Asp58Ala is the Predominant Mutation of the TTR Gene in Korean Patients with Hereditary Transthyretin-Related Amyloidosis
This study demonstrated the distinct phenotypic and genotypic spectra in Korean patents with hereditary ATTR.
Summary
Hereditary transthyretin (TTR)-related amyloidosis (ATTR) seems to be a rare autosomal-dominant inherited form of systemic amyloidosis. Studies indicate considerable heterogeneity in the disease's presentation and genotype; however, there is little data from Korea, where the prevalence of hereditary ATTR is very low. In this study, we investigated the phenotypic and genotypic spectra of hereditary ATTR in Korea. Direct sequencing analysis was performed to detect TTR gene mutations in amyloidosis patients whose results of TTR immunohistochemical staining were positive or equivocal. Clinical presentation was categorized as exclusively cardiac, exclusively neurologic, or mixed phenotype. Of 12 genetic tests performed, seven were positive for TTR mutations. D58A (c.173A>C) was the most common mutation in this study (57%, 4/7). The majority of those patients with hereditary ATTR had the mixed phenotype (86%, 6/7). The patients with D58A mutation had older ages of disease onset (median, 61 years vs. 42 years; P = 0.08), and a higher incidence of gastrointestinal involvement (75% vs. 0%; P = 0.03) than those with other identified TTR mutations. A significant male predominance was also noted in this study (P = 0.01).
Introduction
Amyloidosis comprises a large group of hereditary or acquired disorders caused by the extracellular deposition of amyloid fibrils composed of insoluble, misfolded proteins, which can impair tissue structure and the function in various organs (Ando et al., 2013). Transthyretin-related amyloidosis (ATTR) is a subtype of amyloidosis, composed of transthyretin (TTR), a plasma transport protein for thyroxine, and vitamin A (Ando et al., 2013). There are two distinct types of ATTR: hereditary and wild type. Hereditary ATTR is a relatively rare autosomal dominant disease resulting from the production of an unstable variant of TTR serum protein (Rapezzi et al., 2010). However, wild-type ATTR is a disorder that mainly affects elderly men, resulting from the deposition of wild-type TTR protein (Rapezzi et al., 2010).
Hereditary ATTR is known to be associated with mutations in the TTR gene. The TTR gene encodes the transthyretin protein and spans an approximately 7.3-kb region on Chromosome 18q21.1, where it consists of four exons (Benson & Kincaid, 2007). Currently, more than 120 TTR gene variants are listed in the TTR mutation database (http://amyloidosismutations.com/attr.html, last accessed on May 7, 2014). Hereditary ATTR is generally considered to be mainly a neurological disease, but its clinical spectrum varies heterogeneously from exclusively neurological involvement to a predominantly cardiac presentation (Rapezzi et al., 2010). This heterogeneity is linked to several factors including specific TTR mutations, patient and transmitting parent gender, geographical distribution and endemic/nonendemic aggregation (Ikeda et al., 2002; Bittencourt et al., 2005; Conceicao & De Carvalho, 2007).
In Korea, the prevalence of hereditary ATTR is unknown, but it is estimated to be lower than in Europe, at approximately one in 100,000 individuals. Currently, insufficient data are available on the spectrum of mutations in Korean patients. There are only two documented Korean cases of hereditary ATTR based on molecular genetic studies to date (Ryu et al., 2005; Cho et al., 2012). Our aim in this study was to investigate the phenotypic and genotypic spectra of hereditary ATTR in Korean patients.
Materials and Methods
Patients
Between January 2010 and April 2014, 110 patients were diagnosed with systemic amyloidosis at Samsung Medical Center (a tertiary referral hospital in South Korea). Amyloidosis was confirmed by tissue specimen showing apple-green birefringence under polarized light after Congo red stain. Among them, immunohistochemical staining was performed to determine the type of amyloidosis, and patients who had positive (n = 7) or equivocal (n = 5) results of TTR immunohistochemical staining were screened for TTR gene mutations by direct sequencing analysis involving all coding exons and flanking intronic sequences. The diagnosis of hereditary ATTR was confirmed by the identification of a TTR mutation. Family studies (clinical and genotype) were performed when applicable and feasible. Written informed consent was obtained before the genetic study from the patients and their family members. This study was approved by the Institutional Review Board of Samsung Medical Center (IRB approval 2013-04-087).
