The distribution and immunolocalization of fibroblast growth factors (FGFs) in the rat oviduct during early pregnancy and the post-partum period
Abstract
The mammalian oviduct provides a favourable environment for several reproductive processes, including ovum transport, sperm capacitation, fertilization and pre-implantation embryonic development. This environment is regulated by cyclic ovarian steroids, that is oestrogen, and growth factors. Fibroblast growth factors (FGFs) regulate the differentiation and growth of various cell types in the female genital tract. This study aimed to determine the localization of FGF1, FGF2, FGF receptor 1 (FGFR1) and 2 (FGFR2) in the rat oviduct, by immunohistochemistry, on day 5 of pregnancy and post-partum days 1, 3 and 5, and to demonstrate the possible functions of these proteins during early pregnancy and the post-partum period. On all examination days, cytoplasmic and nuclear FGF1 immunoreactivity was detected in the epithelium lining the infundibulum, ampulla and isthmus of the oviduct. Immunoreactivity was much stronger in the basal bodies of the cilia on the epithelium lining the infundibulum and ampulla. FGF1 immunoreactivity was also detected in stromal cells, myocytes and endothelial cells. Cytoplasmic FGF2 immunoreactivity was observed in the tunica muscularis, vascular myocytes and endothelial cells. While strong cytoplasmic FGF2 immunoreactions were observed in the stromal cells of the lamina propria, the luminal epithelium, some stromal cells and smooth muscle cells displayed a rather weak FGFR1 and FGFR2 immunoreactivity. Immunoreaction intensity did not differ between the periods examined. This study shows that FGF1, FGF2, FGFR1 and FGFR2 are produced by rat oviduct cells during pregnancy and the post-partum period, and reproductive physiology is regulated not only by hormonal mechanisms, but also by growth factors.
1 INTRODUCTION
The mammalian oviduct is an important organ, where fertilization and the initial cell divisions of early embryonic development take place. It is lined by ciliated secretory epithelial cells, the height and activity (ciliation and secretion) of which increase under the effect of oestrogen (Verhage et al., 1979). In healthy animals, the secretory activity of the oviduct decreases during the luteal phase and pregnancy, both of which are characterized by high progesterone levels (Nayak et al., 1976). In a study conducted in the oviduct of goats in the post-partum period when the effect of the progesterone hormone disappears, it is reported that the number of secretory cells containing a large number of secretory granules in the ampulla epithelium increases from the post-partum 21st day (Krajničáková et al., 2002). In another study examining the morphological features of the sheep oviduct in the post-partum period, it is stated that the secretory cells in the early post-partum period are light coloured and do not contain secretory granules. On days 17 and 25 post-partum, it is reported that secretory cells contain many secretory granules of various sizes, shapes and densities, and on day 34 post-partum, secretory cells release their secretions into the oviduct lumen through the pores (Ciganková et al., 1996). The secretion released into the oviduct lumen support the survival of sperm cells (Boilard et al., 2004) and embryonic development (Lai et al., 1996). The secretory epithelial cells of the oviduct are also regulated by local growth factors (Verhage et al., 1979).
Fibroblast growth factors (FGFs), which are one of the major growth factor families, are polypeptides that are highly expressed in the female genital tract and play a substantive role in fertility. Known to exhibit various biological activities, the FGF gene family is composed of 22 members (FGF1-FGF23) that are found in a wide array of organisms, from nematodes and Drosophila to mammals, including humans, mice and rats (Itoh & Ornitz, 2004; Itoh & Ornitz, 2011). Based on phylogenetic analyses, the human FGF gene family is classified into seven subfamilies by their biochemical functions, sequence similarities and phylogenetic relationships. Members of a given subfamily have similar receptor binding capacity. In humans and mice, FGFRs are classified into four types, each encoded by a different gene (Pasquale & Singer, 1989). These are FGFR1 (flg or fms-like gene), FGFR2 (bek or bacterially expressed kinase), FGFR3 and FGFR4.
