Origin and Distribution of the Brachial Plexus Nerves in Northern Tamandua (Tamandua mexicana)
Funding: The authors received no specific funding for this work.
ABSTRACT
Tamandua mexicana is a species of the superorder Xenarthra that is found in many regions of Mexico, Peru, Colombia, Ecuador and Venezuela. This species is important because it is a model for comparative studies of the evolutionary anatomy of xenarthrans. However, there are few anatomical studies on which to base these areas, such as the anatomy of the brachial plexus. Several studies use the brachial plexus anatomy for phylogenetic analysis and medical procedures. Thus, this study aimed to describe the origin and distribution of the brachial plexus in T. mexicana. Twelve specimens of T. mexicana fixed in formaldehyde 4% were dissected. The ventral spinal nerves from C5 to T2 originated the brachial plexus. In most cases, C5–C7 formed the cranial trunk, C6–C7 the middle trunk and C8–T1 the caudal trunk. In all specimens, these trunks joined and formed the common trunk, which distally divided into two divisions: dorsal and ventral. The pectoralis cranialis, pectoralis caudalis, thoracicus longus, thoracicus lateralis and thoracodorsalis nerves supplied the extrinsic thoracic limb muscles. The innervation for the intrinsic thoracic limb muscles was supplied by the suprascapularis, subscapulares, axillaris, musculocutaneus, radialis, medianus and ulnaris nerves. The intercostobrachialis, axillaris, radialis, ulnaris, medianus and caudal cutaneous antebrachial nerves innervated the skin of this species. The trunks and divisions were similar comparatively to those reported in other xenarthrans. However, T. mexicana was more similar to sloths. This suggests a phylogenetic trade in their evolution. The origin and distribution of the brachial plexus nerves resembled those of the other Xenarthras, which can assist in medical procedures within the superorder.
1 Introduction
The superorder Xenarthra is one of the oldest Eutherian clades, which originated in South America about 100 million years ago (Delsuc et al. 2004). Several studies classify this superorder as having 31 species. It includes 21 armadillos, six sloths and four anteaters (Abba et al. 2012). It includes the Tamandua mexicana, or Northern Tamandua (Delsuc et al. 2004). This species is part of the order Pilosa, suborder Vermilingua and family Myrmecophagidae (Rojano et al. 2014). This species ranges widely; it extends from southern Mexico to northeastern Peru, Colombia, Ecuador and Venezuela (Nuñez-Perez et al. 2011). They are territorial mammals and live at altitudes of 0 to 1200 m above sea level (Cuervo et al. 1986).
T. mexicana has a diet based on insects, mainly ants and termites (Superina et al. 2010). They forage in forests and savannahs because of this type of feeding (Toledo et al. 2015); for this reason, this species can move through both arboreal and terrestrial substrates, developing a semi-arboreal locomotion (Englemann 1985; Toledo et al. 2013). This type of locomotion gives this species various anatomical features, such as a prehensile tail (Lubin and Montgomery 1981; Navarrete and Ortega 2011) and some changes in the thoracic limb. The scapula has two spines (Vélez-García et al. 2020), and the third phalanx is more developed (Taylor 1978). The humerus has developed bony reliefs for broad muscle origins and insertions to allow for stronger and more stable movements (Heredia-Díaz et al. 2022). The craniolateral muscles of the forearm are developed, causing constant semisupination (Polania-Guzmán and Vélez-García 2019; Taylor 1978). Also, the extensor muscles of the elbow are more massive than those of the brachium (Torres Suárez et al. 2024), and the flexor digitorum profundus muscle is more developed to be able to carry out species activities such as removing bark from trees (Vélez-García et al. 2021). These adaptations let them perform daily tasks, such as separating hard materials for feeding, defending themselves and digging for ants. The male can also adjust the female's position during mating (Matlaga 2006).
The brachial plexus is a nervous structure formed from the ventral spinal nerves; these nerves innervate the skin and muscles of the forelimb (Dyce et al. 2012). Researchers can use the study of its anatomy for evolutionary purposes (Adami et al. 2013). It is also useful for phylogeny (Figueredo-da-Silva et al. 2021) and its key, the anatomy of this, to perform medical interventions, such as locoregional blocks and surgeries (Barbosa et al. 2024; Silva et al. 2024). Moreover, T. mexicana is a species often found in wildlife rehab centres due to habitat destruction, illegal trade, roadkill and dog fights (Ortega-Reyes et al. 2014). This makes this species need medical-surgical interventions where the basis is the anatomy. Despite its importance in veterinary medicine and evolution, no studies exist on the brachial plexus in T. mexicana. Therefore, the objective is to describe the macroscopic anatomy of the brachial plexus in T. mexicana.
2 Materials and Methods
2.1 Animals
Twenty-four brachial plexuses were used from 12 specimens of T. mexicana, which died of causes other than those of the study. None of the donated specimens had brachial plexus or musculoskeletal system disorders. They were donated by the Centro de Atención y Valoración de Animales Silvestres of the Corporación Autónoma Regional del Tolima (CAV CORTOLIMA) between 2015 and 2021. Eight of these specimens were used in other studies (Heredia-Díaz et al. 2022; Polania-Guzmán and Vélez-García 2019; Torres Suárez et al. 2024; Vélez-García et al. 2020, 2021). This study was approved by the ethics committees for the use of animals (FMVZ) of the University of São Paulo, under the number 7312240820, and the University of Tolima.