Clinical Data
We collected demographic and comprehensive clinical data including neurological and cardiological components, and other relevant findings. Organ involvement was investigated according to the consensus from the 10th International Symposium on Amyloid and Amyloidosis (Gertz et al., 2005). To assess neurological impairment, manifestations associated with autonomic neuropathy such as orthostatic hypotension, diarrhoea and constipation, urinary incontinence and sweating abnormalities were examined. Orthostatic hypotension was defined as at least a 20 mmHg fall in systolic blood pressure and/or a 10 mmHg fall in diastolic blood pressure within 2–5 min of quiet standing after a 5-min supine position rest (Freeman et al., 2011). Conduction abnormalities in the electrocardiography (ECG) included bundle branch block, atrioventricular block, or left anterior/posterior hemiblock. Low voltage in ECG was defined as a QRS voltage <0.5 mV in the limb leads or <1.0 mV in the precordial leads (Quarta et al., 2014). A restrictive left ventricular filling pattern in the ECG was defined in terms of a peak mitral inflow velocity at early diastole (E) to late diastolic mitral inflow velocity (A) ratio >1.5 and an E wave deceleration time <160 cm/s (Nagueh et al., 2009).
According to a previous study (Rapezzi et al., 2013), the clinical phenotype of patients with TTR mutations was divided into: (1) ‘cardiac phenotype’, based on echocardiographic and/or ECG evidence of cardiac amyloidosis in the absence of any signs or symptoms of neurologic involvement; (2) ‘neurologic phenotype’, based on clinical/instrumental evidence of neuropathy in the absence of any evidence of amyloidotic cardiomyopathy; and (3) ‘mixed (cardiac/neurologic) phenotype’.
Mutation Identification in the TTR Gene
Genomic DNA was extracted from peripheral blood leukocytes using the Wizard Genomic DNA Purification Kit following the standard protocols (Promega, Madison, WI, USA). Point mutations were detected by direct sequencing of all TTR coding exons and their flanking intronic regions on the ABI Prism 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using the BigDye Terminator cycle Sequencing Ready Reaction Kit (Applied Biosystems). Each primer set was designed by the authors (available upon request). The sequence chromatograms obtained were compared with the reference sequence of TTR, NM_000371.3. Identified mutations were described following the recommendations by the Human Genome Variation Society (http://www.hgvs.org/mutnomen/), along with the conventional numbering.
Statistical Analyses
Statistical analyses were performed using the χ2 test and Fisher's exact test for categorical variables, and the Mann–Whitney U test or one-way analysis of variance for continuous variables, as appropriate. A difference with a P value <0.05 was considered statistically significant. All statistical analyses were performed using the statistical Software Package for the Social Sciences (IBM SPSS Statistics 21; SPSS Inc., Chicago, IL, USA).
Results
Clinical Characteristics of Study Patients
Among 110 patients of the total amyloidosis cohort, 28 (26%) patients required further TTR gene study to determine the type of amyloidosis; seven patients with positive TTR stain and 21 patients with inconclusive immunohistochemical stain in their biopsy specimen. Among those 28 patients, 12 patients underwent TTR gene analysis in our retrospective cohort. Only the 7 patients with positive TTR stain showed TTR mutation, whereas TTR mutations were not detected in the five patients with inconclusive immunohistochemical stain. The major clinical characteristics of the seven Korean patients with hereditary ATTR are shown in Table 1 (cases TTR-01 to TTR-07). The median age of the first symptom onset was 48 years (range, 39–64 years), and the median time interval from the first symptom to diagnosis was 2 years (range 0–21 years). Among the patients with hereditary ATTR, five patients died at a median age of 64 (range 50–66), and two patients are still in follow-up.
