Genetically predicted retinal vascular occlusion in relation to cardiovascular diseases: A bidirectional two-sample Mendelian randomization analysis
Jun Zhang Yiji Pan, and Hongxia Yang shared the first authorship.
Abstract
Introduction
Increasing evidence implicates retinal vascular occlusions as a susceptibility factor for cardiovascular diseases (CVDs), whereas inconsistent results on the relationship were reported in previous observational studies. This research using a bidirectional two-sample Mendelian randomization (MR) analysis aimed to investigate the potential association between genetically determined central/branch retinal artery and retinal vein occlusions (CRAO/BRAO/RVO) and the risk of CVD.
Methods
Summary statistics of retinal vascular occlusions from the largest available genome-wide association study of European descent were used to investigate their relationship with CVDs, and vice versa. Primary analyses were conducted using the common inverse-variance weighted approach. Several complementary sensitivity analyses were performed to verify the reliability of our results.
Results
Inverse variance weighted method showed suggestive effects of genetically determined RVO on ischemic stroke (IS) (odds ratio [OR] = 1.021, 95% confidence [CI] = 1.004–1.037, p = 0.012), a genetic liability to CRAO increased the risk of myocardial infarction (MI) (OR = 1.014, 95% CI = 1.006–1.023, p = 7.0 × 10−4). In addition, genetic predisposition to BRAO had a positive effect on stroke (OR = 1.008, 95% CI = 1.002–1.013, p = 0.011), IS (OR = 1.007, 95% CI = 1.001–1.014, p = 0.022), and cardioembolic stroke (CES) (OR = 1.018, 95% CI = 1.006–1.031, p = 0.004). The point estimates from sensitivity analyses were in the same direction. Reverse MR analyses found no significant evidence for the effect of CVDs on retinal vascular occlusions.
Conclusion
Our MR study provides potential evidence that retinal vascular occlusions are causally linked to increased risk of CVDs including IS, MI, stroke, and CES. This supports the need for clinical CVD screening in individuals with retinal vascular occlusions. Further investigations are warranted to clarify the effects of CVDs on ocular comorbidities.
1 INTRODUCTION
Retinal vascular occlusions are predominant causes of permanent vision impairment and loss among elderly individuals (Hayreh et al., 2009; Suner et al., 2013). Retinal artery and vein occlusions (RAO/RVO) are two common retinal vascular disorders. The former has a relatively low incidence among the general population, with an incidence rate of 1.8−1.9 per 100,000 person-years for central RAO (CRAO) and an even lower rate for branch type (BRAO) (Leavitt et al., 2011; Park et al., 2014). However, the latter affects about 16 million people globally with a 15-year cumulative incidence rate of 0.5% for central RVO (CRVO) and 1.8% for branch type (BRVO) (Klein et al., 2008; Rogers et al., 2010). Although preliminary data from case series and randomized controlled trials showed promising outcomes, the pathophysiological basis underlying both types of ocular occlusions is still not fully understood.
Retinal vascular occlusions are attributable to the interaction between genetic predisposition and environmental risk factors including, but not frequently limited to, hypertension, hyperlipidemia, diabetes, smoking, oral contraceptive medication, and the pregnancy-puerperal state (Chapin et al., 2015). Over the past decade, there has been substantial interest and discussion in the association of retinal vascular occlusions with the risk of cardiovascular diseases (CVDs). A retrospective nationwide population-based study from Taiwan found no overall association of RVO with stroke (Ho et al., 2009). However, another large population-based study in Korea recently observed an increased risk of stroke development in patients diagnosed with RVO (Rim et al., 2015). Besides, most of the published case series and longitudinal cohort studies investigating the association of RVO with myocardial infarction (MI) demonstrated inconsistent results (Chen et al., 2017; Hu et al., 2009; Rim, Han, et al., 2016; Werther et al., 2011). Additionally, as Hayreh et al. (2009) reported, stroke has previously been identified as significantly associated with RAO. Roskal-Wałek et al. (2022) argued that RAO patients are significantly more likely to experience an ischemic stroke. In contrast, several single-center studies based on direct evaluation did not show any high risk of ischemic stroke following RAO events (Chodnicki et al., 2022; Laczynski et al., 2020; Leisser & Findl, 2020).
Although prior observational studies have explored the potential relationship between retinal vascular occlusions and CVDs, no certain evidence supports the causal link between two kinds of clinical phenotypes. Relatively small sample sizes, incomplete matching, insufficiently representative samples generated from single center, reverse causality, and unmeasured confounding factors could constitute methodological limitations, which remain a cause for concern. Thus, exploring and clarifying the correlation between them are of positive significance. Mendelian randomization (MR) approach can overcome such confounders of conventional observational models by using genetic variants (single-nucleotide variant [SNV]) of genome-wide association studies (GWASs), which are randomly allocated at meiosis as instrumental variables (IVs) (Davey Smith & Hemani, 2014). As genetic phenotypes are postnatally unchanged through lifetime exposure, MR can also reduce the susceptibility to reverse causation, which is analogous to the design of a randomized trial (Richmond & Davey Smith, 2022).
As far as we know, no MR analysis has explored the effect of retinal vascular occlusions on CVDs. In the current research, we conducted a bidirectional two-sample MR study to unearth the potential link between retinal vascular occlusions including RVO, CRAO, and BRAO, and common CVDs at the genetic level.