2.2 Fixation and Dissection
The specimens were fixed by subcutaneous, intramuscular and cavitary infiltrations with a 4% aqueous solution of formaldehyde and 5% glycerine. Subsequently, they were immersed in 4% formaldehyde aqueous solutions for a minimum period of 48 h prior to dissection.
The two thoracic limbs of each specimen were dissected from the superficial to the deep plane with dissecting instruments. Photographs were made with a camera (Canon Power Shot Alph 150 ISO, 18 MP). The names of the structures were determined according to the terminology of the Nomina Anatomica Veterinaria (ICVGAN 2017).
To dissect the brachial plexus, a longitudinal cut was made at the level of the ventral midline from the umbilical region to the laryngeal region, joined by two transverse incisions to reach the dorsal midline, where it was also incised to begin the removal of the skin from the dorsal to the distal part of the hand. Next, to reach the brachial plexus, the pectoral muscles were removed. Once the origin of the brachial plexus was exposed, the ventral branches forming the brachial plexus and its nerves were described. Finally, the distribution of each nerve along the thoracic limb was dissected and identified.
2.3 Limitations of the Study
The distribution of the ulnaris and medianus nerves at the palmar level was not described because these nerves were damaged by the removal of the palmar pad.
3 Results
3.1 Anatomy of the Brachial Plexus in the Tamandua mexicana
3.1.1 Origin and Structure of the Brachial Plexus
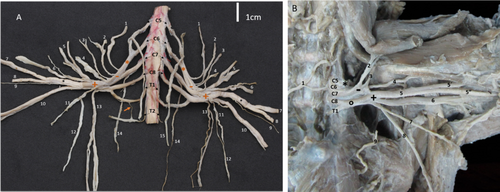
The origin of the brachial plexus in 91.7% of cases was at the level of the ventral spinal nerves from C5 to T1. In the 8.3%, there was a contribution from T2. Three trunks forming the brachial plexus were found: cranial, middle and caudal trunks (Figures 1, 2A). C5, C6 and C7 in 83.3% formed the cranial trunk, and C5 and C6 in the remaining 16.6%. C6 and C7 formed the middle trunk in 100% of the cases. C8 and T1 formed the caudal trunk in 75.1% of the plexus; in 16.6%, there was a contribution from C7 and in 8.3% from T2 (Figure 2A). Once the trunks formed, they joined to form a common trunk (Figure 2A). From it, two divisions emerged: one dorsal and one ventral, from which the nerves of the brachial plexus were formed.
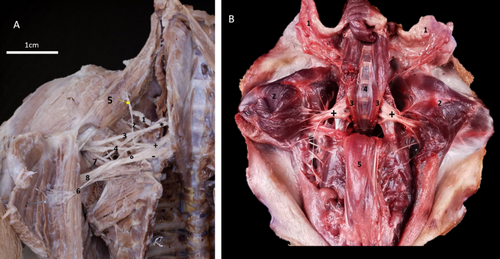
The nerves supplying the extrinsic muscles are divided into cranialis and pectoralis caudalis nerves, thoracicus longus nerve, thoracicus lateralis nerve and thoracodorsalis nerve (Figure 2B). The nerves for the intrinsic muscles of the thoracic limb, namely: suprascapularis nerve, cranialis and caudal subscapularis nerves, axillaris nerve, musculocutaneus nerve, radialis nerve, medianus nerve and ulnaris nerve (Figure 3A,B, Table 1).
| Nerves | Origin | Absolute frequency | Frequency percentage |
|---|---|---|---|
| Suprascapularis | C5 e C6 | 12 | 100 |
| Subscapularis cranialis | C5 e C6 | 12 | 100 |
| Subscapularis caudalis | C5 e C6 | 12 | 100 |
| Pectoral cranialis | C5, C6, C7 | 6 | 50 |
| C5, C6 | 4 | 33,3 | |
| C5–C8 | 2 | 16.6 | |
| Pectoral caudalis | C5, C6, C7 | 6 | 50 |
| C6, C7 | 2 | 16,6 | |
| C7, C8 | 2 | 16,6 | |
| C7, C8, T1 | 2 | 16,6 | |
| Musculocutaneus | C5–C7 | 6 | 50 |
| C5, C6 | 2 | 16,6 | |
| C6–C7 | 2 | 16,6 | |
| C5–T1 | 2 | 16,6 | |
| Medianus | C5–T1 | 11 | 91.7 |
| C5–T2 | 1 | 8.3 | |
| Ulnaris | C5–T1 | 11 | 91.7 |
| C5–T2 | 1 | 8.3 | |
| Radialis | C5–T1 | 11 | 91.7 |
| C5–T2 | 1 | 8.3 | |
| Axillaris | C5, C6, C7 | 4 | 33,3 |
| C5, C6 | 4 | 33,3 | |
| C6, C7 | 2 | 16,6 | |
| C7 | 2 | 16,6 | |
| Thoracodorsalis | C7, C8, T1 | 12 | 100 |
| Cutaneus antebrachii caudalis | C8, T1 | 8 | 66,6 |
| C7–T1 | 4 | 33,3 | |
| Thoracicus longus | C7 | 12 | 100 |
| Thoracicus lateralis | C5–C7 | 6 | 33,3 |
| C7–C8 | 2 | 16,6 | |
| C7 | 2 | 16,6 | |
| C7–T1 | 2 | 16,6 |
- Abbreviations: C, Nerve branch originating in the cervical vertebrae; T, Nerve branch originating in the thoracic vertebrae.
