Gross anatomical study of the thoracolumbar primary dorsal branches and their course from the spinal cord to the skin in the cat
Abstract
The course of spinal nerves and the corresponding cutaneous areas are fundamental for numerous therapeutic approaches used in complementary veterinary medicine. Positive effects of these methods are primarily based on segmental reflex arcs which are associated with the course of the spinal nerves. In this morphological study, the lateral cutaneous branches of the thoracolumbar dorsal branches from Th9 to L7 were examined in cats with special regard to their anatomical course. A four-layer dissection was carried out to reveal the course of nerves between the intervertebral foramina and their point of entry into the skin, starting in the dorsal midline. Dorsal branch courses and covered distances were documented and measured in each layer. The covered distance was evaluated by the Caudal Shift Index (CSIn) on both body sides and within each layer. The ‘back region’ was used as relative dimensional unit, describing the distance between the cranial tips of two consecutive spinous processes. Overall, the mean CSIn for dorsal branches of Th9 to L7 amounted to three back regions from the intervertebral foramen to the skin entry point of a dorsal nerve branch. This provides therapists with clues and should be put into practice, by extending the treatment area up to three segments caudally from the nerve exit point. Furthermore, the results of this study present new data on inferred lumbar dermatomes in cats, data which until now have only been transferred from other species. These results may serve as an anatomical foundation for manual therapies.
1 INTRODUCTION
Based on the embryological development of the spinal cord and vertebral column, dermatomes and myotomes are interconnected with each other through corresponding spinal nerves and the medulla spinalis (Stoffel, 2011; van Cranenburgh, 1990; Wancura-Kampik & Fanghänel, 2009; Zohmann, 1991; Zohmann & Kasper, 1994). The spinal nerves follow the expansion of the growing body surface (DeLahunta & Glass, 2009) and represent the anatomical basis for interactive segmental reflex arcs (Kasper, 2011b; Nickel, Schummer, & Seiferle, 2003b; Wancura-Kampik & Fanghänel, 2009; Zohmann, 1989). These relationships are widely used for diagnosis, therapies and anaesthesia (Kasper, 2011a, 2011b; Nickel, Schummer, & Seiferle, 2003a; Stoffel, 2011).
Dermatomes are skin areas of the trunk innervated by cutaneous branches of spinal nerves’ dorsal and ventral branches. While their existence is well established, their surface locations have not been systematically studied in animals. Some authors like Bailey, Kitchell, Haghighi, and Johnson (1984), Hekmatpanah (1961), Kuhn (1953) and Reid (1970) examined these cutaneous branches and their innervation areas in cats and dogs.
Based on fetal development and the expansion of the body surface, the entry of spinal nerves into the skin is located caudal in relation to their exit at the intervertebral spaces (Arnold & Kitchell, 1957; Bernigau, 2013). In case the caudal lumbar nerve branches are missing (Maigne, Lazareth, Surville, & Maigne, 1989; Salomon, 2015), the overlap between other dermatomes ensures nerve supply to skin areas without an own nerve branch (Bailey et al., 1984).
Sato (1995) and van Cranenburgh (1990), among others, described that skin stimulation can have effects on visceral organs. This interaction is known as cutivisceral reflex (Wancura-Kampik & Fanghänel, 2009) and can be used in therapeutic approaches. It has been shown that placing acupuncture needles in corresponding dermatomes achieves the best results in treating visceral organs, irrespective of their meridian attribution (Sanchez-Araujo & Luckert-Barela, 2014). Moreover, visceral disorders can show superficial neurogenic inflammation spots associated to acupuncture points which are located in corresponding dermatomes (Kim et al., 2017).
Although a defined morphological correlation of acupuncture points is yet to be proven, neurovascular bundles have been described under many acupuncture points (Egerbacher, 1991, 1993; Heine, 1988). Furthermore, studies about influencing visceral organs via skin stimulation, like reducing feline urethral sphincter spasm by stimulating sacral dermatomes, have shown encouraging results (McCoin, Bhadra, & Gustafson, 2013).
Studies of feline thoracolumbar skin innervation are scarce and, to date, have only assessed limited regions (e.g. Kukulinsky & Brown, 1979; Reid, 1970). In addition, to the best of our knowledge, very little is known about the shape and location of feline dermatomes (e.g. Brown & Koerber, 1978). Moreover, none of the studies describe nerve distances covered between their origin at the intervertebral foramen and their skin entry point.
Hence, the goal of the present study was to gain morphological understanding of innervation patterns and the course of cutaneous nerve branches of feline spinal nerves.