2 MATERIALS AND METHODS
The de-identified summary-level data of retinal vascular occlusions and common CVDs from the publicly available integrative epidemiology unit (IEU) GWAS database (https://gwas.mrcieu.ac.uk/about/), GWAS Catalog (https://www.ebi.ac.uk/gwas/), and the FinnGen consortium (R7) (https://finngen.gitbook.io/documentation) were acquired for MR analysis. A schematic overview of the MR design and data sources is presented in Figure 1. The variants were selected as IVs based on three primary MR assumptions, namely, the IVs (i) should be strongly associated with the exposure factor, (ii) would not be correlated with any confounder of the exposure–outcome association, and (iii) should only affect the outcome through the exposure factor(Hartwig et al., 2016).
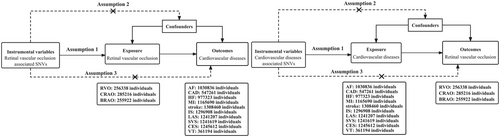
2.1 Data source
Ethical approval and consent to participation acquired by each cohort were obtained in the GWAS studies. To avoid the effects of population stratification, all variants and their associated summary-level datasets were only extracted from studies with participants of European descent.
Genetic association estimates of retinal vascular occlusions were downloaded from a large-scale GWAS performed by the FinnGen consortium. Of which, the GWAS summary statistics for RVO included 572 cases and 255,766 controls, and the RVO subtype information was not available. Genetic associations with RAO provided data on the disease entities including 390 CRAO cases (with 284,826 controls) and 156 BRAO cases (with 255,766 controls). The diagnosis of retinal vascular occlusion was performed by experienced ophthalmologists and all included cases fulfilled the international diagnosis criteria.
All CVD-related genome-wide instrumental variants published in the publicly available IEU GWAS database and GWAS Catalog were also acquired. Summary estimates of atrial fibrillation (AF) were obtained from the latest GWAS including six contributing cohorts, which comprised 60,620 AF patients and 970,216 controls (Nielsen et al., 2018). GWAS data on coronary artery disease (CAD) were downloaded from the recently published genome-wide meta-analysis and constituted 122,733 CAD cases and 424,528 controls from the UK Biobank and CARDIoGRAMplusC4D consortium (Verweij et al., 2017). Summary-level data on heart failure (HF) were leveraged from a GWAS meta-analysis including 26 studies with 47,309 HF cases and 930,014 controls from the HERMES consortium (Shah et al., 2020). Similarly, the resultant MI GWAS dataset includes 181,522 cases and 984,168 controls of predominantly European ancestry (Aragam et al., 2022); the stroke dataset included 73,652 cases and 1,234,808 controls from a summary-level GWAS meta-analysis of the GIGASTROKE consortium (Mishra et al., 2022). Among these stroke individuals, 62,100 could be further classified as ischemic stroke (IS), involving 6399 cases of large-artery atherosclerotic stroke (LAS), 6811 cases of small vessel stroke (SVS), and 10,804 cases of cardioembolic stroke (CES). Additionally, a GWAS summary-level statistical dataset of venous thromboembolism (VT) was available from the Neale lab (https://www.nealelab.is/) containing 4620 cases and 356,574 controls. The individuals contributing to the data all met the clinical consensus criteria for the diagnosis of CVDs.
There was no overlap of participants between the exposure and outcome GWASs. The information of the genetic datasets used in this study is summarized in Table 1. All datasets are publicly available online upon request.
| GWAS/study | Year | Data source | Case | Control | PMID |
|---|---|---|---|---|---|
| Retinal vascular occlusion | |||||
| RVO | 2021 | FinnGen_R7 | 572 | 255,766 | NA |
| CRAO | 2021 | FinnGen_R7 | 390 | 284,826 | NA |
| BRAO | 2021 | FinnGen_R7 | 156 | 255,766 | NA |
| Cardiovascular diseases | |||||
| AF | 2018 | HUNT, UK Biobank, deCODE, DiscovEHR, MGI and AFGen | 60,620 | 970,216 | 30061737 |
| CAD | 2018 | UK Biobank and CARDIoGRAMplusC4D | 122,733 | 424,528 | 29212778 |
| HF | 2020 | HERMES consortium | 47,309 | 930,014 | 31919418 |
| MI | 2022 | CARDIoGRAMplusC4D consortium | 181,522 | 984,168 | 36474045 |
| Stroke | 2022 | GIGASTROKE consortium | 73,652 | 1,234,808 | 36180795 |
| IS | 2022 | GIGASTROKE consortium | 62,100 | 1,234,808 | 36180795 |
| LAS | 2022 | GIGASTROKE consortium | 6399 | 1,234,808 | 36180795 |
| SVS | 2022 | GIGASTROKE consortium | 6811 | 1,234,808 | 36180795 |
| CES | 2022 | GIGASTROKE consortium | 10,804 | 1,234,808 | 36180795 |
| VT | 2022 | UK Biobank (Neale lab) | 4620 | 356,574 | NA |
- Abbreviations: AF, atrial fibrillation; BRAO, branch retinal artery occlusion; CAD, coronary artery disease; CES, cardioembolic stroke; CRAO, central retinal artery occlusion; HF, heart failure; IS, ischemic stroke; LAS, large-artery atherosclerotic stroke; MI, myocardial infarction; NA, not available; RVO, retinal vein occlusion; SVS, small vessel stroke; VT, venous thromboembolism.
