Origin and distribution of facial nerve anatomy in van cats
Abstract
This study aims to reveal the morphological properties of facial nerve and the middle ear in Van cats. Study material was composed of 6 female Van cats. Dissections were performed under a Zoom Stereo Microscope. There was no plexus buccalis in Van cats. The chorda tympani was observed to pass through an opening in the tympanic cavity, emerge through a small opening just behind the retroarticular process, and join the lingual nerve. A rounded anatomical formation with a size of 2.75 ± 0.3 mm was found to be located within the mastoid process of the temporal bone between the facial nerve and the auricular branch of the vagus nerve. The stapes nerve was not present. The geniculate ganglion was very prominent and about 1.00 mm high. The deep petrosal nerve was observed to emerge from the plexus tympanicus. The bulla tympanica was 18.96 ± 0.10 mm long, 13.03 ± 0.20 mm wide and 13.16 ± 0.20 mm high. After leaving the mandibular nerve, the n.tensoris tympani coursed caudally around the a.maxillaris, formed an ansa, entered the tympanic cavity through the canalis musculotubarius and reached an end in the m. tensor tympani. Due to the scarcity of studies on the middle ears of Van cats, it is thought that this study will fill a gap in the field of veterinary anatomy.
1 INTRODUCTION
White cats, especially the blue-eyed ones, are deaf as a result of the effects of the dominant pigment gene [dominant white (W)]—W, which acts by suppressing the melanocytes. Skin and hair are white due to the absence of melanin in melanocytes. When the W gene is strongly dominant, it suppresses the melanocytes in the iris, and as a result, an iris appearing blue occurs due to the absence of melanin. Furthermore, the strong effect of the W gene suppresses the melanocytes in the vascular stria on the outer wall of the cochlea. Thus, the degeneration of stria leads to the degeneration of the hair cells and deafness in the first weeks of life (Strain, 2017).
The facial paralysis caused by n.facialis damage is a relatively common clinical condition. When it is single-sided, it can cause changes in facial symmetry, eye closure, dysphagia and the problems with the pronunciation of some words. Although the surgical techniques have improved, the functional outcome of repairing full injuries to the n.facialis is still unsatisfactory (Choi & Dunn, 2001; De Faria et al., 2006; Mersa et al., 2000).
In case of the impairment of the main trunk of the n.facialis during the surgical procedure or due to a traumatic injury, it is a good result to see the tonus in the facial muscles again when the surgeon realigns the cut ends or places a nerve graft between the two ends. However, it is very important to correctly realign the fibre bundles within the repaired nerve. In the studies on this field, there is very little information about the topographical representation of the fibres in the nerve trunk. The previous studies have concluded that there is no specific fibre arrangement in the n.facialis (Harris, 1968; Sunderland & Cossar, 1953).
Today, the aetiology of ‘Bell's Palsy’ is still an unresolved problem. The role of vasa nervorum has recently been accepted as an important factor. The nerve lesion is attributed to ischaemia that is severe enough to cause necrosis. According to some researchers, ischaemia is caused by intense local vasoconstriction or unspecified vascular changes. On the other hand, some other researchers believe that it is caused by the swelling of the n.facialis triggered by cold, inflammation or circulatory disturbances. It was also asserted that the paralysis may be caused by the pressure on the nerve triggered by vascular stasis (Sunderland & Cossar, 1953).
The middle ear lavage or curette can also cause disease. Although very rare, congenital disorders can also be seen in cats such as Siamese, Burmese and Tonkinese (Paterson & Tobias, 2012). The rate of incidence of Horner's syndrome in the cats undergone bulla osteotomy is about 58%, which is quite high compared to dogs. While the recovery takes place within two weeks in dogs, it is permanent in 25% of cats (Spivack et al., 2013; Zwueste & Grahn, 2019).
