A novel homozygous LRRK1 stop gain mutation in a patient suspected with osteosclerotic metaphyseal dysplasia
Abstract
Osteosclerotic metaphyseal dysplasia (OSMD) is a very rare autosomal-recessive disorder and a distinctive type of osteopetrosis, characterized mainly by skeletal fractures and deformity, osteosclerosis, and sometimes hypotonia, developmental delay, and seizures. Sequence variants in the leucine-rich repeat kinase 1 (LRRK1) gene underlying OSMD have been reported previously. In the present study, we investigated a 14-year-old girl suspected with OSMD in a consanguineous family of Iranian origin segregating the disease in an autosomal-recessive manner. The patient had severe short stature, multiple sclerotic lesions, sandwich vertebrae, Erlenmeyer flask deformity, and looser zones. The multifocal active bony pathology suggested multifocal bony inflammation or multiple looser fractures. Whole-exome sequencing followed by Sanger sequencing confirmation revealed a novel homozygous stop gain mutation (c.G2785T, p.E929X) in the LRRK1 gene. This is the first mutation in the LRRK1 gene, underlying OSMD, in the Iranian population and the third case worldwide. The mutation is located in the C terminal of the Roc domain, distinct from domains affected in the previous two LRRK1 mutations. Additionally, a new group of clinical indications different from the two previous cases is discussed.
1 INTRODUCTION
Osteosclerotic metaphyseal dysplasia (OSMD; OMIM 615198) is a rare disease that was first described by Nishimura and Kozlowski in 1993 (Nishimura & Kozlowski, 1993). The main clinical characteristic of such sclerosing bone disorder is skeletal dysplasia and, in some cases, associated with hypotonia, developmental delay, and seizures. Radiographic illustrations show that osseous changes are an inseparable feature of this disease. Osteosclerosis localizes predominantly to the metaphysis of the long bones, whereas the shafts are osteopenic. Sclerotic involvement of other skeletal structures such as epiphyses of the extremity long bones, margin of the pelvic bones, ends of the ribs and clavicles, vertebrae, talus, and calcaneus is common. Some studies have demonstrated that the skull may be spared (Guo et al., 2017; Iida et al., 2016; Kasapkara et al., 2012; Mennel & John, 2003; Nishimura & Kozlowski, 1993; Zheng, Cai, Wang, & He, 2015). Paraclinical findings that are seen in OSMD include increased amounts of alkaline phosphatase, creatine kinase, and aspartate aminotransferase in serum. Other laboratory manifestations show high levels of urinary pyridinoline and deoxypyridinoline (Kasapkara et al., 2012; Mennel & John, 2003; Zheng et al., 2015).
Only nine cases of OSMD have been studied and identified so far, all suggesting an inheritance pattern of for this autosomal-recessive disorder (Nishimura & Kozlowski, 1993). Iida et al. (2016) reported a homozygous LRRK1 frameshift deletion (c.5938_5944delGAGTGGT) in a Japanese OSMD patient, causing an elongated LRRK1 protein (p.E1980Afs*66) (Iida et al., 2016). The same research group has also reported the second case of LRRK1 mutation, a homozygous frameshift mutation (c.5971_5972insG) occurring in Indian siblings with OSMD. Similar to the first case, the mutation resulted in an elongated protein (p.A1991Gfs*31) (Guo et al., 2017).
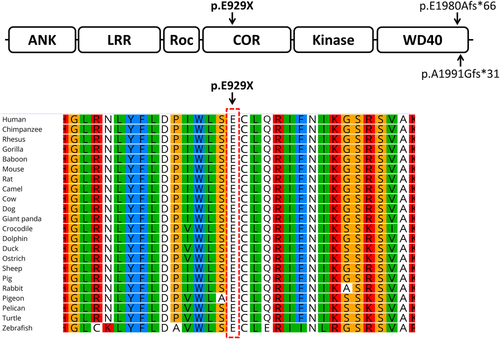
The LRRK1 (leucine-rich repeat kinase 1) gene (OMIM 610986) locates on chromosome 15q26.3 and contains 37 exons (Guo et al., 2017; Iida et al., 2016). The LRRK1 protein consists of several N-terminal ankyrin and leucine-rich repeats, a RAS-like GTPase domain (also known as a Roc domain), a C terminal of Roc (COR) domain, and a serine/threonine kinase domain followed by a number of C-terminal WD40 repeats (Figure 1) (Guo et al., 2017; Iida et al., 2016). Osteopetrosis in knockout mice lacking the LRRK1 gene provides an extra proof supporting the role of LRRK1 in negative regulation of bone mass (Xing et al., 2013).
In the current study, we report the third LRRK1 mutation, a novel homozygous stop gain mutation (c.G2785T, p.E929X), affecting the COR domain of the protein in an Iranian patient suspected with OSMD.