Recent research has demonstrated that growth factors have an important regulatory role in the differentiation and growth of various cell types in the female genital tract (Alan et al., 2015; Berisha et al., 2004; Chegini et al., 1994; Gabler et al., 1998; Sağsöz et al., 2015; Watson et al., 1992). It has been shown that in the ovarian tissue, growth factors are involved in folliculogenesis, oocyte maturation, and the proliferation, differentiation and steroidogenesis of granulosa and theca cells (Schomberg, 1989). Previous research on the local production and expression of growth factors in the oviduct has mainly focussed on the sexual cycle. The presence of TGF-α mRNA (Morishige et al., 1993), EGF and EGFR mRNA (Lei & Rao, 1992), IGF1, IGF2 and IGFR1 mRNA and specific immunoreactivity (Pfeifer & Chegini, 1994), and immunoreactive EGF, EGFR and TGF-α (Chegini et al., 1994) has been demonstrated in the human oviduct. Furthermore, research in mammals has shown the expression of IGF1 mRNA in the epithelial cells of the porcine oviduct (Wiseman et al., 1992), IGF1, IGF2 and IGFR1 in the ovine oviduct (Kincy et al., 1992; Stevenson & Wathes, 1996), FGF1, FGF2 and FGFR2 in the marmoset oviduct (Gabler et al., 1998) and PDGFA (Eriksen et al., 1993), IGF1 (Schmidt et al., 1994), PDGFB, IGF1 and FGF2 (Viuff et al., 1995) in the bovine oviduct. Furthermore, mRNA encoding FGF2, TGF-α, TGF-β, PDGFA and IGF2 has been detected in epithelial cell cultures of the bovine oviduct (Watson et al., 1992).
Most of the previous studies investigating the possible effects of FGFs on the female genital tract have focussed on the ovarian and uterine tissues (Ben-Haroush et al., 2005; Berisha et al., 2004; Katsahambas & Hearn, 1996; Schams et al., 1994; Welter et al., 2004; Wollenhaupt et al., 2004). Only very few studies have been performed on the oviduct, which provides the setting required for gamete transport, oocyte maturation, sperm capacitation, fertilization and early embryonic development (Ellington, 1991). In view of the paucity of information in this area, the present study was designed to immunohistochemically determine the localization of the paracrine factors FGF1, FGF2, FGFR1 and FGFR2 in the rat oviduct. The rat is described as an optimum animal model for research on the reproductive system of humans and several domestic animals (Hamid & Zakaria, 2013; Lee & DeMayo, 2004; Mitchell & Taggart, 2009; Paixao et al., 2017; Walker et al., 2003). This study was also aimed at identifying the possible functions of these proteins during the post-partum period (days 1, 3 and 5), which is particularly significant for early pregnancy and the maintenance of fertility.
2 MATERIALS AND METHODS
2.1 Animals and tissue preparation
The 6- to 8-week-old female Wistar rats used in the present study were of the species Rattus norvegicus, weighed 250–300 g, and were supplied from the Hakan Çetinsaya Experimental and Clinical Research Centre of Erciyes University. The rats were given standard feed and water, and kept in individual cages. The stage of the oestrous cycle was determined by examining vaginal smears taken in the morning (09.00–10.00 hr). Four female rats, which were determined to be in oestrus, were mated with a male rat. The day on which spermatozoa and vaginal mucus were observed in the smears was considered as the first day of pregnancy. On day 5 of pregnancy (Group I) and post-partum days 1 (Group II), 3 (Group III) and 5 (Group IV), five rats per group were anaesthetized by the intraperitoneal injection of 1.5 g/kg urethane. Following the sampling of the right and left oviducts of each rat, the animals were euthanized by cervical dislocation. This study was conducted pursuant to the approval (Decision 10/27 dated 10.03.2010) of the Ethics Board for Experimental Animals.
2.2 Histological analysis
After being fixed in 10% formalin–alcohol solution for 18 hr, the oviduct tissue samples were passed through graded alcohol, methyl benzoate and benzol solutions, and embedded in paraffin. Four-micron-thick cross sections were cut from the paraffin blocks, and five series of sections were prepared. Each series included at least six cross sections of both oviducts of each animal. These were mounted on polylysine-coated slides and dried in an incubator at 37°C. The first series was stained with Crossman's triple stain (Crossman, 1937) to demonstrate the general structure of the different regions of the oviduct tissue (infundibulum, ampulla and isthmus). The other three series were immunostained with the streptavidin–biotin complex to determine the localization of the proteins FGF1, FGF2, FGFR1 and FGFR2.