3.1.2 Origin and Distribution of the Brachial Plexus Nerves That Innervate the Intrinsic Muscles of the Thoracic Limb
3.1.2.1 Suprascapularis Nerve
The origin of the suprascapularis nerve was between C5 and C6 in 100% of the plexuses studied, originating directly from the cranial trunk (Figures 1A and 3A). The nerve pathway is in the scapular region. It runs cranially to the subscapularis and supraspinatus muscles and proximally to the neck of the scapula. It innervates the supraspinatus muscle via a muscle branch. Finally, it passes through the supraspinatus muscle and by the foramen of the suprascapular nerve of the scapula to supply the infraspinatus muscle (Tables 1 and 2).
| Nerves | Innervated muscles |
|---|---|
| Suprascapularis | Supraspinatus and infraspinatus |
| Subscapulares (cranialis and caudalis) | Subscapularis |
|
Axillaris Cutaneus brachii lateralis and cutaneus antebrachii cranialis |
Teres major, teres minor and deltoideus (Pars scapularis and acromialis) Skin |
|
Musculocutaneus |
Coracobrachialis, biceps brachii (Capita breve and longum) and brachialis |
|
Radialis Ramus superficialis Ramus lateralis Digitales dorsalis communes II et IV Ramus medialis Digitales dorsalis commun I Ramus profundus |
Triceps brachii (Capita longum, longum accesorium, laterale and accessorium), anconeus lateralis, anconeus medialis and tensor fasciae antebrachii. Skin Dorsal structures of the hand (fingers II-IV) Axial and abaxial surface of the finger I Brachioradialis, extensor digitorum communis, extensor digitorum lateralis, extensor carpi radialis, extensor carpi ulnaris, supinator, abductor digiti I longus, extensor digiti I et II and extensor digiti III et IV muscles. |
| Medianus | Palmaris longus, flexor digitorum superficialis, flexor carpi ulnaris, epicondylar humeral head of the flexor digitorum profundus, interflexorius and pronator quadratus muscles |
| Ulnaris | Capitis Anconeus medialis and humerale brachialis of the flexor digitorum profundus muscles |
| Cutaneus antebrachialis caudalis | Caudal skin of the forearm |
3.1.2.2 Subscapulares Nerves
Two subscapular nerves were found. Their positions led to naming them subscapularis cranialis and subscapularis caudalis. Both arose from the dorsal division of the brachial plexus, with contributions from C5 and C6 (Figures 1A and 3A, Table 1). They were limited to the axillary region. They innervated the pars cranialis and caudalis of the subscapularis muscle (Figure 3B).
3.1.2.3 Axillaris Nerve
The axillaris nerve originated in the dorsal division. This nerve had four origins. The most common were C5–C7 and C5–C6. Some were at C6 and C7, and only at C7 (Table 1). It passed through the axillary region (Figure 3B) and moved towards the subscapular muscle on the ventral side. It continues to the neck of the humerus, where it travelled medially to innervate the teres minor muscle. Its course continues between the teres minor muscle, caput laterale, caput longum and caput accesorius of the triceps brachii muscle. At the lateral level of the humeral joint, it sent out two muscular branches innervating the two parts of the deltoideus muscle (Figure 3B, Table 2). Finally, it passes between the brachiocephalicus and the pars acromialis of the deltoideus muscles. It then gives rise to the cutaneus brachialis cranialis laterale nerve (Figure 4C). This nerve was at the origin and the most proximal part of the brachioradialis muscle, at the cranial level of the deltoid tuberosity. It continues down the forearm as the cutaneus cranialis antebrachii nerve, which runs along the craniomedial aspect of the arm to the proximal third of the forearm at the medial level of the brachioradialis muscle (Figure 4C). These nerves innervate the skin in these regions.
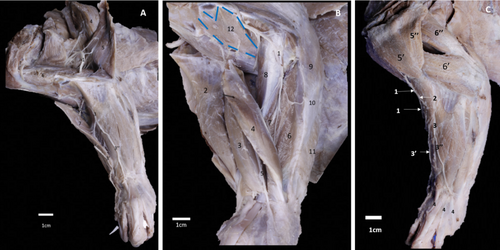
3.1.2.4 Musculocutaneus Nerve
It originated in the ventral division of the common trunk. In 50% of the dissected plexuses, it originated at the level of C5–C7; in 16.6%, it was formed by branches from C5 to T1; in 16.6%, it came from C6 and C7; and in the remaining 16.6%, it originated from C5 and C6 (Figures 2, 3A, 5, Table 1). This nerve passed caudally to the axillaris nerve and cranially to the common trunk for the medianus and ulnaris nerves (Figures 3A and 5). It reached the middle third of the caput brevis of the biceps brachii and coracobrachialis muscles, where it divided into three thinner branches for its innervation. The main branch continued deep to the caput longum of the biceps brachii muscle at the mid-arm and supplied the brachialis muscle, where it ended (Table 2).