The n.facialis is a nerve consisting of motor, sensitive and parasympathetic fibres. The motor fibres are thick, and their origin is the motor n.facialis nucleus located in the pons. The motor fibres innervate the facial muscles and the caudal portion of the stylohyoid and digastric muscles. The origin of sensitive fibres is the ganglion geniculi (Çalışlar, 1995).
The most recent and detailed descriptions of the topographical anatomy of the ear and cranial nerves in cats are found in some doctoral thesis (Hartmann, 1992; Schleip, 1992). Nevertheless, these descriptions do not provide quantitative data. More recently, another thesis focused on the histology of the middle and inner ear of cats (Heitmann, 2018).
Since various damage to n.facialis can cause serious functional disorders, it is one of the clinically important cranial nerves. For example, superficial trauma of the face, parotid gland tumours/infections, and chronic middle ear infections, surgical procedures in the area of the parotid salivary gland may cause various levels of paralysis in the n.facialis (Harris, 1968; Sunderland & Cossar, 1953). Therefore, it is of capital importance to understand the course and distribution of this nerve.
The subject of this study is that deafness is a common phenomenon in Van cats due to their genetic characteristics. Therefore, the purpose of this study is to reveal differences or similarities in terms of anatomical structure.
2 MATERIALS AND METHODS
2.1 Supply of cadavers
Six female cats were used in this study. The anatomical study was conducted using 6 adult female Van cat cadavers with a mean weight of 2.5–3.0 kg. The cats, which were referred to the Veterinary Faculty clinics at Yüzüncü Yıl University, Van, Turkey for various reasons, died without treatment, and these animals were delivered to the department of anatomy and used for this study. The Van cats kept in 10% formaldehyde for about a year were used. The heads were removed away as near as possible to the chest area. In order to soften the subcutaneous connective tissue, they were kept in water for 3 months. The facial skin was then carefully removed. 20,000 cycle drills were used for the course of the n.facialis in the temporal bone. This study was carried out using the digital calipers with a sensitivity of 0.01 mm, and the samples were photographed using a camera (Samsung NX300-Japan, 20.3 Mp). Zoom Stereo Microscope (Olympus SZ-PT—Japan—Auxiliary objective 100ALO 0,5X, eyepiece GSWH 10X/22 ) was used. Nomina Anatomica Veterinaria (NAV, 2017) was used for the nomenclature. This experimental study was approved on 29 August 2019 (approval code:19/155) by the Committee for Ethics of Animal Research (ERÜHADYEK) at the Erciyes University, Kayseri, Turkey.
3 RESULTS
3.1 Origin of the N.facialis and its course in the canalis facialis
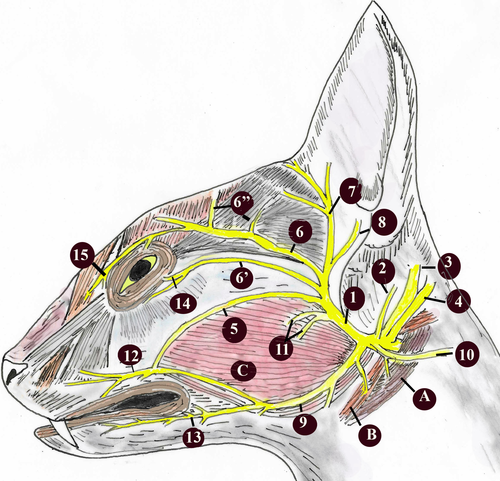
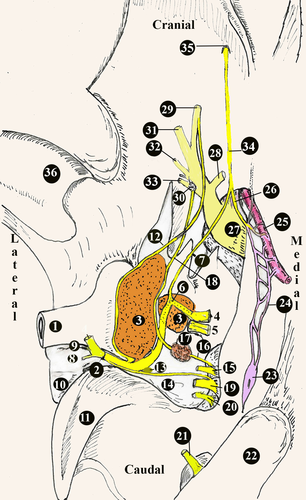
A diagram of general distribution of the origin of the facial nerve is given in Figures 1 and 2, and the diameters of its branches are given in Table 1.