2.3 Immunohistochemical analysis
The sections prepared for immunohistochemical examination underwent routine histological processing. Accordingly, they were deparaffinized, rehydrated and immersed in 3% hydrogen peroxide in methanol for 15 min. Next, the sections were washed twice, in a 0.01 M phosphate buffer solution (PBS, pH 7.4). Then, the sections were boiled in 80°C citrate buffer (0.01 M, pH 6.0) for 30 min, and were cooled for 20 min. Subsequently, the sections were treated with a blocking solution (Thermo Fisher Scientific, TA-125UB) for 5 min in a humidifier. After being washed twice in PBS, the sections were incubated for overnight at 4°C in the humidity chamber with the primary antibodies [FGF1 (rabbit polyclonal, Abcam, ab9588, 1:150 dilution), FGF2 (mouse monoclonal, Santa Cruz Biotechnology, sc-74412, 1:50 dilution), FGFR1 (FLG) (mouse monoclonal, Santa Cruz Biotechnology, sc-57132, 1:50 dilution) and FGFR2 (BEK) (mouse monoclonal, Santa Cruz Biotechnology, sc-6930, 1:50 dilution)] diluted in antibody diluent solution (Invitrogen corporation, 00-3118; see Table 1). The next day, the sections were washed 4 times in PBS, and were treated with biotinylated goat anti-rabbit secondary antibodies (Thermo Fisher Scientific, TR-125-BN) for FGF1 and with biotinylated anti-mouse secondary antibodies (ScyTec Laboratories, UHM-125) for FGF2, FGFR1 and FGFR2, at room temperature for 20 min. After being washed in PBS, the sections were treated with streptavidin peroxidase (Thermo Fisher Scientific, TS-125-HR) for 20 min. Then, the sections were washed in PBS and treated with 3-3′-diaminobenzidine solution (DAB Plus Substrate System, Thermo Scientific, Lab Vision, TA-125-HDX) for 5–10 min to demonstrate immunoreactivity. For nuclear staining, the sections were first washed in distilled water and treated with Gill's haematoxylin for 5–10 min, and then washed under running tap water until a blue colour developed. After being passed through graded alcohol and xylol solutions, the sections were mounted in Entellan.
| Antibody | Host | Immunogen | Supplier/ Catalogue number | Dilution | Reactivity |
|---|---|---|---|---|---|
| FGF1 antibody | Rabbit polyclonal | Highly pure (>98%) recombinant FGF1 (human Fibroblast Growth Factor-acidic) | Abcam, ab9588 | 1:150 | Rat and human |
| FGF2 antibody (C-2) | Mouse monoclonal | raised against amino acids 10–140 of FGF-2 of human origin | Santa Cruz Biotechnology, sc-74412 | 1:50 | Mouse, rat and human |
| FGFR1 (Flg) antibody (M2F12) | Mouse monoclonal | raised against the ectodomain of Flg isoform α of human origin | Santa Cruz Biotechnology, sc-57132 | 1:50 | Mouse, rat and human |
| FGFR2 (Bek) antibody (C-8) | Mouse monoclonal | specific for an epitope mapping between amino acids 805–821 at the C-terminus of Bek of human origin | Santa Cruz Biotechnology, sc-6930 | 1:50 | Mouse, rat and human |
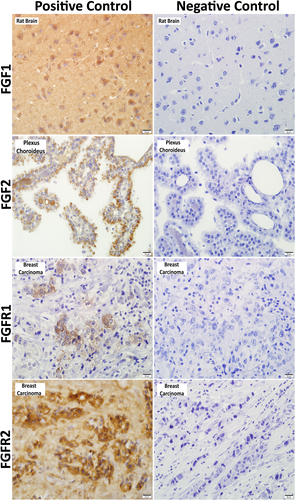
The specificity of the immunohistochemical procedure was checked using negative and positive controls. The positive controls were applied in accordance with the manufacturer's recommendations indicated in the primary antibody data sheets. Accordingly, rat brain sections were used for FGF1 (Ito et al., 2005) and FGF2 (Gonzales et al., 1995), and human breast carcinoma sections were used for FGFR1 (Jacquemier et al., 1994) and FGFR2 (Martin et al., 2011). The tissue samples taken for use as negative controls were incubated with non-immune rabbit IgG (Santa Cruz, sc-2027) and non-immune mouse IgG (Santa Cruz, sc-2025) without the primary antibody. The slides were examined under a light microscope at different magnifications (10×, 20×, 40× and 100×), and were photographed (BX51, Olympus).