3.1.2.5 Radialis Nerve
It originated from the dorsal division of the common trunk, through contributions from C5 to T1 in 91.7% and C5–T2 in 8.3% of the plexuses studied (Figure 1A, Table 1). It was located laterally to the common trunk for the medianus and ulnaris nerves. It passed at the mid-arm level, through the brachial groove of the humerus. It reached the lateral aspect of the brachial region, where it sent branches to the triceps brachii, anconeus lateralis, tensor fasciae antebrachii muscles and proximal end of the anconeus medialis muscle. After giving rise to these muscle branches, it continued along the distal third of the humerus and perforated the brachioradialis muscle but did not innervate it. It then divided into a ramus superficialis, which passed laterally to the forearm, between the brachioradialis and extensor carpi radialis muscles (Figures 4A and 5C). It divided into a ramus medialis and a ramus laterale. The latter passed through the craniolateral region of the forearm; at the level of the carpus, it gave rise to the dorsal common digital nerves. The ramus medialis reached the carpus by passing over the brachioradialis muscle on the forearm's cranial aspect. It then continued on the hand as the digitales dorsales communes (Figures 4A,C and 5C).
The deep branch of the radialis nerve originated in the forearm, above the elbow. It sent a branch to innervate the extensor carpi radialis and brachioradialis muscles. It continued laterally on the forearm and passed deep to the supinator muscle and supplied the extensor digitorum communis muscles. It then went over the extensor carpi ulnaris and abductor digiti I longus muscles (Figure 4B). At this level, it sent muscular branches to innervate all the craniolateral muscles, including the extensor digitorum communis, extensor digitorum laterale, extensor carpi ulnaris, extensor digiti III et IV, supinator, abductor digiti I longus and extensor digiti I et II.
3.1.2.6 Medianus Nerve
It originated from the ventral division, via a common trunk with the ulnaris nerve. This trunk was formed by contributions from C5 to T1 in 91.7% and 8.3% of the dissected plexuses (Figures 1A and 2, Table 1). This trunk divides at the proximal third of the arm, over the tendon of the latissimus dorsi. It gives rise to two nerves. The medianus nerve passed cranially to the ulnar nerve and brachial artery, reached the caudomedial part of the forearm, at the level of the distal coracobrachialis muscle. It then crossed the supracondylar foramen with the brachialis artery and vein and reached the proximal third of the forearm, where it sent a small branch to innervate the palmaris longus muscle. Distally, it innervated the caput humerale and caput ulnaris of the flexor carpi ulnaris muscle and the flexor digitorum superficialis. The main branch of this nerve continued through the craniomedial forearm. It innervated the pronator teres muscle (Figure 5). Finally, it continued to the caput humeral epicondilaris of the flexor digitorum profundus muscle and the interflexorius and pronator quadratus muscles to innervate them (Table 2).
3.1.2.7 Ulnaris Nerve
It originated in the ventral division, through a common trunk with the medianus nerve. Contributions from C5 to T1 formed this trunk in 91.7% of the cases and C5–T2 in 8.3% of the dissected plexuses (Figures 1A and 2, Table 1). This nerve passed caudally to the medianus nerve and brachialis artery, reached the caudal margin of the coracobrachialis muscle, and cranially to the tensor fasciae antebrachii muscle. It sent three muscular branches to innervate the caput humerale brachialis of the flexor digitorum profundus muscle and continued along the medial aspect of the olecranon, covered by the anconeus medialis muscle, which innervated its distal end through a muscular branch. It continued its course along the forearm in a caudomedial direction, penetrating the flexor digitorum superficialis and flexor carpi ulnaris muscles, reaching the caput humerale brachialis of the flexor digitorum profundus muscle (Figure 5). Then, it sent muscle branches to innervate the caput ulnare, the caput humerale brachialis and the caput humerale lateralis supracondylaris lateralis of the flexor digitorum profundus. It also innervated the flexor carpi ulnaris muscle (Table 2).
3.2 Nerves That Innervate the Extrinsic Muscles of the Thoracic Limb
3.2.1 Pectoralis Cranialis Nerve
It originated from the ventral division of the common trunk. In 50% of the dissected plexuses, it originated from C5 to C7, while in 33.3%, it was from C5 to C6, and in 16.6% from C5 to C8 (Table 2). It supplied the pectoralis transversus and pectoralis descendens muscles (Figure 2, Table 2).
3.2.2 Pectoralis Caudalis Nerve
It originated in the ventral division. In 50% of the dissected plexuses, the pectoralis caudalis nerve originated from C5 to C7; in 16.6% of the specimens, it originated from C7 to T1, while in the remaining 16.6%, it came from C6 and C7 (Table 1). It innervated the pectoralis profundus cranialis and pectoralis profundus caudalis muscles (Figure 2, Table 2).