2.2 Selection of IVs
Since there was no variant associated with retinal vascular occlusions reaching the genome-wide significant threshold (p < 5 × 10−8), we extracted all variants predicting an exposure with a relaxed threshold of p < 1 × 10−5 for retinal vascular occlusions and p < 5 × 10−8 for CVDs as common IVs, which have been adopted in several previous MR studies (Liu et al., 2022; Wootton et al., 2021; Yeung et al., 2021). From these sets of IVs, linkage disequilibrium (LD) in the selected SNVs was assessed using the European 1000 Genome Project reference panel, and SNVs were removed if they had a measured LD greater than 0.01 with a window size of 5000 kb. Independent variants with the smallest p-value were retained, and the variants unavailable in the outcome dataset were replaced (LD threshold: r2 > 0.8) by their corresponding suitable proxy variants obtained by searching on an online website (https://ldlink.nci.nih.gov/). To avoid violations of the MR assumptions, we also searched the PhenoScanner (www.phenoscanner.medschl. cam.ac.uk/) and GWAS Catalog (www.ebi.ac.uk/gwas/) for all variants associated with exposures to explore whether there was any variant linked to confounding variables or outcomes (p < 5 × 10−8). In addition, any palindromic variant with intermediate allele frequencies would be removed.
2.3 Instrumental strength and power calculation
F-statistics were calculated (F = βexp2/SEexp2) to be an indicator of the strength of the genetic IVs. If the IVs used to proxy the exposure have a mean F-statistic greater than 10, it indicates that the MR analysis should not suffer from weak instrument bias. The proportions of variance (R2) in the MR study explained by exposures associated with each selected variant were calculated as previously described (Zeng & Zhou, 2019). Subsequently, statistical power calculations were performed using an online tool for binary outcomes (https://sb452.shinyapps.io/power/), where the alpha level was set to 0.05.
2.4 Statistical analysis
The two-sample bidirectional MR methods were used to explore the potential causal inference of retinal vascular occlusions (such as RVO, CRAO, and BRAO) in CVDs, including AF, CAD, HF, MI, VT, stroke, and ischemic stroke subtypes.
In the primary analysis, the conventional inverse-variance weighted (IVW) method (Burgess et al., 2017) was employed to estimate the causal associations between genetically predicted retinal vascular occlusions and various CVD events. In addition, five complementary methods including weighted median (Bowden et al., 2016), maximum likelihood (Nguyen et al., 2015), MR–Egger regression (Burgess & Thompson, 2017), MR-robust adjusted profile score (MR-RAPS) (Hemani et al., 2018), and MR-pleiotropy residual sum and outlier (MR-PRESSO) (Verbanck et al., 2018) were further applied to examine the robustness of the results and possible pleiotropy. Detailed information on each sensitivity analysis conducted in the present study can be found in Supporting Information 1. Moreover, the heterogeneity tests for individual variant estimates were performed to detect the heterogeneity of the IVW method. The I2 statistic was calculated to assess the degree of heterogeneity among variant estimates in each analysis. p-Values <0.05 in Cochran's Q test indicated obvious heterogeneity. In addition, a p-value less than 0.05 for the MR–Egger intercept test indicated the presence of pleiotropic bias. Finally, a leave-one-out sensitivity analysis was also applied to examine whether a single variant could bias the estimate.
For multiple testing corrections, associations with a p-value less than the Bonferroni adjusted threshold of 1.7 × 10−3 (0.05/30) were considered statistically significant. A p-value above the corrected significance threshold but <0.05 was also considered suggestive evidence. All MR analyses were two-sided and implemented using the R-based packages “MR-PRESSO,” “TwoSampleMR,” and “MR-RAPS” in R statistical software (version 4.1.2).
3 RESULTS
We investigated the effect of retinal vascular occlusions on AF, CAD, HF, MI, VT, stroke, and ischemic stroke and its three subtypes (LAS, CES, and SVS). Power calculations for our bidirectional two-sample MR analyses were conducted for the genetically predicted exposure level. Our sample provided sufficient statistical power (greater than 80%) to detect the effect of retinal vascular occlusions on common CVDs.
3.1 Effect of genetic liability to retinal vascular occlusions on cardiovascular diseases
The full characteristics of the selected variants for each exposure are presented in Table S1. The F-statistic of all selected IVs was above the threshold of 10, indicating that no weak instrument bias existed. Our genetic analysis showed that these variants explained 0.05%−1.06% of the variance in retinal vascular occlusions.
The results of the conservative analysis based on the IVW method are shown in Figure 2. Overall, we found weak evidence that a higher genetic liability to RVO increases the risk of IS (odds ratio [OR] = 1.021, 95% confidence interval [CI] = 1.004−1.037, p = 0.012). However, no associations were observed for the effects of genetically predicted RVO on the other nine CVDs. Regarding CRAO, there was some evidence suggesting that one unit increase in the genetic liability to CRAO had a positive effect on MI but not on the other nine CVDs (for CRAO and MI: OR = 1.014, 95% CI = 1.006−1.023, p = 7.0 × 10−4). Moreover, there was evidence suggesting that genetic liability to higher BRAO showed an increased risk of stroke (OR = 1.008, 95% CI = 1.002−1.013, p = 0.011). Subgroup analyses showed that a higher genetic liability to BRAO increases the risk of IS and CES (OR = 1.007, 95% CI = 1.001−1.014, p = 0.022; OR = 1.018, 95% CI = 1.006−1.031, p = 0.004). Primary analysis revealed no evidence of genetic liability to BRAO on the risk of other CVDs.