| This study, total | Ryu et al., | Cho et al., | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TTR-01 | TTR-02 | TTR-03 | TTR-04 | TTR-05 | TTR-06 | TTR-07 | n/Na (%) | 2005 | 2012 | |
| Gender (male:female) | Female | Male | Male | Male | Male | Male | Male | (6:1) | Male | Male |
| TTR mutation | D38E | D58A | D58A | G67R | D58A | E109K | D58A | K55N | D58A | |
| First symptom | Oedema | Diarrhoea | Paraesthesia | Diarrhoea | Diarrhoea | Dyspnea | Diarrhoea | Diarrhoea | Diarrhoea | |
| Age of symptom onset, years (median) | 48 | 64 | 58 | 42 | 63 | 39 | 47 | (48) | 32 | 51 |
| At diagnosis | 48 | 66 | 63 | 63 | 65 | 39 | 51 | (63) | NA | NA |
| At last follow-up | 50 | 70 | 65 | 64 | 66 | 41 | 52 | (64) | NA | NA |
| Survival at last follow-up | Dead | Alive | Dead | Dead | Dead | Alive | Dead | NA | NA | |
| Autonomic involvement | − | + | + | + | + | + | + | 6/7 (86) | + | + |
| Orthostatic hypotension | − | + | + | + | + | + | + | 6/7 (86) | + | + |
| Diarrhoea | − | + | + | + | + | − | + | 5/7 (71) | + | + |
| Constipation | − | − | + | − | − | − | − | 1/7 (14) | + | − |
| Urinary incontinence | − | − | + | − | − | + | − | 2/7 (29) | + | − |
| Sweating abnormalities | − | − | + | − | − | − | − | 1/7 (14) | NA | − |
| Sensorimotor polyneuropathy | − | − | + | + | + | + | + | 5/7 (71) | + | + |
| Carpal tunnel syndrome | − | + | − | − | − | − | − | 1/7 (14) | NA | + |
| Cardiac involvement | + | + | + | + | + | + | + | 7/7 (100) | − | + |
| ECG | − | |||||||||
| Conduction disturbance | + | + | − | − | + | + | + | 5/7 (71) | − | + |
| Low voltage | + | + | − | + | − | + | + | 5/7 (71) | − | − |
| Echocardiographic findings | ||||||||||
| IVS thickness (mm) | 13 | 14 | 15 | 12 | 23 | 18 | 26 | NA | NA | |
| Restrictive LV filling pattern | + | + | − | − | − | + | + | 4/7 (57) | − | − |
| Ejection fraction (%) | 42 | 58 | 42 | 56 | 53 | 47 | 43 | NA | NA | |
| Classificationb | Cardiac | Mixed | Mixed | Mixed | Mixed | Mixed | Mixed | Neurologic | Mixed | |
| Organ involvement | H | H, N, G | H, N | H, N | H, N, G | H, N, F | H, N, G | N | H, G |
- Abbreviations: NA, not available; H, heart; G, gastrointestinal; N, nerve; F, fat tissue; ECG, electrocardiogram; IVS, interventricular septum.
- a n/N indicate the positive numbers/total numbers of subjects.
- b Clinical phenotype at presentation. ‘Cardiac’, based on echocardiographic and/or ECG evidence of cardiac amyloidosis in the absence of any sign or symptom of neurologic involvement; ‘neurologic’, based on clinical/instrumental evidence of neuropathy in the absence of any echocardiographic, ECG, or clinical evidence of amyloidotic cardiomyopathy; ‘mixed’ (cardiac/neurologic).
Six patients were male, showing a male predominance (a male-to-female ratio of 6:1). At the time of diagnosis, six hereditary ATTR patients showed a mixed phenotype (86%, 6/7; cases TTR-02, -03, -04, -05, -06 and -07), and one had a cardiac phenotype (14%, 1/7; case TTR-01). All the patients showed autonomic dysfunction except for the individual with a cardiac phenotype (case TTR-01). Among the manifestations associated with autonomic impairment, orthostatic hypotension was the most common symptom (86%, 6/7), followed by diarrhoea (71%, 5/7). In patients with neurologic involvement, sensorimotor polyneuropathy was also frequently seen (71%, 5/7). Only one patient had a history of carpal tunnel syndrome (14%, 1/7). However, because seven patients with peripheral neuropathy visited the hospital only after significant sensorimotor polyneuropathy had developed, the true incidence of carpal tunnel syndrome could not be identified. All of the patients had cardiac involvement (100%, 7/7), and the LV ejection fraction was mildly decreased with a mean value of 48.7% ± 6.9%. Conduction abnormalities and low voltage on the ECG were observed in 71% (5/7) of the patients, respectively. A restrictive filling pattern in echocardiographies was observed in 57% (4/7) of the patients.