2 MATERIALS AND METHODS
2.1 Sampling and genomic DNA extraction
The informed consent was obtained from the family studied in this manuscript. Genomic DNA (gDNA) was then extracted from their peripheral blood using a standard protocol.
2.2 Whole-exome sequencing
Using the patient's gDNA, SureSelectXT Library Prep Kit, and Illumina HiSeq 4000 System, whole-exome sequencing (WES) was performed generating 101-bp paired-end reads (Macrogen, Seoul, Korea). WES data were then analyzed as described previously (Frans et al., 2017; Shirkani et al., 2017). BWA (Version 0.7.12) was used to map the short reads to the genome reference sequence (version b37). SAMtools (Version 1.3) was used to further process the BAM files (Li et al., 2009) and duplicated reads were marked using Picard (Version 2.6.0) (https://broadinstitute.github.io/picard). The Genome Analysis Toolkit (Version 3.6) was used for variant calling and filtration based on best practices (https://software.broadinstitute.org/gatk/best-practices). Our in-house annotation pipeline (ExoMiner) was employed to annotate, filter, and prioritize the called variants.
2.3 Sanger sequencing and in silico comparative studies
A pair of primers (forward: 5′-AGGACTTTGGCAAGAGTTACCA-3′; reverse: 5′-CAGACCCCAACTCCCTCTTTC-3′) was designed for amplification of target LRRK1 mutation using Geneious version 10.0.6. PCR conditions, purification of the PCR product and Sanger sequencing were performed based on standard protocols. To retrieve protein sequences, the NCBI blastp was employed (https://blast.ncbi.nlm.nih.gov). Geneious (Version 10.0.6) was used to perform multiple alignment.
3 RESULTS
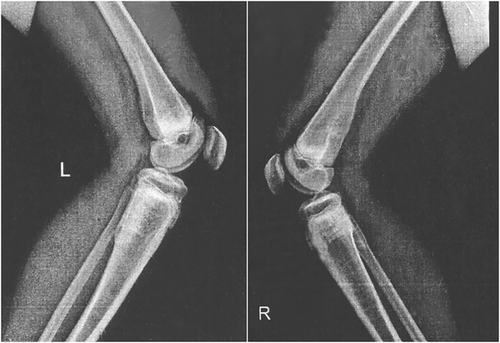
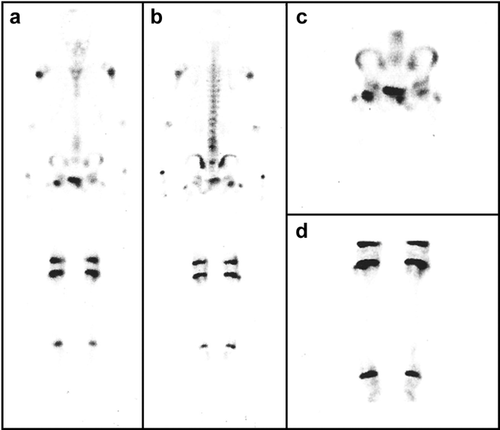
The proband is a 14-year-old girl from consanguineous healthy parents (first cousin marriage). She presented with bone pain, particularly abnormal gait, and lower limb pain. She suffered from severe short stature with problems walking. Additionally, she had a leg fracture at the age of 10. The patient was subjected to a magnetic resonance imaging scan. Perfusion, blood pool, static phase, and anteroposterior whole-body images were obtained using three-phase bone imaging by technetium-99m methylene diphosphonate. Multiple sclerotic lesions were observed, including osteosclerotic lesions in femur and spine, thickening of the cortex in the outer portion of the proximal femoral shaft associated with bone marrow edema, Erlenmeyer flask deformity, and looser zones as well as the sandwich vertebrae (Figure 1). The whole-body bone scan demonstrated an abnormal increased radiotracer activity in the upper part of the left humerus, L3-L4 spinal segment, right femoral head, upper third of the left femur, midshaft of the right femur, and lower part of both legs (Figure 2). This multifocal active bony pathology of the above-mentioned areas suggests multifocal bony inflammation or multiple looser fractures. Finally, increased density at the epiphyseal line of the distal radius and ulna was noticed.
All biochemical and serological tests such as complete blood count, blood glucose, creatinine, cholesterol, calcium, sodium, iron, phosphorous, and alkaline phosphatase were within the normal range, except for the C-reactive protein, which was high, indicating inflammation in the body.