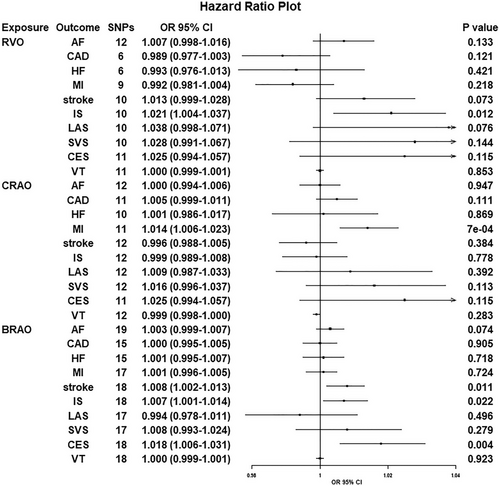
There was no evidence to suggest horizontal pleiotropy in most MR analyses. Although Cochran's Q statistics showed substantial heterogeneity in the estimates of HF (p = 0.003), the intercept from the MR–Egger regression analysis did not reach statistical significance in all outcomes (p for intercept > 0.05) (Table 2). Potential outliers in our MR were identified by the MR-PRESSO method, which resulted in potential pleiotropy assessed by the global test. However, the results remained to be similar to MR-RAPS estimates after outlier correction (Table S2). The results of the leave-one-out analyses were again concordant with our primary findings.
| Exposure | Outcome | Heterogeneity | Weighted median | Maximum likelihood | MR-Egger regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | p for intercept | ||
| RVO | AF | 10.24 | 0.509 | 1.005 | 0.994–1.017 | 0.386 | 1.007 | 0.998–1.016 | 0.141 | 1.002 | 0.987–1.017 | 0.787 | 0.446 |
| CAD | 4.03 | 0.546 | 0.989 | 0.973–1.006 | 0.205 | 0.989 | 0.976–1.003 | 0.122 | 0.989 | 0.968–1.011 | 0.389 | 0.989 | |
| HF | 8.17 | 0.147 | 1.003 | 0.980–1.026 | 0.797 | 0.993 | 0.975–1.010 | 0.411 | 1.001 | 0.959–1.045 | 0.972 | 0.147 | |
| MI | 10.99 | 0.202 | 0.993 | 0.976–1.011 | 0.449 | 0.992 | 0.980–1.005 | 0.220 | 0.988 | 0.947–1.031 | 0.609 | 0.982 | |
| Stroke | 5.07 | 0.749 | 1.017 | 0.997–1.038 | 0.095 | 1.013 | 0.998–1.029 | 0.072 | 1.006 | 0.977–1.035 | 0.710 | 0.566 | |
| IS | 8.42 | 0.394 | 1.016 | 0.992–1.039 | 0.185 | 1.021 | 1.005–1.039 | 0.012 | 1.009 | 0.977–1.043 | 0.587 | 0.543 | |
| LAS | 10.09 | 0.259 | 1.014 | 0.961–1.070 | 0.610 | 1.035 | 0.997–1.073 | 0.068 | 0.983 | 0.907–1.065 | 0.686 | 0.214 | |
| SVS | 2.33 | 0.676 | 1.012 | 0.969–1.056 | 0.595 | 1.001 | 0.966–1.037 | 0.966 | 0.971 | 0.885–1.065 | 0.575 | 0.537 | |
| CES | 9.23 | 0.323 | 1.030 | 0.987–1.075 | 0.169 | 1.029 | 0.997–1.061 | 0.072 | 1.032 | 0.960–1.109 | 0.420 | 0.898 | |
| VT | 13.33 | 0.206 | 1.000 | 0.999–1.001 | 0.919 | 1.000 | 0.999–1.001 | 0.847 | 1.000 | 0.999–1.001 | 0.156 | 0.064 | |
| CRAO | AF | 20.59 | 0.038 | 1.003 | 0.995–1.011 | 0.512 | 1.000 | 0.994–1.006 | 0.944 | 1.004 | 0.994–1.016 | 0.442 | 0.295 |
| CAD | 11.28 | 0.336 | 1.004 | 0.995–1.013 | 0.412 | 1.005 | 0.999–1.011 | 0.115 | 1.005 | 0.996–1.013 | 0.329 | 0.957 | |
| HF | 25.11 | 0.003 | 1.003 | 0.989–1.015 | 0.695 | 1.001 | 0.992–1.011 | 0.777 | 1.016 | 0.992–1.041 | 0.226 | 0.172 | |
| MI | 8.84 | 0.547 | 1.015 | 1.003–1.027 | 0.013 | 1.015 | 1.006–1.024 | 0.0006 | 1.009 | 0.989–1.031 | 0.374 | 0.624 | |
| Stroke | 8.86 | 0.354 | 0.987 | 0.972–1.003 | 0.108 | 0.991 | 0.979–1.003 | 0.134 | 0.972 | 0.948–0.996 | 0.054 | 0.110 | |