| Anatomical structures | Diameters (mm) and SD |
|---|---|
| Ganglion geniculi (high) | 1.00 ± 0.11 |
| N.petrosus major | 0.67 ± 0.15 |
| N. canalis pterygoidei | 0.42 ± 0.10 |
| Canalis pterygoideus (long) | 7.64 ± 0.20 |
| Chorda tympani | 0.23 ± 0.15 |
| Ganglion-like structure | 2.75 × 2.17 × 1.00 ± 0.30 |
| Bulla tympanica (long) | 18.96 ± 0.10 |
| Bulla tympanica (wide) | 13.03 ± 0.20 |
| Bulla tympanica (high) | 13.16 ± 0.20 |
| Round window (inner) | 1.36 ± 0.20 |
| Round window (outer) | 3.52 ± 0.15 |
| Oval window (long) | 1.01 ± 0.15 |
| Oval window (wide) | 0.60 ± 0.20 |
| N. facialis (inner of facial canal) | 0.92 ± 0.12 |
| N. Facialis (outer of facial canal) | 1.32 ± 0.20 |
- Abbreviation: SD, standard deviation.
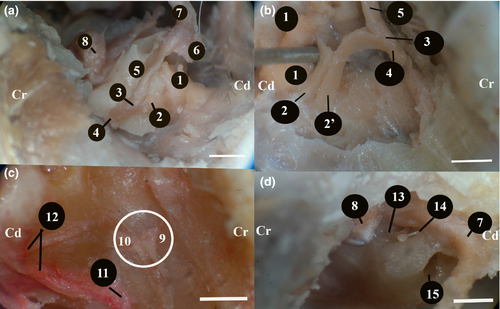
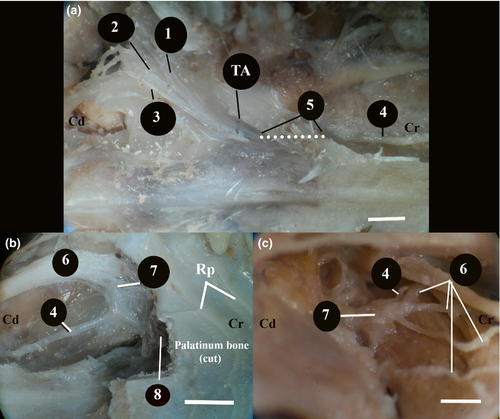
In the Van cats, along with the n.vestibulocochlearis, the n.facialis originated at the level of the corpus trapezoideum in the lateral of the medulla oblongata (Figure 3). The n.facialis was observed to emerge in a bundle. It entered the petrous part of the temporal bone through the meatus acusticus internus in the lateral of the n.vestibulocochlearis. The n.facialis reached the level of the medial wall of the fossa for the m.tensor tympani when it progressed approximately 2.69 ± 0.15 mm in the petrous part of the canal in the petrous bone. At this level, it was observed that the n.facialis formed the first genu, that is the geniculum of the canalis facialis and the ganglion geniculi at the height of 1.00 ± 0.11 mm.
It was observed that the canalis facialis took a straighter course after this first genu and formed its second genu in the immediate vicinity of the vestibular window (oval window) and reached the middle ear through an opening at this level. The second genu was the opposite of the first, and the entire canal was in the shape of ‘S’. The fossa for the stapedial muscle and the stapedial muscle were found to lie on the dorsomedial wall of where the n.facialis communicated with the middle ear (Figure 2d). Then, the n.facialis emerged from the stylomastoid foramen and left the skull. During its course in the petrous part of the temporal bone, the n.facialis first gave off the major petrosal nerve and then the tympanic chord.