3.2.3 Thoracodorsalis Nerve
It originated in the ventral division. C7–T1 contributed 100% to the plexuses studied (Table 1). It innervated the latissimus dorsi muscle (Figure 2, Table 2).
3.2.4 Thoracicus Longus Nerve
It originated from the ventral branch of C7 in 100% of the plexuses studied. It passed through the lateral part of the serratus ventralis thoracis muscle and emitted muscular branches for its innervation (Table 2).
3.3 Innervation of the Skin of the Thoracic Limb
The intercostobrachialis nerve (Figure 2) originated from T1. It innervated the portion of the skin located in the caudomedial region of the arm. The craniolateral brachial cutaneous nerve originated from the axillaris nerve, supplied the skin of the craniolateral region of the arm, near the middle third, by the deltoid tuberosity. The cutaneus antebrachii cranialis nerve innervated the forearm skin; it ran to the craniomedial part of the arm and reached the proximal third of the forearm at the medial level of the brachioradialis muscle (Figure 3C). Also, the radial nerve gave rise to a superficial branch, which runs laterally on the forearm, between the brachioradialis and extensor carpi radialis muscles. This branch has two branches; the medialis one innervates the skin of the cranial-medial forearm. The lateralis one innervates the skin of the craniolateral forearm (Figure 3C). The cutaneus antebrachii caudalis nerve originated from the ventral division of C8–T1 in 66.6% of the plexuses, and C7–T1 in 33.3%, and supplied the caudal part of the forearm. Finally, the thoracicus lateralis nerve originated from a common trunk with the pectoralis caudalis nerve. It was formed by contributions from C5 to C7 in 33.3% of the dissected plexuses, and received a single contribution from C7 in 16.6% of the dissected pieces, from C7 to C8 in 16.6%, and from C7 to T1 in 16.6% of the remaining plexuses (Table 1). The craniolateral skin of the thorax is supplied by this nerve (Table 2).
4 Discussion
4.1 Origin and Structure of the Brachial Plexus
The origin of the brachial plexus at C5–T1 in the studied specimens was like that in some myrmecophagous species. These include Tamandua tetradactyla and Myrmecophaga tridactyla (Cruvinel et al. 2012; Cruz et al. 2012; Souza et al. 2014). It was also like that in the armadillo species Priodontes maximus (Fernandes et al. 2015). In sloths, the number of cervical vertebrae varies, which changes the origin of a plexus. Alcântara et al. (2020) reported contributions from C5 to T2 in Bradypus variegatus, as occurs in 8.3% of T. mexicana. In Choloepus didactylus, the contribution of T2 is a variant (Silva et al. 2024), such as reported in this study. In Bradypus torquatus, nine cervical vertebrae were found; thus, the plexus formed from C8 to T1, with a C10 spinal branch (Adami et al. 2013).
The brachial plexus has four nerve trunks: cranial, middle, caudal and common; it then divides into two parts. Its structure is like that in sloths, such as B. torquatus, B. variegatus and C. didactylus (Adami et al. 2013; Alcântara et al. 2020; Medeiros-do-Nascimento et al. 2019; Silva et al. 2024). For Tamandua tetradactyla, different nerve arrangements have been described. One has a common trunk that splits into lateral and medial trunks (Cruvinel et al. 2012). Another has three trunks: cranial, middle and caudal (Cruz et al. 2012). The brachial plexus nerves originate from these trunks. The different conformations of the brachial plexus in Xenarthra may be due to an embryological factor. In humans, this formation is mediated by chemoattractants and chemorepellents, such as neutrophil growth factor, neutrophin-1, neutrophin-2, c-kit ligand and neutrophin-2 (Hamilton et al. 1972). This alters signalling in mesenchymal cells and neuronal growth cones; any change in this signalling can affect stem formation and divisions (Benes et al. 2021). These variations may be due to external factors, like genetic differences among species and environmental adaptations.
4.2 Nerves Related to the Intrinsic Muscles of the Thoracic Limb
The suprascapularis nerve forms from the C5 and C6 nerve roots and directly originates from the cranial trunk. This is like what is described in armadillos, such as Dasypus novemcinctus (Miles 1941) and P. maximus (Fernandes et al. 2015). It is also like myrmecophagous species, like T. tetradactyla (Cruvinel et al. 2012). In M. tridactyla, this arrangement is less common (Souza et al. 2014); the C5–C7 contributions are more common for this species. In the case of sloths, in B. variegatus, its origin is only from C6 and in B. torquatus only from C7 (Adami et al. 2013; Alcântara et al. 2020). However, in another study in B. variegatus and C. didactylus reports origin from the cranial trunk (Silva et al. 2024) like in T. Mexicana. The muscles innervated by the suprascapularis nerve are the supraspinatus and infraspinatus muscles, which are similar to some domestic mammals, primates, and domestic carnivores (Demiraslan et al. 2015; Dyce et al. 2012; Evans and De Lahunta 2013; Monroy-Cendales et al. 2023; Silva et al. 2024; Vélez-García et al. 2021). This nerve innervates only the supraspinatus muscle in T. tetradactyla, due to the infraspinatus muscle being innervated by the axillaris nerve (Cruz et al. 2012). This arrangement was not found in the specimens studied.