| IS | 6.06 | 0.641 | 0.992 | 0.976–1.009 | 0.360 | 0.994 | 0.981–1.006 | 0.333 | 0.976 | 0.951–1.002 | 0.109 | 0.161 | |
| LAS | 8.46 | 0.584 | 1.009 | 0.980–1.038 | 0.555 | 1.013 | 0.991–1.035 | 0.260 | 1.018 | 0.988–1.049 | 0.278 | 0.622 | |
| SVS | 3.62 | 0.305 | 0.989 | 0.948–1.032 | 0.611 | 0.999 | 0.966–1.033 | 0.955 | 0.999 | 0.899–1.111 | 0.996 | 0.991 | |
| CES | 3.24 | 0.987 | 1.014 | 0.990–1.039 | 0.249 | 1.012 | 0.995–1.029 | 0.182 | 1.013 | 0.989–1.038 | 0.309 | 0.882 | |
| VT | 9.27 | 0.597 | 0.999 | 0.998–1.000 | 0.105 | 0.999 | 0.998–1.000 | 0.284 | 0.999 | 0.998–1.000 | 0.417 | 0.937 | |
| BRAO | AF | 20.73 | 0.293 | 1.003 | 0.997–1.009 | 0.318 | 1.004 | 0.999–1.007 | 0.077 | 1.005 | 0.998–1.012 | 0.212 | 0.670 |
| CAD | 17.39 | 0.236 | 0.997 | 0.990–1.004 | 0.475 | 1.000 | 0.995–1.005 | 0.905 | 0.992 | 0.982–1.003 | 0.184 | 0.119 | |
| HF | 13.49 | 0.488 | 1.002 | 0.994–1.010 | 0.672 | 1.001 | 0.995–1.007 | 0.709 | 1.003 | 0.991–1.016 | 0.584 | 0.664 | |
| MI | 13.11 | 0.729 | 0.997 | 0.987–1.007 | 0.587 | 1.001 | 0.994–1.008 | 0.722 | 0.999 | 0.987–1.013 | 0.971 | 0.792 | |
| Stroke | 9.91 | 0.624 | 1.009 | 1.000–1.017 | 0.046 | 1.008 | 1.002–1.014 | 0.012 | 1.003 | 0.992–1.013 | 0.641 | 0.217 | |
| IS | 10.23 | 0.595 | 1.010 | 1.002–1.019 | 0.017 | 1.007 | 1.001–1.014 | 0.023 | 1.003 | 0.992–1.014 | 0.589 | 0.364 | |
| LAS | 10.15 | 0.859 | 1.007 | 0.987–1.026 | 0.510 | 1.001 | 0.987–1.015 | 0.891 | 1.005 | 0.978–1.033 | 0.723 | 0.739 | |
| SVS | 9.54 | 0.299 | 1.012 | 0.992–1.033 | 0.247 | 1.009 | 0.994–1.025 | 0.220 | 0.997 | 0.959–1.036 | 0.877 | 0.517 | |
| CES | 10.92 | 0.861 | 1.022 | 1.005–1.039 | 0.011 | 1.018 | 1.006–1.031 | 0.004 | 1.015 | 0.994–1.036 | 0.181 | 0.469 | |
| VT | 14.02 | 0.666 | 1.000 | 0.999–1.001 | 0.755 | 1.000 | 0.999–1.001 | 0.924 | 1.000 | 0.999–1.001 | 0.618 | 0.505 | |
- Abbreviations: AF, atrial fibrillation; BRAO, branch retinal artery occlusion; CAD, coronary artery disease; CES, cardioembolic stroke; CI, confidence interval; CRAO, central retinal artery occlusion; HF, heart failure; IS, ischemic stroke; LAS, large-artery atherosclerotic stroke; MI, myocardial infarction; OR, odds ratio; RVO, retinal vein occlusion; SVS, small vessel stroke; VT, venous thromboembolism.
3.2 Effect of genetic liability to cardiovascular diseases on retinal vascular occlusions
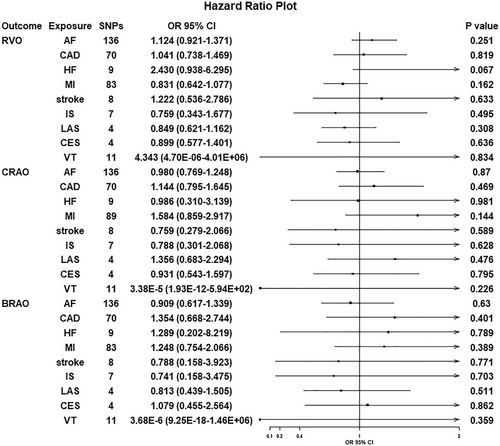
We further examined the causal effects of CVDs on three types of retinal vascular occlusions. The detailed genetic variants used in the conservative analysis are displayed in Table S3. The F-statistic of all selected variants was greater than 10, indicating that they strongly predicted various CVDs in the MR analysis. The selected independent IVs could explain about 0.05%−1.62% of the total variation for each exposure risk.