3.1.1 Major petrosal nerve (N.petrosus major)
The diameter of the major petrosal nerve was about 0.67 ± 0.15 mm (Figures 2 and 3). After leaving the anterior edge of the ganglion geniculi, the major petrosal nerve proceeded rostroventrally in the small canal for the major petrosal nerve in the dorsal of m.tensor tympani and reached the middle ear cavity by emerging from a small opening in the immediately distal of where the m.tensor tympani communicated with the middle ear cavity. It was observed that the major petrosal nerve formed the n. canalis pterygoidei (vidian nerve) by receiving the branch of deep petrosal nerve coming from the plexus caroticus plexus and plexus tympanicus formed by the nn.caroticotympanici on the internal carotid artery in the middle ear. Along with the major petrosal nerve, the deep petrosal nerve was observed to course cranioventrally on the dorsal wall of the cartilaginous part of the Eustachian tube on the medial wall of the Eustachian tube and to leave the middle ear.
The n. canalis pterygoidei (vidian nerve) was found to have a thickness of about 0.42 ± 0.10 mm. It was determined that this nerve passed through the canalis pterygoideus, which was approximately 7.64 ± 0.20 mm long, reached the fossa pterygopalatina, and joined the ganglion pterygopalatinum on located on the medial wall of a.maxillaris and in the dorsal of the m. pterygoideus medialis (Figure 4).
3.1.2 Stapedial nerve (N.stapedius)
No separate stapedial nerve was observed to exist in the dissection. It was observed that the n.facialis was very close to the head of stapes in the middle ear and found to lie on the stapedial muscle located in the fossa for the stapedial muscle, and it was in close contact with this small muscle (Figure 3d).
3.1.3 Tympanic chord (Chorda tympani)
It was found that the chorda tympani emerged from cranial face approximately 2.00 ± 0.12 mm before the n.facialis emerged from the stylomastoid foramen (Figures 5a and 6a,b). The diameter of the chorda tympani was about 0.23 ± 0.15 mm. It reached the middle ear cavity through the canal in the tympanic bone. When it reached the middle ear cavity, it was in the lateral of the anterior ligament of the malleus. Later on, it was observed to proceed towards the end of this ligament. It was found that the chorda tympani was covered with the middle ear mucosa and run between the handle of malleus and the long limb of the incus, afterwards headed first towards the dorsal and then towards the cranioventral, and entered through a small opening, also known as the petrotympanic fissure, on the rostrodorsal wall of the tympanic cavity.
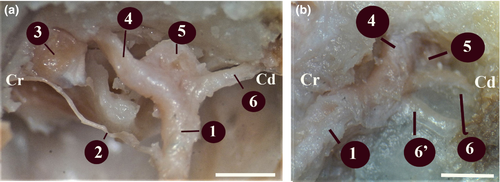

The chorda tympani was observed to emerge from an opening in the ventromedial of the retroarticular process of the temporal bone and the caudodorsal of the oval foramen. Approximately 10 ± 0.15 mm after emerging, the chorda tympani was found to progress by joining the lingual nerve, a branch of the trigeminal nerve emerging from the oval foramen.
After giving off its branch running to the anterior 2/3 level of the tongue (level of fungiform papillae), the chorda tympani progressed, along with the lingual nerve, to the muscles in the ventral of the tongue. No prominent submandibular ganglion was detected. After giving off its branch named ‘branches communicating with hypoglossal nerve’, the lingual nerve was found to head towards the dorsal and reach an end by giving off many branches in the sublingual region. It was observed that during its course along with the lingual nerve, the chorda tympani gave off branches to the mandibular gland and the sublingual gland.
3.1.4 Auricular branch (Ramus auricularis of N.vagus)
This was a branch given off by the n.vagus at the level of the jugular foramen (Figure 5a,b). It was observed that this branch coursed first caudodorsally and then rostrally towards the back of the bulla tympanica and merged into the n.facialis embedded in the mastoid process.
It was observed that the level of this merging was approximately 6.20 ± 0.50 mm before the n.facialis emerging from the stylomastoid foramen. In the cranial of the point of merging, a round-shaped anatomical formation with a diameter of 2.75 × 2.17 × 1.00 ± 0.30 mm, which could be defined as a ganglion was observed between the n.facialis and the auricular branch. This ganglion-like structure was within the mastoid process of the temporal bone.