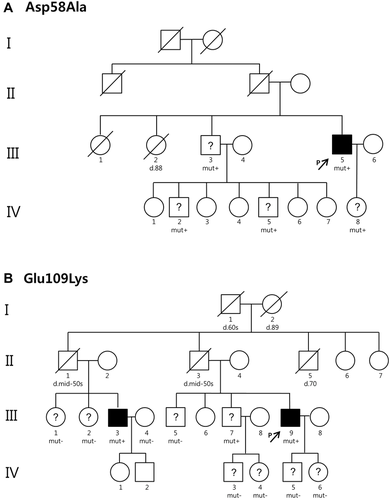
Of the seven patients with hereditary ATTR, a genetic study of family members was performed in only two families (cases TTR-02 and TTR-06), and the pedigrees are shown in Figure 1. In the family history of TTR-02 patient with D58A, all the family members (III-3, IV-2, IV-5 and IV-8) who underwent TTR genetic testing were heterozygous for the D58A mutation (Figure 1A). Unfortunately, information on their clinical phenotype was unavailable. In the family of TTR-06 patient with mutation E109K, his first paternal uncle (II-1) and father (II-3) had died in their mid-50s of unknown causes (Figure 1B). His 49-year-old first nephew (III-3) was diagnosed with amyloidosis by an abdominal fat tissue biopsy. He (III-3) was also confirmed as a case of cardiac amyloidosis with sensorimotor polyneuropathy and autonomic dysfunction.
TTR Mutation Analysis
The mutational analyses of seven Korean patients with hereditary ATTR are summarized in Table 2. Four mutations were identified in the sequence analysis; D38E, D58A, G67R and E109K. All mutations are heterozygous and known mutations. The D58A (c.173A>C) was recurrent and was observed in four patients (57%, 4/7). Three of the identified mutations were found in exon 2; the E109K mutation was located in exon 3.
| Exon | Nucleotide change | Amino acid change | Patients, n/Na (%) | Mutation type | Reference |
|---|---|---|---|---|---|
| 2 | c.114T>G | p.D38E | 1/7 (14) | Known, missense | (Connors et al., 2004) |
| 2 | c.173A>C | p.D58A | 4/7 (57) | Known, missense | (Kishikawa et al., 1999, Tachibana et al., 1999, Yazak et al., 2000, Yazaki et al., 2000) |
| 2 | c.199G>A | p.G67R | 1/7 (14) | Known, missense | (Murakami et al., 1992, Ferlini et al., 2000) |
| 3 | c.325G>A | p.E109K | 1/7 (14) | Known, missense | (Nakamura et al., 2000) |
- Abbreviations: ATTR, transthyretin-related amyloidosis
- a n/N indicate the positive numbers/total numbers of subjects.
Genotype–Phenotype Correlations
We compared demographical and clinical features between the D58A mutation and other mutations including D38E, G67R and E109K (Table 3), because D58A was identified as a recurrent mutation in this study. A comparison of the different ethnic groups of D58A was also performed (Table 3). In comparison with other mutations, Korean patients with D58A mutation showed a later onset of the disease (median age, 61 years vs. 42 years; P = 0.08), and a higher incidence of gastrointestinal involvement (75% vs. 0%, P = 0.03). All reported Japanese patients with D58A were female, but the Korean population showed a male predominance (P = 0.01). Other clinical features between the groups did not show any significant differences.