Since the Cochran's Q test revealed heterogeneity (p > 0.05), we applied the IVW approach in a fixed-effects model. The IVW results of the MR analysis estimates for the effect of CVDs on the risk of retinal vascular occlusions are displayed in Figure 3. In addition, we found that genetic predisposition to CVDs had no effect on retinal vascular occlusions. The OR of AF on RVO was estimated to be 1.124 (95% CI = 0.921−1.371, p = 0.251). Furthermore, the results of other methods were almost the same as those of the IVW method (Table 3).
| Outcome | Exposure | Heterogeneity | Weighted median | Maximum likelihood | MR–Egger regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | p for intercept | ||
| RVO | AF | 15.38 | 0.128 | 1.037 | 0.729–1.474 | 0.841 | 0.125 | 0.920–1.374 | 0.251 | 1.114 | 0.728–1.704 | 0.621 | 0.963 |
| CAD | 91.48 | 0.036 | 1.255 | 0.771–2.043 | 0.361 | 1.042 | 0.771–1.409 | 0.788 | 0.839 | 0.411–1.714 | 0.633 | 0.502 | |
| HF | 4.67 | 0.792 | 1.508 | 0.435–5.227 | 0.517 | 2.46 | 0.940–6.442 | 0.067 | 1.534 | 0.111–21.30 | 0.759 | 0.724 | |
| MI | 33.81 | 0.112 | 0.848 | 0.557–1.290 | 0.440 | 0.829 | 0.639–1.077 | 0.160 | 1.556 | 0.664–3.645 | 0.319 | 0.221 | |
| Stroke | 27.93 | 0.032 | 2.111 | 0.769–5.796 | 0.147 | 1.827 | 0.923–3.616 | 0.083 | 1.248 | 0.003–461.3 | 0.943 | 0.907 | |
| IS | 23.73 | 0.127 | 1.266 | 0.515–3.115 | 0.607 | 1.728 | 0.921–3.243 | 0.089 | 0.341 | 0.007–17.17 | 0.598 | 0.426 | |
| LAS | 3.51 | 0.319 | 1.167 | 0.612–2.226 | 0.64 | 0.94 | 0.560–1.578 | 0.814 | 3.736 | 0.125–111.9 | 0.527 | 0.504 | |
| CES | 1.24 | 0.744 | 0.987 | 0.615–1.583 | 0.956 | 0.952 | 0.616–1.469 | 0.823 | 1.204 | 0.409–3.548 | 0.768 | 0.687 | |
| VT | 11.67 | 0.307 | 3.2 × 105 | 3.9 × 10−3–2.6 × 1013 | 0.173 | 4.459 | 4.6 × 10−6–4.4 × 106 | 0.832 | 9.4 × 106 | 9.1 × 10−4–9.8 × 1016 | 0.205 | 0.155 | |
| CRAO | AF | 15.03 | 0.174 | 0.878 | 0.571–1.351 | 0.554 | 0.979 | 0.768–1.250 | 0.871 | 0.975 | 0.584–1.626 | 0.922 | 0.981 |
| CAD | 6.86 | 0.491 | 1.676 | 0.939–2.993 | 0.081 | 1.147 | 0.795–1.654 | 0.463 | 1.937 | 0.914–4.105 | 0.089 | 0.121 | |
| HF | 6.87 | 0.551 | 0.493 | 0.102–2.383 | 0.379 | 0.986 | 0.307–3.171 | 0.982 | 1.492 | 0.061–36.71 | 0.814 | 0.794 | |
| MI | 22.52 | 0.606 | 1.331 | 0.747–2.375 | 0.332 | 1.051 | 0.698–1.582 | 0.812 | 2.541 | 1.032–6.260 | 0.054 | 0.042 | |
| Stroke | 11.42 | 0.783 | 0.851 | 0.273–2.653 | 0.78 | 1.032 | 0.455–2.343 | 0.939 | 1.759 | 0.009–343.4 | 0.837 | 0.843 | |
| IS | 11.52 | 0.828 | 1.638 | 0.589–4.553 | 0.344 | 1.446 | 0.677–3.091 | 0.341 | 1.449 | 0.027–78.91 | 0.858 | 0.996 | |
| LAS | 1.07 | 0.783 | 0.985 | 0.469–2.069 | 0.969 | 1.231 | 0.661–2.293 | 0.512 | 6.247 | 0.174–224.1 | 0.421 | 0.462 | |
| CES | 6.17 | 0.104 | 1.223 | 0.633–2.362 | 0.549 | 1.069 | 0.627–1.820 | 0.807 | 2.959 | 0.52–16.54 | 0.342 | 0.333 | |
| VT | 7.07 | 0.719 | 4.5 × 10−3 | 1.1 × 10−12–1.9 × 107 | 0.632 | 3.9 × 10−5 | 2.1 × 10−12–7.2 × 102 | 0.234 | 7.3 × 102 | 7.0 × 10−10–7.5 × 1014 | 0.652 | 0.168 | |
| BRAO | AF | 13.29 | 0.536 | 0.901 | 0.493–1.647 | 0.735 | 0.909 | 0.616–1.341 | 0.63 | 0.816 | 0.377–1.768 | 0.607 | 0.751 |
| CAD | 91.72 | 0.006 | 1.937 | 0.765–4.909 | 0.163 | 1.362 | 0.756–2.453 | 0.303 | 2.636 | 0.610–11.39 | 0.199 | 0.312 | |
| HF | 11.09 | 0.196 | 3.729 | 0.215–64.59 | 0.366 | 1.299 | 0.198–8.551 | 0.785 | 0.105 | 2.3 × 10−4–48.97 | 0.496 | 0.419 | |
| MI | 36.53 | 0.063 | 1.792 | 0.657–4.887 | 0.255 | 1.282 | 0.666–2.470 | 0.457 | 3.436 | 0.613–19.27 | 0.173 | 0.219 | |
| Stroke | 26.24 | 0.051 | 0.406 | 0.058–2.873 | 0.367 | 1.295 | 0.343–4.883 | 0.703 | 25.56 | 3.9 × 10−4–1.6 × 106 | 0.574 | 0.599 | |
| IS | 23.18 | 0.143 | 0.587 | 0.101–3.422 | 0.554 | 1.012 | 0.297–3.449 | 0.985 | 8.293 | 0.004–17101 | 0.594 | 0.589 | |