Most Xenarthra have two subscapulares nerves (Adami et al. 2013; Cruvinel et al. 2012; Cruz et al. 2012; Miles 1941). However, B. torquatus has three (Adami et al. 2013) and in B. variegatus and C. didactylus, reports only one (Silva et al. 2024). The origin of the subscapulares nerves at C5 and C6, found in the dissected plexuses, is reported in T. tetradactyla. However, in this species, the subscapularis caudalis nerve originates in the lateral trunk (Cruvinel et al. 2012). In the studied T. mexicana, it originates in the dorsal division. In other myrmecophagous species, such as M. tridactyla, this nerve originates at C5–C7 (Adami et al. 2013). This nerve innervates the subscapularis muscle in T. tetradactyla, B. torquatus and D. novemcinctus (Adami et al. 2013; Cruvinel et al. 2012; Miles 1941). In the latter, it also innervates the teres major muscle, which does not occur in T. mexicana.
The different origins of the axillaris nerve found through the nerve branches of C5–C7, C6–C7 and C5–C6 are described in M. tridactyla and D. novemcinctus (Miles 1941; Rosa 2012; Souza et al. 2014). The dorsal division forms this nerve, as in B. torquatus (Adami et al. 2013). In T. tetradactyla, this nerve comes from the cranial and middle trunks, or the lateral trunk (Cruvinel et al. 2012; Cruz et al. 2012). In B. variegatus, it comes only from the caudal trunk (Alcântara et al. 2020). Such dispositions were not found in T. mexicana. The axillaris nerve innervates the deltoideus, teres major and teres minor muscles in some myrmecophaga species and armadillos (Cruz et al. 2012; Miles 1941; Rosa 2012). The formation of the cranial cutaneous antebrachial nerve from the axillary nerve is described in M. tridactyla (Rosa 2012); however, the presence of the lateral cutaneous brachial nerve is not described in any of the species consulted.
The origin of the musculocutaneous nerve is more frequent at C5–C7. This has also been reported in M. tridactyla and D. novemcinctus (Miles 1941; Souza et al. 2014). In B. torquatus, B. variegatus, C. didactylus and D. novemcinctus, this nerve fuses at the arm with the median nerve (Adami et al. 2013; Miles 1941; Silva et al. 2024), which was not found in T. mexicana. However, its distribution to the brachialis, biceps brachii and coracobrachialis muscles was similar to that found by Cruz et al. (2012) and Miles (1941) for anteaters and armadillos, respectively.
The median nerve origin from C5–T1 is like that of M. tridactyla (Souza et al. 2014). In B. torquatus, it is formed mainly by C8 (Fernandes et al. 2015). In D. novemcinctus, it originates through C6-T1 contributions (Miles 1941). In other Xenarthra species such as T. tetradactyla, this nerve originates in the medial trunk (Cruvinel et al. 2012) or in the cranial, middle and caudal trunks (Cruz et al. 2012), as occurs in M. tridactyla, in which it originates in all the trunks that compose the brachial plexus (Souza et al. 2014). The distribution of this nerve is not reported in the described species. In T. mexicana, this nerve innervates some caudomedial forearm muscles, like reports on some domestic mammals and wild carnivores. In those species, it innervates the flexor and pronator muscles (Allam et al. 1952; Evans and De Lahunta 2013). Its course through the supracondylar foramen with the brachial artery has been reported in some carnivores (Demiraslan et al. 2015; Ghoshal and Magilton 1972).
Despite their genetic differences, these families have similar anatomy. This may be due to conserved traits in mammals with a supracondylar foramen. The origin of the radialis nerve at the dorsal division, described in this study, was similar to that in B. torquatus (Adami et al. 2013), C. didactylus and B. variegatus (Silva et al. 2024). In T. tetradactyla and M. tridactyla, this nerve came from all the trunks forming the plexus (Cruz et al. 2012; Souza et al. 2014). In B. variegatus, it came from the common trunk (Alcântara et al. 2020). In armadillo species such as D. novemcinctus, C5-C8 forms it (Miles 1941) and in P. maximus, it is only by C7 (Fernandes et al. 2015). The distribution of this nerve and the arm muscles were like those in anteaters and armadillos (Cruz et al. 2012; Miles 1941). The same occurred for the forearm muscles, being reported in T. mexicana (Polania-Guzmán and Vélez-García 2019). However, in C. didactylus, reports a connection with the musculocutaneus nerve (Silva et al. 2024), a disposition not found in this study. The consulted Xenarthra species did not describe the skin on the fingers and branches. However, it was like that in domestic mammals (Dyce et al. 2012; Evans and De Lahunta 2013; ICVGAN, 2017).