| D58A | Other | D58A | |||
|---|---|---|---|---|---|
| Variable | n = 4 (%) | mutationsa, n = 3 (%) | n = 3 (%) | P valueb | P valuec |
| Ethnicity | Korean | Korean | Japanese | ||
| Male | 4 (100) | 2 (67) | 0 (0) | 0.21 | 0.01 |
| Age of onset | |||||
| Median, years (range) | 61 (47–64) | 42 (39–48) | 61 (48–78) | 0.08 | 0.86 |
| Older than 60 | 2 (50) | 0 (0) | 2 (67) | 0.43 | 1.00 |
| Neurologic involvement | 4 (100) | 2 (67) | 3 (100) | 0.43 | 1.00 |
| Cardiac involvement | 4 (100) | 3 (100) | 3 (100) | NA | NA |
| Gut involvement | 3 (75) | 0 (0) | 3 (100) | 0.03 | NA |
| Clinical classificationd | |||||
| Cardiac | 0 (0) | 1 (33) | 0 (0) | 0.43 | 1.00 |
| Mixed | 4 (100) | 2 (67) | 3 (100) | 0.43 | 1.00 |
| Neurologic | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| References | This study | This study | (Kishikawa et al., 1999, Tachibana et al., 1999, Yazak et al., 2000) |
- Abbreviations: NA, not applicable.
- a Other mutations included: D38E, G67R and E109K.
- b D58A versus other mutation in Koreans.
- c D58A between Koreans and Japanese.
- d Clinical phenotype at presentation. Cardiac, exclusively cardiac; Neurologic, exclusively neurologic; mixed, cardiac and neurologic
- P values for the comparison between groups were calculated using the χ2 test and Fisher's exact test for categorical variables and the Mann–Whitney U test for continuous variables, as appropriate. Significant P values are shown in bold.
Discussion
This study is the first investigation into the phenotypic characteristics and molecular genetics of hereditary ATTR in Korea. Based on a comprehensive review of this study and previous Korean reports (Cho et al., 2012, Ryu et al., 2005), the majority of the Korean patients with hereditary ATTR had a mixed (cardiac/neurologic) phenotype (78%, 7/9; Table 1). Although the number of hereditary ATTR patients identified in this study was limited, the data supported the finding that the D58A genetic variant is a predominant mutation of the TTR gene among Koreans (56%, 5/9; Table 1).
The clinical phenotype associated with hereditary ATTR is quite variable from an exclusively neurological involvement to a predominantly cardiac presentation (Bittencourt et al., 2005; Conceicao & De Carvalho, 2007; Rapezzi et al., 2006). The most studied TTR mutation is V50M (valine-to-methionine at amino acid position 50, previously described as V30M), which causes familial amyloid polyneuropathy type 1 (Coelho et al., 2013). The characteristic presentation of V50M mutation includes prominent symptoms of sensorimotor polyneuropathy and autonomic neuropathy, but signs of cardiomyopathy are rarely observed (Rapezzi et al., 2010). On the contrary, several TTR mutations, such as V142I, T80A, L131M and I68L, have been shown to be responsible for a predominantly cardiac phenotype (Rapezzi et al., 2013). In this study, hereditary ATTR patients predominantly showed a mixed (cardiac/neurologic) phenotype. This heterogeneity might be explained by the difference in ethnic background, geographical distribution and specific TTR mutation. Carpal tunnel syndrome is not a frequent clinical manifestation in this study (14%, 1/7). Our finding is contrary to that of a previous study which found that carpal tunnel syndrome occurred frequently and may occasionally be the only clinical manifestation of hereditary ATTR (Tojo et al., 2010; Koyama et al., 2012). However, this result needs to be interpreted cautiously considering that a large portion of the patients in this study visited the hospital only after a significant sensorimotor polyneuropathy had developed; the presence of carpal tunnel syndrome might be masked due to the presence of severe sensorimotor neuropathy. Hereditary ATTR is a rare autosomal dominant disease with variable penetrance in different regions of the world and among families (Munar-Ques et al., 1999; Ando et al., 2013). Because penetrance is incomplete, carriers of the gene may live to an advanced age without symptoms of the disease. In our study, however, clinical information was insufficient to derive a conclusion for the incomplete penetrance and interfamilial differences, although family screening for several members of two kindreds were performed.