| LAS | 3.53 | 0.317 | 1.416 | 0.405–4.950 | 0.586 | 0.958 | 0.349–2.633 | 0.934 | 17.42 | 0.026–11606 | 0.479 | 0.468 | |
| CES | 4.14 | 0.247 | 0.858 | 0.328–2.245 | 0.755 | 0.849 | 0.362–1.995 | 0.708 | 0.146 | 0.018–1.207 | 0.216 | 0.216 | |
| VT | 17.15 | 0.071 | 2.1 × 10−6 | 4.5 × 10−23–9.4 × 1010 | 0.503 | 3.5 × 10−6 | 7.7 × 10−18–1.6 × 106 | 0.359 | 5.1 × 109 | 1.4 × 10−14–1.8 × 1033 | 0.439 | 0.149 | |
- Abbreviations: AF, atrial fibrillation; BRAO, branch retinal artery occlusion; CAD, coronary artery disease; CES, cardioembolic stroke; CI, confidence interval; CRAO, central retinal artery occlusion; HF, heart failure; IS, ischemic stroke; LAS, large-artery atherosclerotic stroke; MI, myocardial infarction; OR, odds ratio; RVO, retinal vein occlusion; SVS, small vessel stroke; VT, venous thromboembolism.
The MR-PRESSO method and MR–Egger regression were further used for sensitivity analyses to reanalyze the association observed in primary IVW analyses. We found that the association patterns remained directionally consistent in most statistical models, and stable estimates were found in the MR-RAPS and MR-PRESSO methods (Table S4). Weak evidence of directional pleiotropy was found in the MR–Egger intercept tests (p > 0.05, Table 3). The leave-one-out analyses suggested that the associations were unlikely to be influenced by certain extreme variants.
4 DISCUSSION
To our knowledge, this is the first bidirectional study to assess the effect of genetically determined retinal vascular occlusions (subtypes) on CVDs using the MR method based on the newest summary GWAS databases. In the present MR study, we found that retinal vascular occlusions causally increased the risk of CVDs, such as IS, MI, stroke, and CES. However, no significant causal effect of CVDs on retinal vascular occlusions was observed.
Previous observational studies have suggested that RVO might be a risk factor for CVDs. Martin et al. (2002) reported that patients with RVO were at increased risk of CVDs. Several population-based retrospective cohort studies proposed a causal link between RVO and developing MI, HF, and stroke (Bakhoum et al., 2022; Chen et al., 2017; Rim, Han, et al., 2016; Rim, Oh, et al., 2016). Similarly, a retrospective observational study revealed that patients with CRAO were at a very high risk of ischemic stroke (Raber et al., 2022). A retrospective analysis of 292 consecutive patients confirmed that CRAO portends a high-risk population for AF (Vonderlin et al., 2021). These findings were consistent with two published systematic reviews and meta-analyses, which reported a positive association of RVO with HF, stroke, and MI (Li et al., 2016; Wu et al., 2019). By contrast, two observational studies failed to find such an association between RVO and stroke risk (Cugati et al., 2007; Ho et al., 2009). Likewise, RAO is reported to be associated with a higher risk of stroke in a previous meta-analysis. In the reverse direction, a multi-center case–control study with a large sample concluded that AF and MI did not increase CRVO risk (Trovato Battagliola et al., 2022). A retrospective study showed that patients with stroke did not increase CRAO risk (Lo et al., 2022). Data obtained from Danish nationwide registries suggested that AF is not involved in the pathophysiology of RAO (Orskov et al., 2022a). Besides, a retrospective registry study indicated that patients with a CVD history tended to have a significantly higher risk of BRAO (Cho et al., 2016). Up to now, the only meta-analysis, involving a total population of 12,305 subjects with retinal vessel occlusion, showed that AF is significantly associated with an increased risk of CRAO and CRVO (Kewcharoen et al., 2019).