2.4 Semi-quantitative analysis
Immunohistochemical staining was assessed semi-quantitatively on the basis of intensity scores (IS; Alan et al., 2015). The intensity of positive cytoplasmic and nuclear immunostaining for FGF1, FGF2, FGFR1 and FGFR2 in the luminal epithelial, stromal, smooth muscle and endothelial cells of the infundibulum, ampulla and isthmus of the oviduct was scored as (−) negative, (+) weak, (++) moderate or (+++) strong. The intensity scores of the cellular immunoreactions were assessed by two independent researchers (E.A., N.L.), and the mean value of the scores given by the two assessors was calculated.
3 RESULTS
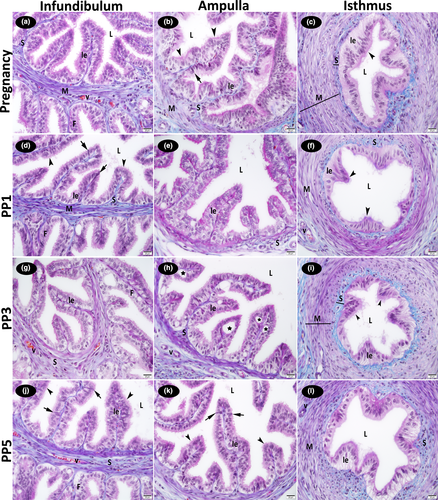
3.1 Histological examination
On all examination days during pregnancy and the post-partum period, it was determined that the luminal epithelium of the oviduct was simple columnar; and in some locations, pseudostratified columnar epithelium. The ciliated cells and non-ciliated cells (sporadic secretory cells) were found in the luminal epithelium of the oviduct infundibulum and ampulla. It was observed that the cytoplasm of the ciliated cells was lighter, and the nuclei were round or oval. In contrast, the cytoplasm of the non-ciliated cells was darker, and the nuclei were less regular. It was determined that the number of non-ciliated cells was high during pregnancy and partially decreased on post-partum days. This decrease was most obvious in the infundibulum and in the ampulla. The presence of numerous cilia was observed on the free border of ciliated cells of the oviduct mucosa. On post-partum days 1st, 3rd and 5th, the cilia on many cells had decreased in number and length. Cells with a vacuolized cytoplasm were present in the ampulla of oviduct on day 3 post-partum. Stromal cells, collagen fibres and blood vessels were found under the basal membrane in the connective tissue (stroma). The microscopic appearance of the oviduct on day 3 post-partum was similar to that on day 5 of the post-partum period observed (Figure 1).
3.2 Immunohistochemical examination
Immunoreactivities were detected in the rat brain (FGF1), rat brain plexus choroideus (FGF2) and human breast carcinoma (FGFR1 and FGFR2) sections, which were used as positive controls for immunohistochemical analysis. On the other hand, immunoreactivity was negative in the tissue samples, which were used as negative controls and were incubated with non-immune rabbit and mouse IgG serum without the primary antibody (Figure 2).