In two cats, it was observed that there was a second branch extending caudally at the junction of the auricular branch and the n.facialis. At the level of the tympano-occipital fissure (jugular foramen), the auricular branch also received branches from the proximal ganglion of the n.vagus (jugular ganglion) and the glossopharyngeal nerve right next to it.
3.1.5 Tympanic bulla (Bulla tympanica)
The bulla tympanica was about 18.96 ± 0.10 mm long, 13.03 ± 0.20 mm wide and 13.16 ± 0.20 mm high. It had a smooth surface. Its posterior part was limited by the mastoid bone. The tympanic cavity was divided into two separate cavities, dorsolateral (outer) and ventromedial (inner), by a bony curtain called septum bullae. The dorsolateral (outer) was smaller than the other. It was 6.68 ± 0.25mm high. The dorsal part of the septum bullae was not closed at the level of promontorium. Both sides were in contact with each other through a small opening.
The ventromedial cavity (inner) was bigger than the dorsolateral one. It was 12.17 ± 0.15 mm high. It was observed that in this cavity there were the half-cylinder-shaped promontorium and the round window (fenestra cochlea) lying on the promonotorium in the caudodorsal direction. The inner and outer diameters of the round window were about 1.36 ± 0.20 mm and 3.52 ± 0.15 mm, respectively. It was found that the n.tympanicus branch of the glossopharyngeal nerve entered the tympanic cavity through the foramen fissure and formed the plexus tympanicus with the branches coming from the carotid plexus. After forming this plexus, the tympanic nerve was found to proceed towards the dorsomedial and then the cranioventral under the name ‘ n. petrosus minor (Jakobsen nerve)’. Then, the n. petrosus minor passed through the ventral wall of the fibrous canal of the Eustachian tube on the medial wall of the m.tensor tympani, left the tympanic cavity, and reached an end in the otic ganglion in the oval foramen. It was found that during its course, the n. petrosus minor gave off branches to the tympanic cavity mucosa and the Eustachian tube mucosa. It was also observed that some branches of the plexus tympanicus extended to the secondary tympanic membrane on the round window (Figure 7).
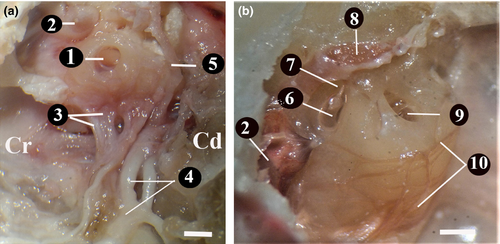
The oval window (vestibular window) was found to be in the craniodorsal of the round window and closed by the base of stapes. The widest diameter of the oval window was approximately 1.01 ± 0.15 mm long and 0.60 ± 0.20 mm wide. It was found that the stapedial ligament was connected to the head of stapes, and the stapedial foramen between the crura of the stapedial bone had a smooth circle shape like a ring.
3.2 The branches given off by the N.facialis after leaving the stylomastoid foramen
The diameter of the n.facialis in the canalis facialis was determined to be different from its diameter when it was out of the canalis facialis. While its diameter in the canalis facialis was approximately 0.92 ± 0.12 mm, its diameter after going out of the canal was approximately 1.32 ± 0.20 mm. In dissection, it was observed that the nerve diameter increased even more.
3.2.1 Internal auricular branch (Ramus auricularis internus)
This is the first branch given off by the n.facialis right after leaving the stylomastoid foramen. This branch was observed to disperse to the skin of the lateral and medial part of the rostral concavity of the auricle and the small ear muscles on the outer face of the auricle.
3.2.2 Caudal auricular nerve (N.auricularis caudalis)
This is a strong branch leaving the n.facialis behind the ear. This nerve was found to proceed towards the back of the ear, and then reach an end after dispersing to the muscles in the convex part of the auricle and the muscles behind the ear. Furthermore, it gave off branches to the platysma on the dorsum of the neck.