The above results were not confirmed by single-center studies based on a direct patient evaluation, and some of them even contradicted the findings of the previous large analyses. Several pathogenic links between retinal vascular occlusions and CVDs have been suggested because of the shared common immunological response and inflammation mediator (Ritzel et al., 2016; Zuo et al., 2023). Furthermore, studies have shown that the association between CVD, such as atrial fibrillation and stroke, may indicate that emboli arising from the heart was the primary causes of RAO (Hayreh et al., 2009). Some investigators concluded that the atherothrombotic embolism in several mechanisms of stroke has contributed to the same extent in the development of RAO (Orskov et al., 2022b). Our bidirectional MR study extends a bit from prior research in supporting the significant causal relationship between retinal vascular occlusions and CVDs but not vice versa. These findings are partially contrary to the report of the observational studies and systemic meta-analyses that in particular, patients with CVD have a greater risk of developing a retinal vascular occlusion. The relatively small number of patients with retinal vascular occlusion in the FinnGen consortium may account for this null association. Besides, the previously observed associations or reviews between CVDs and retinal vascular occlusions may be coincidental or thwarted by an unknown confounder. Our null findings refute the role of CVDs in the etiology of retinal vascular occlusions, providing a less confounded causal estimate compared to previous observational studies. More studies are warranted to identify these potential confounders and elucidate their complex relationship.
Even with the wide recognition of the causal association of retinal vascular occlusions with CVDs from our MR study and previous observational studies, the precise mechanisms by which retinal vascular occlusions play a direct or indirect contributory role in the risk of CVDs remain ambiguous and are known to be multifactorial. First, retinal vascular occlusions are usually associated with certain cardiovascular risk factors, such as hypertension, diabetes mellitus, hyperlipidemia, renal disease, smoking habits, high body mass index, and age, which are also known as risk factors for CVDs (Chang et al., 2015; Li et al., 2016). Second, several macrovascular pathogenic processes including embolic occlusion, hemorrhage into an atherosclerotic plaque, intraluminal thrombus, vasculitis, and vasospasm that are present in retinal vascular occlusions are also a potential risk for CVDs (Chang et al., 2015). Our MR study did not corroborate the findings from the previous meta-analysis which provided consistent estimates of the associations between CRAO/BRAO and the incidence of cardiovascular events. There may be differences in the pathogenic processes of arterial atherosclerosis between RVO and RAO. As reported, RAO is more commonly associated with vascular inflammation, cholesterol emboli, and enhanced platelet aggregation, while RVO is associated with traditional venous thrombosis risk factors such as thrombophilia, venous stasis, and endothelial injury. Additionally, our MR results concluded that the risk of cardiovascular events differs between CRAO and BRAO, inconsistent with the currently published single-center retrospective study (Roskal-Walek et al., 2021). Lauda et al. (2015) speculated that not only the size and composition of the embolic material but also the unrecognized etiology may differ in the pathophysiology between CRAO and BRAO. Further investigation into the potential mechanisms is thus required.
Several strengths of this MR study deserve special emphasis. First, to our knowledge, we are the first to systematically assess a causal association between retinal vascular occlusions and CVDs and vice versa using a bidirectional two-sample MR approach, which could exclude environmental cofounders and reverse causation bias seen in observational studies. Second, the availability of the most recent large-scale GWASs and multiple MR models ensured a relevantly reliable and lifelong causal estimation. Although previous studies have indicated that both RVO and RAO exhibit similar associations with CVDs, there are variations in their effects on CVDs. Besides, we discovered that CRAO and BRAO had fundamentally distinct relationships with various CVDs. Third, we conducted several sensitivity analyses to ensure the consistency of causal estimates and confirm the robustness of the present findings.
Notwithstanding the explicit strengths, several limitations should be noted. First, only European patients were included in our study; thus, the findings cannot be directly extrapolated into other ethnic groups. Second, the sample size of retinal vascular occlusions was relatively small, which might have resulted in ambiguous positive or negative results. Third, the genetic factors could only account for a small proportion of the variation in RVO, RAO, and CVDs and hence had insufficient statistical power to show an effect. The remaining variation is likely explained by pathways that are not influenced by genetic factors. Finally, these included RVO patients were not divided into two main sub-entities, CRVO and BRVO. Therefore, it is uncertain whether a specific type of RVO could increase the risk of CVDs. Based on these, it is urgent to collect another independent population to validate the findings in future studies.
In conclusion, our findings have corroborated evidence that retinal vascular occlusions are causally linked to an increased risk of CVDs, which may help guide clinical decisions in the management of ocular diseases in patients with CVD, especially regarding RVO in IS; CRAO in MI; and BRAO in stroke, IS, and CES. In addition, our study did not support a causal effect of CVDs on retinal vascular occlusions. MR studies with larger population-specific sample sizes are still needed to confirm the potential effect for various CVD subtypes.
AUTHOR CONTRIBUTIONS
Tao He and Jun Zhang conceived and designed the study. Hongxia Yang, Shuqiong Hu, and Yiji Pan collected the data. Sheng Zheng and Shuqiong Hu analyzed and interpreted the data. Tao He and Jun Zhang wrote the initial draft. Tao He, Jun Zhang, and Hongxia Yang revised the manuscript. Tao He supervised the study. All authors provided a final review and approved the manuscript before submission.
ACKNOWLEDGMENTS
The authors thank all the investigators of the UK Biobank and the MRC IEU Open GWAS Project for providing the data publicly.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data used in our Mendelian randomization analysis can be obtained from the UK Biobank, GWAS Catalog, and the MRC IEU Open GWAS Project. We also want to acknowledge the participants and investigators of FinnGen study. Further inquiries can be directed to the corresponding author.