3.2.1 The immunohistochemical localization of FGF1
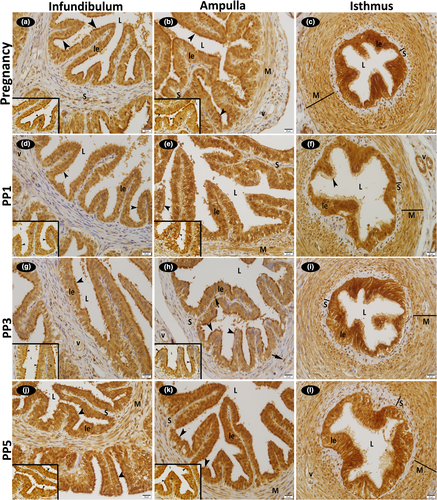
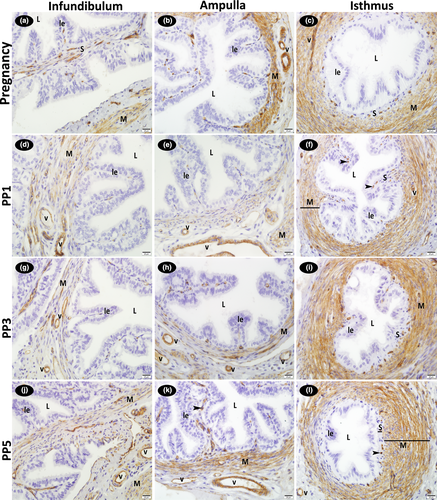
On all examination days during pregnancy and the post-partum period, the luminal epithelial and some stromal cells localized to the connective tissue of the infundibulum, ampulla and isthmus of the oviduct displayed both cytoplasmic and nuclear immunoreactions for FGF1. Immunoreactivity was stronger in the basal bodies of the cilia in the epithelium lining, particularly the infundibulum and ampulla regions of the oviduct and in the luminal epithelium of the all regions of the oviduct. Strong FGF1 immunoreactivity was observed in the cytoplasm and nucleus of the smooth muscle cells in the rather thick muscle layer of the ampulla and isthmus wall. Strong positive FGF1 immunoreactions were also detected in the cytoplasm and nucleus of the vascular endothelial and medial smooth muscle cells in the infundibulum, ampulla and isthmus regions of the oviduct. No difference existed between pregnancy and the post-partum period for FGF1 immunoreactions (Figure 3; Table 2).
| FGF1 | FGF2 | FGFR1 | FGFR2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preg | PP1 | PP3 | PP5 | Preg | PP1 | PP3 | PP5 | Preg | PP1 | PP3 | PP5 | Preg | PP1 | PP3 | PP5 | |
| Infundibulum | ||||||||||||||||
| Luminal epithelium | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Stromal cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Smooth muscle cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Endothelial cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- |
| Ampulla | ||||||||||||||||
| Luminal epithelium | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Stromal cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Smooth muscle cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Endothelial cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- |
| Isthmus | ||||||||||||||||
| Luminal epithelium | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/++, n/- | c/++, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Stromal cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/++, n/- | c/++, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Smooth muscle cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/++, n/- | c/++, n/- | c/++, n/- | c/++, n/- | c/+, n/- | c/+, n/- | c/+, n/- | c/+, n/- |
| Endothelial cells | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/+++ | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/+++, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- | c/-, n/- |
3.3 The immunohistochemical localization of FGF2
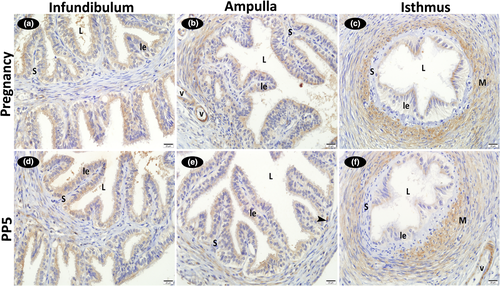
Strong positive FGF2 immunoreactions were detected in the cytoplasm of the vascular endothelial and medial smooth muscle cells in the infundibulum, ampulla and isthmus regions of the oviduct. Strong FGF2 immunoreactivity was also observed in the cytoplasm of the smooth muscle cells in the rather thick muscle layer of the ampulla and isthmus wall. While positive reactions were determined in the cytoplasm of the stromal cells localized to the lamina propria throughout the oviduct, nuclear reactions were absent in these cells. Reactions were also negative in the luminal epithelium. No difference was detected between the pregnancy and post-partum examination days for immunoreaction intensity (Figure 4; Table 2).
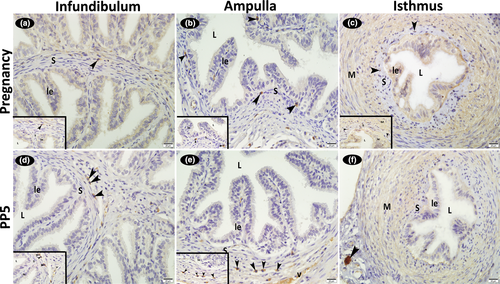
3.3.1 The immunohistochemical localization of FGFR1 and FGFR2
Immunoreactivity for FGFR1 and FGFR2 was weak in the cytoplasm of the luminal epithelial, some stromal cells, smooth muscle cells and vascular media of the infundibulum, ampulla and isthmus; nuclear reactions were absent in these cells. Also, reactions were negative in the endothelial cells (Figures 5 and 6; Table 2). When a comparison was made in terms of FGFR1 immunoreactivity between the days examined, it was seen that it was stronger in the luminal epithelium in early pregnancy. It was also noteworthy that the FGFR1 immunoreaction is stronger in the muscular layer of the isthmus compared to the infundibulum and ampulla in both early pregnancy and involution periods (Figure 5; Table 2).