The origin of the ulnaris nerve at C5–T1 was analogous to that described in M. tridactyla (Souza et al. 2014). For other Xenarthra, such as D. novemcinctus, the origin is C7-T1 (Miles 1941) and P. maximus reports origins from C7 and C8 (Fernandes et al. 2015). The origin of this nerve from the ventral division in the studied plexuses is like that in some sloth species, such as B. variegatus and B. torquatus, and C. didactylus (Adami et al. 2013; Alcântara et al. 2020; Silva et al. 2024). In T. tetradactyla, this nerve arises from all the trunks of the brachial plexus. It forms a common trunk with the median nerve (Cruz et al. 2012). The specimens studied also had this common origin. In species such as D. novemcinctus, C. didactylus and T. tetradactyla, this nerve supplies the caudomedial forearm muscles and innervates the triceps brachii (Cruz et al. 2012; Miles 1941; Silva et al. 2024). In T. mexicana, the innervation of the anconeus medialis muscle by the ulnaris and radialis nerves indicates fusion with the caput mediale of the triceps brachii and the anconeus medialis muscles (Torres Suárez et al. 2024).
The nerve arrangement in the intrinsic muscles is very similar to that of myrmecophagous animals and sloths. This may be due to the phylogenetic relationship of the superorder. Xenarthra species are monophyletic (Delsuc et al. 2004); they share a single ancestor. Despite their different locomotion, they share anatomical similarities. Other mammals, including humans, show a link between their form and evolutionary traits (Diogo and Molnar 2014; Diogo and Wood 2012; Monroy-Cendales et al. 2023; Pretterklieber and Pretterklieber 2020; Singh et al. 2020).
4.3 Nerves Related to the Extrinsic Muscles of the Thoracic Limb
The presence of two pectoral nerves, as occurs in the T. mexicana studied, has been described in sloths such as B. variegatus and B. torquatus with origin in the common trunk and ventral fascicle, respectively (Adami et al. 2013; Alcântara et al. 2020). However, in these species, the name of the individual nerves is not specified. In M. tridactyla, two pectoral nerves are described; the pectoralis cranialis nerve originates in the cranial trunk in 60% of cases, formed by contributions from C5–C8, and the pectoralis caudalis nerve originates in C8–T1 (Souza et al. 2014). This differs from T. mexicana, where the most common origin for both nerves is C5–C7. The innervation of the superficial pectoral muscles is through the pectoralis cranialis nerve. The pectorales profundus muscles are innervated by the pectoralis caudalis nerve. This is the same as in domestic mammals and some wild carnivores (Demiraslan et al. 2015; Dyce et al. 2012; Evans and De Lahunta 2013; Ghoshal and Magilton 1972). Regarding the thoracodorsalis nerve, its origin through C5–T1 or more frequently through C7–T1was not reported in any of the Xenarthra species consulted; however, the closest is in P. maximus, where it originates from C8–T1 (Fernandes et al. 2015).
In the case of M. tridactyla, the origin is C5–C8 (Souza et al. 2014), and in T. tetradactyla, it is at the level of the lateral trunk or the middle and caudal trunks (Cruvinel et al. 2012). In sloths, such as B. torquatus, it originates from the dorsal fascicle (Adami et al. 2013). In B. variegatus, it comes from the caudal trunk (Alcântara et al. 2020). These dispositions were not found in the specimens studied. The distribution in the latissimus dorsi muscle is described in some species, such as T. tetradactyla and D. novemcinctus (Cruvinel et al. 2012; Miles 1941).
The origin of the thoracicus longus nerve solely through the contribution of C7 and its origin from the cranial trunk was described in sloth species such as B. variegatus (Alcântara et al. 2020; Silva et al. 2024). In the case of myrmecophagous species such as T. tetradactyla, the origin of this nerve is from C6 to C7 (Cruvinel et al. 2012). In M. tridactyla, the origin was described from C5 to C7 (Souza et al. 2014). In B. torquatus, it originates from C9 (Adami et al. 2013) and, in armadillos of the species D. novemcinctus, the origin is from C6 to C7 (Miles 1941). Its distribution through the serratus ventralis cervicis and thoracis muscles was like that in T. tetradactyla, D. novemcinctus and B. variegatus (Cruz et al. 2012; Miles 1941; Silva et al. 2024).
The most frequent origin of the thoracicus lateralis nerve by C5–T1 was not reported in the described Xenarthra species. However, in M. tridactyla, C7–T1 contributed to its origin (Souza et al. 2014). This was similar in 16.6% of the studied tamanduas. In other species such as P. maximus a single origin in T1 is reported (Fernandes et al. 2015) and in D. novemcinctus this nerve is referred to as ‘anterior thoracic nerve’ with origin in C6–C8 and sometimes with contributions from T1 (Miles 1941). In T. tetradactyla, this nerve originates in the middle and caudal trunks (Cruz et al. 2012). However, it may be absent in some specimens (Cruvinel et al. 2012). This nerve innervates the cutaneous trunci muscle in some Xenarthra. (Cruz et al. 2012; Miles 1941; Souza et al. 2014). However, in D. novemcinctus, this nerve also innervates the pectoralis abdominalis and pectorales superficiales muscles (Miles 1941). In T. mexicana studied, the cutaneous trunci muscle was not found and this nerve only supplied the skin.