3.2.3 Digastric branch
This muscle emerged from the n.facialis near the stylomastoid foramen. It was observed that this branch proceeded from the inferior of the parotid gland to the cranioventral and innervated the caudal part of the digastric muscle. During its course, the digastric branch was also found to give off branches to the jugulohyoid muscle and the stylohyoid branch to the stylohyoid muscle.
3.2.4 Auriculopalpebral nerve (N.auriculopalpebralis)
This nerve branched from the n.facialis on the rostral edge of the circular cartilage of the external ear near the caudal edge of the mandible. It emerged from the rostral–dorsal edge of the parotid gland to the outer surface. On the lateral surface of the zygomatic arch, this nerve gave off two branches called ‘zygomatic branch’ and ‘rostral auricular branches’. The course of the auriculopalpebral nerve on the zygomatic arch was found to be subcutaneous; therefore, it is noteworthy that this nerve was in a position sensitive to traumatic injuries.
3.2.5 The zygomatic branch (Ramus zygomaticus)
This branch was observed to shape the rostral auricular plexus between the eye and the ear. It was observed that it gave off branches from this plexus to the rostral auricular muscle, nasolabial muscle, orbicularis oculi muscle, retractor muscle of lateral angle and levator muscle of medial angle (Figure 8a,b).

On the other hand, the rostral auricular branch reached an end in the superficial and deep scutuloauricular muscles.
3.2.6 Buccal branches (Rami buccales)
After leaving the n.facialis as a thick branch, the buccal branches split into two branches (dorsal and ventral buccal) in the parotid gland.
3.2.7 Ventral buccal branch
This branch emerged as a single branch from the rostral end of the n.facialis in the parotid gland immediately after the origin of the cervical branch (Figure 8a,b). After proceeding approximately 8.00 ± 0.15 mm to the rostral direction in the parotid gland, it reached the masseter muscle. After advancing approximately 35 ± 0.30 mm rostrally on the masseter muscle, it reached the rostral edge of masseter muscle. Here, it was observed to give off branches to the orbicularis oris, buccinator, depressor labii maxillaris muscles and the facial cutaneous muscle. It was found that the ventral buccal branch connected to the dorsal buccal branch through a branch it gave off from its dorsal wall.
3.2.8 Dorsal buccal branch
It was observed that after the n.facialis gave off the ventral buccal branch and proceeded 10.00 ± 0.12 mm rostrodorsally between the external acoustic opening and the masseter muscle in the parotid gland, the dorsal buccal branch emerged from the rostral edge of the parotid gland and reached the surface of the masseter muscle (Figure 8a,b). After rostrally advancing approximately 38 ± 0.10 mm, inferiorly to the facial cutaneous muscle, it reached the rostral edge of the masseter muscle. At this level, it was observed to receive a communicating branch from the ventral buccal branch. It was observed that the dorsal buccal branch reached an end by dispersing to the following muscles: malaris, zygomatic, orbicularis oris, buccinator, caninus, nasolabial levator, maxillary levator labii and the facial cutaneous muscles.
3.2.9 Cervical branch (Ramus colli)
After originating from the n.facialis, it was observed to proceed towards the ventral, in the medial of the parotid gland, and emerge from the ventral edge of the gland and reach the lateral surface of the mandibular gland (Figure 8a,b). In its course, the cervical branch gave off a thin branch to the parotid-auricular muscle as well as joining the ventral branch of the second cervical nerve and giving off branches to the cervical sphincter muscles and platysma.
4 DISCUSSION
Van cat is a species with a dominant W gene. In this study, the structure of hair cells in the internal ear was not examined. The incidence of facial paralysis as a result of n.facialis injuries is very high. As reported in the literature, Horner's syndrome may be permanent as a result of the ventral bulla osteotomy carried out for the treatment of tympanic tumours and abscesses in cats (Spivack et al., 2013; Zwueste & Grahn, 2019).