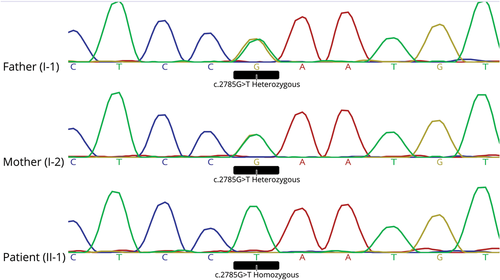
Using WES, a total read bases of 7,122,981,152 (bp) was obtained. In total, around 90,000 variants were detected. Allele frequency-based filtration resulted in about 1,500 variants. The homozygous variations (about 350) were prioritized mainly according to the severity of pathogenicity including splicing, stop gain, and frameshift mutations. Then, several amino acid change predictors were used to prioritize the pathogenic severity of nonsynonymous mutations. At the end, a homozygous stop gain mutation (c.G2785T, p.E929X) was detected in LRRK1, a gene related to skeletal disorders. The detected mutation was also fitting with the patient phenotype. Using Sanger sequencing, presence of the stop gain mutation was confirmed as homozygous in the patient and detected as heterozygous in her parents (Figure 3) as expected. Multiple sequence alignment of LRRK1 from very diverse organisms indicated that the glutamic acid 929 located in the COR domain of the LRRK1 protein is highly conserved. This is the first report of a pathogenic mutation in the COR domain of the LRRK1 protein. The two other previously reported mutations in LRRK1 were both located in the WD40 domain (Figure 4).
4 DISCUSSION
Using WES, a novel homozygous stop gain mutation (c.G2785T, p.E929X) in LRRK1 affecting the COR domain of the protein was detected in an OSMD suspected Iranian girl from a consanguineous family. To our knowledge, there have only been two OSMD-causing LRRK1 mutations reported so far, both affecting the WD40 domain of the LRRK1, which is different from the protein domain (COR) that is affected in our patient.
About 225 organisms have orthologs with human LRRK1. The glutamic acid number 929 of LRRK1's COR domain that is affected from the c.G2785T mutation and its adjacent amino acids are very conserved among various organisms ranging from higher animals to fish, birds, reptiles, etc.
The COR domain (Pfam: PF16095), along with the Roc, act as a putative regulator of LRRK1 kinase activity. It operates as a proper GTP-binding protein, possessing a low GTPase activity that can stimulate the kinase activity (Gotthardt, Weyand, Kortholt, Van Haastert, & Wittinghofer, 2008). In fact, Roc and COR domains are always in tandem and observed both in bacteria and eukaryotes.
Several mutations in Roc and COR-encoding regions of LRRK2, a paralogue of LRRK1, have been linked to Parkinson's disease (PD) (Daniëls et al., 2011; Janković et al., 2015; Purlyte et al., 2018), indicating the essential cellular functions of both genes’ products. Interestingly, Schulte and colleagues have suggested that several rare variants in LRRK1 are also associated with PD (Schulte et al., 2014). However, the LRRK1- and LRRK2-associated PD is mediated through different mechanisms. While the LRRK1-specific interactor is an epidermal growth factor receptor, LRRK2 specifically interacts with 14-3-3 proteins (Reyniers et al., 2014). The two proteins also differ in autophosphorylation and the phosphorylation ability of various substrates (Civiero et al., 2012). In addition, LRRK2 has an extra ARM domain and a very dissimilar WD40 domain, compared with LRRK1. Collectively, LRRK1 is functionally different from LRRK2 (Araki, Ito, & Tomita, 2018), which is consistent with their low amino acid sequence identity. For instance, the COR domain of LRRK1 and LRRK2 has less than 41% identity. Although the current study is the first report suggesting that the COR mutation is linked to a bone disorder, functional differences between LRRK1 and LRRK2 might collectively result in different pathogenicity of the mutated LRRK1 that potentially affects the bones while LRRK2 mutations seem to result in PD. Nevertheless, further studies are required for a better understanding of functional differences as well as similarities of the two proteins.
The first reported patient affected from LRRK1 mutation was suffering from hypotonia and psychomotor developmental delay (in addition to sclerosing bone disorder) (Iida et al., 2016), Those phenotypes were absent in our patient, as well as the second reported case of LRRK1 mutation (Guo et al., 2017). However, our patient has severe short stature, similar to the first case of LRRK1 mutation. Although those observations suggest the phenotypic heterogeneity of OSMD, identifying more patients will provide a more complete phenotypic spectrum of OSMD with LRRK1 mutation.
ACKNOWLEDGMENTS
We thank the proband and her family for their cooperation. A. Nikfar was supported by a fellowship from the Welfare Organization of Zanjan Province. Author contributions: S. Zarvandi, T. Bahrami, and M. Changi-Ashtiani analyzed WES data. M. Shahrooei, M. Miryounesi, H. Dinmohammadi, and M. Momenilandi recruited patients and managed clinical study protocol and sample collection. H. Rokni-Zadeh and T. Shahani planned the experimental steps and supervised the data analysis. M. Miryounesi and A. Nikfar wrote the manuscript. All authors revised the manuscript.
CONFLICTS OF INTEREST
All authors have confirmed the content of the manuscript and declared no conflict of interest.
INFORMED CONSENT
Informed consent was obtained from the family studied in this manuscript.