4 DISCUSSION
Previous research has demonstrated that FGFs, which are an important family of growth factors, are expressed in the oviduct of avians (Harem et al., 2013) and mammals (cattle, marmoset monkey and pigs; Gabler et al., 1997, 1998; Wollenhaupt et al., 2004). The prototypes of the FGFs, the most extensively studied growth factors in the female genital tract, are of acidic (FGF 1, aFGF) and basic (FGF 2, bFGF) forms. Diverse results have been reported on whether the expression of FGFs alters with the sexual cycle, early pregnancy and implantation period. While Katsahambas and Hearn (1996) reported no alteration in FGF2 expression during the sexual cycle and early pregnancy, Wollenhaupt et al. (2004) determined the presence of FGF2 and its receptors (FGFR1IIIc, FGFR2IIIc) in the porcine oviduct and endometrium during the sexual cycle and implantation period. Also, Wollenhaupt et al. (2004) suggested a critical role for maternal steroids in regulating the expression and bioactivation of FGFs, which are essential to the success of conception and continuance of pregnancy in pigs. All components of the FGF system have been shown to be also expressed in the epithelium of the bovine oviduct. It has been reported that in the bovine oviduct, when compared to the luteal phase, ovulation is associated with significantly higher FGF1 protein levels. This suggests FGF1 to be regulated in relation to the sexual cycle. On the other hand, no cyclic alteration has been detected for FGF2 and FGFR2 mRNA. Thus, it is suggested that the FGF system could be responsible for an autocrine or a paracrine mechanism in the bovine oviduct, including the oviduct epithelium and cumulus–oocyte complexes (Gabler et al., 1997). The investigation of the oviduct of the marmoset monkey revealed that the expression of FGF1, FGF2 and FGFR2 mRNA was highest during the late proliferative and early secretory phases of the sexual cycle. This demonstrated that the autocrine and paracrine effects of the growth factors investigated in the marmoset monkey were regulated by oestrogen (Gabler et al., 1998). Although there are many studies in the sexual cycle period, the study examining the effect of FGFs on the female genital system in the post-partum period is quite limited. In the study investigating the possible effects of FGFR on endometrial involution in goats, it was found that epithelial, glandular and stromal cells showed FGFR immunoreactivity, and there was no difference between caruncular and intercaruncular epithelium in terms of staining intensity. In addition, it was stated that the highest level of FGFR expression was found on post-partum 10th and 16th days when epithelial and stromal cellular regeneration was observed, and therefore, uterine involution in goats was associated with changes in FGFR expression (Sánchez et al., 2002). In the present study, which investigated the rat oviduct during early pregnancy and the post-partum period, similar to the results of Katsahambas and Hearn (1996), no difference was detected between the two periods for FGF1, FGF2, FGFR1 and FGFR2 immunoreactivity scores. Furthermore, no difference was observed between the different regions of the oviduct (infundibulum, ampulla and isthmus) for immunoreaction intensity. However, the presence of FGF1, FGF2, FGFR1 and FGFR2 in luminal epithelium, some stromal cells, smooth muscle cells and endothelial cells of the rat oviduct during the post-partum period indicates that it plays an important role in processes of cellular regeneration, healing and neovascularization, as has previously been stated in other studies (Burgess & Maciag, 1989).
