table 2). This result is presumably explained by the higher richness and an even distribution of wild host plants of noctuid stemborers in the more disturbed site (Table 1) which implies a higher abundance of host eggs on which parasitoids build up their populations and spillover to cropped fields than in less disturbed sites (Tscharntke et al., 2007). Although the abundance of noctuid stemborer eggs in the wild habitats was not assessed for a formal comparison between the two habitats in our study, several other studies reported grasses such as Panicum maximum (Le Ru et al., 2006a,b; Matama-Kauma et al., 2008; Ong'amo et al., 2014; Kankonda et al., 2014) and Paspalum paniculatum (Mubenga Kankonda, in prep.) to harbour noctuid stemborers among which B. fusca, S. calamistis and Manga nubifera. In a laboratory study, Telenomus isis (Polaszek), an egg parasitoid common in Western and Central Africa, was found to accept and parasitize twelve noctuid stemborers species found in crop fields and wild habitats including S. calamistis and B. fusca (Chabi-Olaye et al., 2001). Our observations are in line with that from Alignier et al. (2014) who reported a positive correlation between the parasitism of aphids and the density of grasslands and hedge in the vicinity of spring winter fields. Likewise, in a review, Bianchi, Booij & Tscharntke (2006) reported grasslands to favour the presence of natural enemies and a reduction in aphid abundance in adjoining fields as a consequence.
Multiparasitism generally occurs as a consequence of resources limitation (Boivin & Brodeur, 2006). After oviposition, parasitoids mark the parasitized egg batches with appropriate pheromones to deter competitors (Ruther, 2013; Montovan et al., 2015). When the amount of eggs is insufficient for a large number of females, some competitors happen to overcome the deterrent pheromones and oviposit in egg batches already parasitized by other females. In our case, however, multiparasitism appears not to be related to an overcrowding effect as it is positively correlated to the quantity of eggs available in the field (Table 3). In addition, our results showed that trichogrammatid parasitoids abundance increased with the landscape disturbance while scelionids abundance decreased (Table 3). This aspect needs more investigation as multiparasitism can have an important impact on biological control potential.
Maize infestation decreased with increasing disturbance of the agricultural landscape (Table 2). Fields in the most forested sites are generally small (Ndemah, 1999) and surrounded by tall trees. Thus, when a stemborer population happens to settle in a field, female moths oviposit eggs in high density on a limited number of host plants as grasses are scarce and dispersal to nearby habitats is hampered by the surrounding vegetation. In the highly disturbed agricultural landscape, however, stemborer populations are diluted within a large habitat patch which encompasses crop fields and the surrounding wild host plants stands as there is no discrete edges in the most disturbed landscape (Tylianakis, Tscharntke & Lewis, 2007; Thies, Steffan-Dewenter & Tscharntke, 2008; Cronin, 2009; Tscharntke et al., 2012). This observation is in line with the results obtained in the forest zone of Cameroon where the abundance of grasses in the vicinity of fields was significantly negatively related to the percentage of maize plants infested with B. fusca egg batches (Ndemah et al., 2003). As such, we concluded that spread of grasses in the rainforest lead to a reduction of noctuid stemborer pressure on maize crop through a source–sink effect.
Finally, our results showed that maize infestation and egg parasitism were higher in second than during the first season (Table 2). This effect may be attributed to an increase in the density of host plants in the wild and cultivated fields. In the rainforest of Kisangani, the first cropping season is preceded by a long, nearly totally dry season lasting from December to March. The scarcity of host plants in the wild as well as the cultivated habitats during this period negatively affects stemborer populations build-up. In contrast, the second cropping season is preceded by a short, less stringent dry season during which host plants are available. Moreover, a continuous cultivation of maize from March to December favours noctuid stemborer populations build-up. Heisswolf et al. (2009) reported the population density of the beetle C. canaliculata Laich. (Coleoptera: Chrysomelidae) and the occurrence probability of its egg parasitoid Foersterella reptans Nees (Hymenoptra: Tetracampidae) to increase with increased population density of the herbivores’ host plant in the landscape. Nevertheless, our data showed that although both maize infestation and parasitism rates increased from the first towards the second cropping season, maize infestation increased fivefold whereas mean egg parasitism increased twofold and discovery efficiency only increased by 67% (Table 2). This unbalanced increase between infestation and parasitism rates suggests that noctuid stemborer population build up gradually from the first to the second seasons to possibly overcome control by their egg parasitoids.
In conclusion, our results showed that anthropogenic disturbance of the rainforest leads to the enhancement of egg parasitism of noctuid stemborers on maize. This effect proceeds from an improved habitat quality through spread of wild host plants evenly distributed in the landscape. These results raise the important question of how to reconcile sustainable maize production through enhanced natural control of stemborers and a greater conservation of the rainforest in DR Congo. Hence, the effect of factors such as the extent of cleared areas and dimensions of fields needs to be clarified as it is thought that these variables influence the level of noctuid stemborers egg parasitism. In addition, the suitability of eggs laid by nonpest stemborers such as Manga nubifera should be tested as to their role in the perennation of egg parasitoids in the landscape.
Acknowledgements
This research was financially supported by the French Institut de Recherche pour le Développement through the Noctuid Stemborer Biodiversity project. Henoc Talukadi, late Esaïe Uyikur, Steve Ngoy, Collinet Lotumbe, Claude Lomangi, Joseph Atende and Bienvenu Ndjoku helped with field collection of data. Claire Capdevielle-Dulac helped with molecular identification of egg parasitoids. We thank Sylvain Ntambo Mbuya for reading an earlier draft of this manuscript.