We identified four mutant alleles of TTR from the seven Korean patients with hereditary ATTR (Table 2). One known missense mutation, D58A, was recurrent, and accounted for 57% of the mutations (4/7). It is conceivable that D58A is a founder mutation in Korea. The remaining three different mutations were also missense; D38E, G67R and E109K. This is in accordance with previous studies on TTR mutations which found that the mutations are mostly missense or nonsense. Our data also showed that exon 2 is the mutation hotspot in TTR because almost all of the identified mutations were observed there, except for one (E109K).
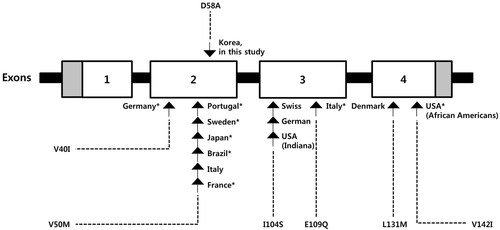
A noteworthy characteristic of the mutational spectrum of the Korean TTR gene is the absence of mutations that are common in the endemic and nonendemic regions. Compared to previous studies of hereditary ATTR in several ethnic and geographical regions (Benson & Dwulet, 1985; Jacobson et al., 1997; Zolyomi et al., 1998; Palacios et al., 1999; Svendsen et al., 1999; Ikeda et al., 2002; Suhr et al., 2003; Conceicao & De Carvalho, 2007; Coelho et al., 2013; Rapezzi et al., 2013), there were remarkable differences in mutation spectrum of the TTR gene in patients from Korea (Figure 2). In each of the main reported geographical foci, such as Portugal (Conceicao & De Carvalho, 2007), Sweden (Suhr et al., 2003) and Japan (particularly in the districts of Arao city and Ogawa village; Ikeda et al., 2002), V50M is the most prevalent mutation in the classical type of ATTR. The V50M mutation was also identified in all Brazilian and 25% of Italian patients with ATTR (Palacios et al., 1999; Rapezzi et al., 2013). The V142I mutation, where isoleucine substitutes for valine at position 142 is one of the most common variants, and it causes late onset cardiac amyloidosis in patients of African American origin (Jacobson et al., 1997). Other mutations associated with hereditary ATTR have been reported, such as I104S in Swiss, German and Indian patients and L131M in Denmark (Benson & Dwulet, 1985; Zolyomi et al., 1998; Svendsen et al., 1999). Although V50M and V142I are the most frequently identified mutations worldwide, no such mutations were detected in this study. Notably, the D58A variant is the predominant mutation in Korean patients, supporting that the mutation spectrum of the TTR gene is highly heterogeneous and varies among populations.
The D58A mutation was previously described in Japanese patients with ATTR (Kishikawa et al., 1999; Tachibana et al., 1999; Yazak et al., 2000a, b). According to the published cases, the D58A mutation is clinically characterized by progressive cardiac impairments, and peripheral and autonomic neuropathy (Kishikawa et al., 1999; Tachibana et al., 1999; Yazak et al., 2000a, 2000b). When compared to Japanese patients with D58A, Korean subjects exhibited a higher male predominance. Although all the relevant patients were male in our study, Japanese cases report that all their subjects were female (P = 0.01). Our other clinical findings were similar to those observed in Japanese patients, that Korean patients also have both amyloid cardiomyopathy and neuropathy accompanied by gastrointestinal involvement. Further accumulation of data from continuous studies is needed to clarify this point.
Our study has the limitation that not all of the patients with equivocal immunohistochemical stain underwent TTR gene analysis. However, based on our results, TTR mutation was detected only in the patients who presented positive TTR stain in their specimen. Those patients who showed inconclusive results of pathologic immunohistochemical stain may belong to the other rare type of amyloidosis resulting from factors such as apolipoprotein AI, fibrinogen, or lysozyme (Kebbel & Rocken, 2006).
Taken together, the results from our work support the hypothesis that the mutation spectrum of the TTR gene is highly heterogeneous and varies between populations. Notably, D58A was the predominant causative mutation of hereditary ATTR in Korea.
Acknowledgement
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2013-E63011-11).
Conflict of Interest
The authors have no conflicts of interest to declare.