Previous reports indicate that heparin sulphate, one of the main glycosaminoglycans (GAGs) of the extracellular matrix, is localized to the cell surface, and the biological activation of FGF1 and FGF2 is initiated by their binding to heparin sulphate. It is also reported that heparin sulphate protects FGFs from being degraded and acts as a cell surface receptor (Rapraeger et al., 1991; Einspanier et al., 1996). As is the case in cattle (Gabler et al., 1997), the presence of heparin sulphate-like structures on the surface of the luminal epithelium in the primate oviduct indicates that FGF1 and FGF2 can readily bind to the surface of the oviduct, and that this setting serves as a reservoir for growth factors, which are also mediated by FGFR. The presence of these proteins on the luminal surface of epithelial cells shows that they can also be secreted into the lumen at the time of ovulation (Gabler et al., 1998). Different from the findings reported by Gabler et al. for cattle (1997) and the marmoset monkey (1998), in the rat oviduct, the luminal epithelium of the infundibulum, ampulla and isthmus displayed immunoreactivity for FGF1, FGFR1 and FGFR2. The FGFR1 and FGFR2 immunoreactions were weaker, and no immunoreaction was detected for FGF2. Furthermore, the basal bodies of the cilia in the epithelium lining the infundibulum and ampulla displayed stronger immunoreactivity for FGF1. This suggests that positive immunostaining for FGF1, FGFR1 and FGFR2 in these regions of the oviduct could be related to heparin sulphate.
Throughout the rat oviduct, FGF2 immunoreactions were strongly positive in the cytoplasm of the vascular endothelial and smooth muscle cells as well as in the stromal cells. In agreement with the present study, Schams et al. (1994) detected immunoreactive FGF2 in the endothelial cells of bovine capillaries, and positive immunoreactions in the basal membrane. The positive FGF2 immunoreactivity detected in the endothelial cells indicates that FGF2 plays a stimulating role in angiogenesis. FGF2 induces angiogenesis by increasing the levels of VEGF and its receptors in the endothelium (Pepper et al., 1992). In agreement with the present study, which demonstrated FGF1, FGF2, FGFR1 and FGFR2 immunoreactivity in the stromal cells of the rat oviduct, Welter et al. (2004) detected positive immunoreactivity for FGF2 and its receptor FGFR1 in the stromal cells of the porcine endometrium during pregnancy. These researchers suggested that the FGF system served as a stromal growth factor system and mediated the conversion of the endometrium to decidua during pregnancy, a period characterized by high progesterone levels. The different localizations of FGF2 are attributed to the presence of different FGF2 molecules, known to be associated with endothelial or epithelial cells (Schams et al., 1994). Schams et al. (1994) described two forms of FGF2, including a higher molecular weight of 18 kDa, and lower molecular weight of 16 kDa, which were variously expressed through pregnancy. It has also been shown that FGF2, which is expressed in the majority of adult and fetal human tissues, mediates the proliferation of fibroblasts and endothelial cells (Smith, 1995).
Based on previous research, it has been reported that functional growth factors, such as FGF and IGF, affect early embryonic development in the oviduct of the marmoset monkey, and their expression and bioactivation have a significant role in embryogenesis. The autocrine and paracrine interactions between growth factors and oviduct epithelial cells are involved in embryo-related epithelial physiology (Gabler et al., 1998). In this context, it has been reported that FGF2 induces the in vitro development of the mesoderm in rabbits (Hrabe de Angelis & Kirchner, 1993), and the development of bovine morulae into blastocysts. Thus, it has been suggested that FGF2 acts as a stimulant of embryonic differentiation in the mammalian oviduct (Larson et al., 1992). Similar to reports for the marmoset monkey (Gabler et al., 1998) and cattle (Larson et al., 1992), the present study, in which FGF2 immunoexpression was detected in the rat oviduct during early pregnancy, suggests that owing to its effects on early embryonic development, bioactivated FGF could have an important role in embryogenesis.
In conclusion, this study documents for the first time the localizations and distributions of FGF family members such as FGF1, FGF2, FGFR1 and FGFR2 in the rat oviduct during the early pregnancy and the post-partum period. Our data were limited to a semi-quantitative immunohistochemical analysis; therefore, we could not determine the exact function of the fibroblast growth factors in the post-partum process. However, considering the cellular localizations of these proteins (FGF1, FGF2, FGFR1 and FGFR2) in the oviduct cells, it seems now that the process of tissue remodelling such as proliferation, differentiation and angiogenesis during early pregnancy and the post-partum period in the rat oviduct may be precisely regulated not only by hormonal mechanisms, but also by growth factors. But still, further experiments will be necessary to understand the specific roles of fibroblast growth factors at the intracellular and molecular level and to determine how and if these proteins control biological activities such as proliferation, differentiation and angiogenesis during early pregnancy and the post-partum period.
CONFLICT OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.