The middle ear of cats has more resemblance to that of humans than the guinea pig in terms of osseous orientation and shape, n.facialis, and tympanic muscles (Judkins & Li, 1997). The data obtained in the present study supported the literature.
Likewise reported in the literature (Dursun, 2000; Godinho & Getty, 1975) in this study, the n.facialis emerged from the caudal edge of pons with n.vestibulocochlearis, entered the internal auditory meatus, passed through the stylomastoid foramen via the facial canal, and then left the skull. Likewise reported by (Barone & Simoens, 2010; Godinho & Getty, 1975), the parasympathetic sensitive fibres of the n.facialis were found to emerge from the ganglion geniculi.
The diameter of the n.facialis in the canalis facialis was different from its diameter after emerging from the stylomastoid foramen. The n.facialis was about 30% thinner in the canal. This was in line with the results reported by some authors (Balkany, 1986; Kumar, 2015) for cats and dogs.
The n.facialis was observed to course approximately 2.69 ± 0.15 mm in the petrosal canal. The findings of this study were similar to the findings reached in a study on rabbits (Ozcan et al., 1993) which reported that the canalis facialis in the petrosal bone was about 10 mm and the diameter of the n.facialis in the canal was 1.2–1.3 mm.
It was reported that in its course in the canal, the n.facialis gave off the major petrosal nerve, stapedial nerve and the chorda tympani (Barone & Simoens, 2010; Godinho & Getty, 1975). However, as reported by Balkany (1986), since the stapedius nerve was found to be almost adherent to the n.facialis, no separate stapedial nerve was observed.
It was asserted that after originating from the ganglion geniculi, the major petrosal nerve emerged from the foramen lacerum, received a branch from the carotid plexus, and formed the n. canalis pterygoidei, and then reached an end at the ganglion pterygopalatinum (Barral & Croibier, 2008). Also in the present study, it was observed that the major petrosal nerve originated from the ganglion geniculi at a distance of 6.00 ± 20 mm from the stylomastoid foramen and, likewise asserted in the literature, received a branch from the plexus caroticus. It coursed towards the cranioventral on the dorsal wall of the cartilage structure of the Eustachian tube, and then, as the n. canalis pterygoidei, it joined the ganglion pterygopalatinum located in fossa pterygopalatina after passing through the canalis pterygoideus with a length of approximately 7.64 ± 0.20 mm.
It was reported that the chorda tympani left 2.00 mm before the n.facialis emerged from the stylomastoid foramen and then entered the tympanic cavity (Ellsworth & Jennings, 2008). It was also reported that it left the middle ear cavity through the fissura petrotympanica (Dursun, 2000; Nur & Teke, 2000). In our study, it was observed that the chorda tympani left the n.facialis approximately 2–3 mm before the stylomastoid foramen, passed through the lateral of the anterior ligament of the malleus, first headed towards the dorsal and then towards the cranioventral, passed through a canal on the medial wall (the canal in the tympanic bone; not through a fissure or groove formed between two separate bones as reported by Kay & Sparks, 2012), and emerged from an opening in the ventromedial of the retroarticular process of the temporal bone (in the caudodorsal of the oval window). Then, after approximately 10 mm, the chorda tympani was observed to join the lingual nerve, a branch of the trigeminal nerve. In the literature, it was reported that there are small tympanic bone spicules within the tympanic cavity (Beck et al., 2020; Heitman et al., 2016; Parzefall et al., 2015). According to the findings reached in this study, small tympanic bone spicules were not seen.