The most common origin of the cutaneus antebrachii caudalis nerve from C8–T1 is described in C. didactylus, but its origin is not reported (Silva et al. 2024). In T. tetradactyla, the medial antebrachial and medial brachial cutaneous nerves are from C8–T1 (Cruvinel et al. 2012) or only T1 (Cruz et al. 2012). However, human anatomical terminology uses the medial antebrachial cutaneous nerve only. The Nomina Anatomica Veterinaria (ICVGAN, 2017) describes it as the caudal cutaneous antebrachial nerve. The cutaneus antebrachii medialis nerve in mammals originates from the musculocutaneus nerve. This was not observed in the studied specimens. Its distribution was similar to some myrmecophagous and domestic mammals (Cruz et al. 2012; Evans and De Lahunta 2013).
The nerves innervating the extrinsic muscles show considerable variation among Xenarthra species, despite their close phylogenetic relationship. Possible anatomical differences in the extrinsic muscles may explain this. During the embryonic stage, nerves form by two processes: neurotropism and a contact guidance mechanism (Weiss 1941 apud. Satyanarayana et al. 2009). The latter is related to muscle formation. Muscles develop from the paraxial mesoderm's mesenchyme during embryonic growth. The growth cones of axons contact the mesenchyme to form nerves (Morgan and Tabin 1994). This contact phase is essential for fully differentiating these structures (Satyanarayana et al. 2009). Thus, muscle formation and their different arrangements may cause nerve variations.
This same pattern of muscle development in the embryo is closely related to the formation of brachial plexus trunks and divisions. During the process of embryological development, two muscle layers originate and act as barriers for the axons, contributing to the organisation of the brachial plexus divisions (Kania and Jessell 2003; Luria and Laufer 2007). Moreover, the development of trunks is not only exclusively determined by the fusion of ventral spinal nerve roots but also modulated by factors such as the development of cartilage, blood vessels and muscles (Leijnse et al. 2020). In the case of T. mexicana, it is reasonable to hypothesise that the formation of the brachial plexus follows embryological mechanisms comparable to those observed in other species. The presence of variants and differences in the morphology of these structures can be attributed to the distinct muscular and vascular configurations present in the analysed species.
4.4 Applied Anatomy for Nerve Blocks
The brachial plexus in T. mexicana shows key similarities to that in C. didactylus (Silva et al. 2024). Therefore, paravertebral blocks can be performed at the same levels, C5–T1, in both species. However, not all Xenarthrans species can follow this standard. In the case of B. variegatus, paravertebral blocks were done at C7–T2 (Barbosa et al. 2024). The block at this level in T. mexicana could generate an incomplete block of the whole brachial plexus. Paravertebral blocks in dogs and cats are often done at the C6 to T1 vertebrae. This technique could also be applied (Rioja et al. 2012; Evangelista et al. 2018).
In C. didactylus, supraclavicular and infraclavicular blocks have been reported (Silva et al. 2024). However, these blocks are not applicable to T. mexicana due to the absence of the clavicle. The axillary block in B. variegatus has a significant risk due to the arteriovenous plexus (Silva et al. 2024). In species like C. didactylus and some domestic mammals, just the axillary artery and vein accompany the brachial plexus near the axillary fossa. This decreases the risk of perforations and bleeding (Budras et al. 2007; Dyce et al. 2012; Evans and De Lahunta 2013). The present study found the same arrangement. So, the blockages described in the earlier species can serve as references to T. mexicana.
The distribution of the radial, ulnar, median and musculocutaneous nerves resembles that of anteaters, sloths, cats and dogs. Blocks at the level of the arm, such as the RUMM block in cats and dogs, may be the basis of the technique in T. mexicana. In the cat, a blocking technique at the proximal level of the humerus is described, having the humeral joint as a reference point (Pratt and Martinez-Taboada 2021). For this block, it is important to consider the anatomical differences in Xenarthrans. The fusion of the median and musculocutaneous nerves might vary. While this fusion was not seen in the studied T. mexicana, it could still exist in other specimens.
Xenarthrans such as B. variegatus and C. didactylus show blockages at the carpal level. These blockages can affect the median musculocutaneous nerve (Silva et al. 2024). In T. mexicana, the two nerves did not fuse. So, the blockage on the palmar side affected only the ulnar and median nerves. In cats, the arrangement of the two nerves at the carpal level is similar. Yet, they use surface anatomy points as a guide for the block. For example, they identify the carpoaccessory bone (Enomoto et al. 2016). In T. mexicana, palpation of this bone is difficult due to the thickness of its skin. Thus, we recommend the use of ultrasound.
5 Conclusions
In T. mexicana specimens, the brachial plexus arose from the spinal nerves C5–T1 mainly, and like a variant from T2 contributions. These formed four trunks, then two divisions. They gave rise to the nerves that form the plexus. The nerves innervating the extrinsic and intrinsic muscles were like those in other Xenarthra species. In this species, the intercostobrachialis, thoracicus lateralis, axillaris, radialis, and caudal cutaneous antebrachial nerves innervate the skin. Finally, the brachial plexus of T. mexicana is similar to that of other Xenarthra species. However, it has species-specific characteristics, mainly in the formation of trunks, being more similar to sloths.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.