In the literature, it has been reported that in cats, the tympanic cavity is elliptical (Wilder & Gace, 2011) and divided into two compartments by a thin bony septum along a line extending from the mid-rostral to the mid-lateral (Little & Lane, 1986). According to Wysocki (2006), the length of the tympanic cavity is about 14.8–18.6 mm and its height is about 13.2–16 mm. In this study, it was observed that the tympanic cavity was divided into two by the septum bullae and its structure was elliptical. In our study materials, the plexus tympanicus was found to be 18.96 ± 0.10 mm long, 13.03 ± 0.20 mm wide and 13.16 ± 02 mm high. Our finding was in line with those reported by both studies in the literature (Wysocki, 2006).
The deep petrosal nerve was reported to join the major petrosal nerve after originating from the plexus of the nn. caroticotympanici which was in a foamy structure in the internal carotid artery wall (Nur & Teke, 2000). In this study, the deep petrosal nerve was found to originate from the plexus tympanicus and leave the middle ear cavity over the ventromedial wall of the Eustachian tube, and then reach an end in the otic ganglion.
Likewise reported by Rosen (1950), it was observed that the glossopharyngeal nerve and the caroticosymphatetic branches on the wall of the internal carotid artery came together and formed the plexus tympanicus. The n. petrosus minor was found to originate from the plexus tympanicus. Furthermore, the plexus tympanicus was found to extend to the secondary tympanic membrane covering the round window.
It was reported that the m.tensor tympani was innervated by either the n.pterygoideus medialis (Dursun, 2000) or the n.tensoris tympani which entered through the tuba auditiva (Barone & Simoens, 2010). In this study, likewise reported by Barone and Simoens (2010), it was found to be innervated by the n.tensoris tympani, a branch of the mandibular nerve. It was found that the n.tensoris tympani coursed towards the caudal by forming an ansa around the a.maxillaris artery, entered the middle ear through the tuba auditiva and then reached an end in the m.tensor tympani.
According to Godinho and Getty (1975), the ramus auricularis of n.vagus given off by the jugular (proximal) ganglion entered the temporal bone, merged with the n.facialis in the facial canal and also innervated the stapedial muscle (Bossy, 1957). According to the findings reached in this study, the ramus auricularis originated from the jugular ganglion but did not give off a branch to the stapedial muscle. Furthermore, a quite large circular ganglion-like structure with a diameter of 2.75 × 2.17 × 1.00 ± 0.30 mm was detected at the anterior part of the junction of the n.facialis and the auricular branch.
In the literature, it was reported that the mandibular ganglion had an irregular appearance in cats and resembled a necklace hanging with two thin nerve filaments on the ventral edge of the lingual nerve (Nur & Teke, 2000); it may be very small in dogs and did not exist in cats at all (Barone & Simoens, 2010); or a prominent mandibular ganglion could not be seen in cats (Godinho & Getty, 1975). Also in this study, no prominent mandibular ganglion was detected. In addition, although it was also reported in the literature that the ganglion pterygopalatinum was a plexus formed as a result of the fusion of 2 to 3 dot-sized ganglionic structures and the fine nerve filaments emerging from these ganglionic structures (Nur & Teke, 2000), in this study, it was found to have a quite large structure in Van cats.
The zygomatic branch of the n.facialis was reported to give off branches to not only the cheek muscles but also to both eyelids (Tomo et al., 2002). In our study, it was observed that the buccal branch and the zygomatic branches gave off branches to the cheek muscles and the orbicular muscles around the eyes, respectively.
5 CONCLUSION
As can be understood from the explanations above, it was observed that, in Van cats, there were some structures (tympanic plexus) similar to the structures which were reported not in other species but humans. It was found that a ganglion-like structure existed at the junction of the auricular branch and the n.facialis; and major petrosal, minor petrosal, and deep petrosal nerves left the pharyngotympanic tube whereas the tensoris tympani nerve entered the pharyngotympanic tube. We could not see a structure different from the information in the literature. To the best of our knowledge, this is the first study examining domestic cats using various measurements in details.
ACKNOWLEDGEMENTS
The authors would like to thank Osman Aydın Kara, veterinary physician, and radiology technician, for their technical help in obtaining the osteological pictures in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.