2 MATERIAL AND METHODS
2.1 Dissection and collection of data
Fifteen European shorthair cats were dissected for this study. All cats were donated to the Institute of Veterinary Anatomy for educational and scientific purposes by their owners. All animals had been euthanised by their referring veterinarian based on medical indications or due to terminal illness, that is for reasons unrelated to this study. Euthanasia was done in compliance with the national and European animal welfare regulations. Each animal was injected with 4%-formalin solution through the femoral artery within 2 hr after euthanasia, immediately upon arrival at the Institute. Mean fixation time was 3 days, according to height, size and physical constitution of the cat.
After fixation, the dissection started in the dorsal midline between scapula and tail root. Cutaneous branches of the thoracolumbar dorsal branches on their way between the skin and the intervertebral foramina from the ninth thoracic vertebra (Th9) to the seventh lumbar vertebra (L7) were examined. This region (Th9–L7) was chosen based on preparation techniques and given anatomical conditions.
One specimen (Cat 7) was excluded from the study after dissection revealed a shortened lumbar spine. After exclusion of this cat, a total of 14 cats were left for investigation.
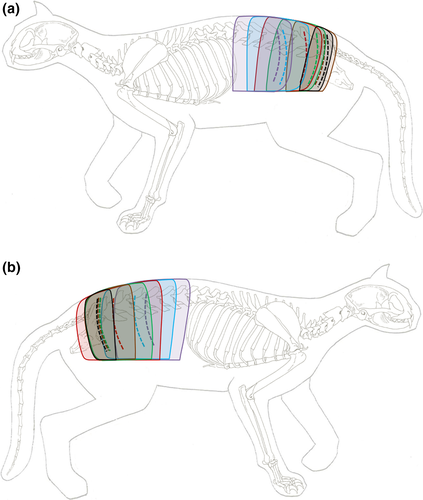
Dissection extended from superficial to deep tissue and was carried out in four layers (Figure 1). The first layer exposed the skin entry points of the dorsal branches (Layer 1—skin). The second layer revealed the dorsal branches of the thoracolumbar fascia (Layer 2—fascia) and was followed by the third layer, which showed nerve passage through the back muscles (Layer 3—musculature). Finally, the intervertebral origin of the dorsal branches was found in the fourth layer (Layer 4—intervertebral foramen).
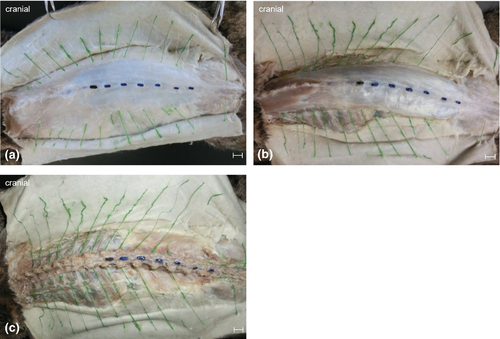
Anatomical landmarks were visualised with acrylic paint. All dissected dorsal branches were painted green. The first lumbar vertebra (L1) was painted black while L2 to L7 were coloured blue. Entry and exit points of the dorsal branches within the examined region were recorded. For this purpose, transverse planes were used to allocate specific back regions based on the respective spinous process as they were easily palpable (Figure 2). A ‘back region’ was defined as the distance between the cranial borders of two consecutive spinous processes (Bernigau, 2013).
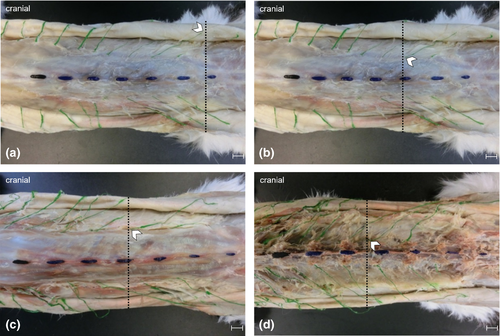
In order to identify the cutaneous branches of the dorsal branches in Layer 1, subcutaneous fat and connective tissue were removed. Subsequently, the cutaneous trunk muscle was detached and placed aside along with the skin. Following this step, nerve branches were visualised and their location within the back regions was recorded (Figure 2a). In Layer 2, the cutaneous branches´ passage through the thoracolumbar fascia was traced and recorded as in Layer 1 (Figure 2b).
To continue tracing the dorsal branches towards the spine, it was necessary to remove the thoracolumbar fascia in Layer 3. The fascia was cut open at the nerves’ point of emergence. In addition, trapezius and latissimus dorsi muscles were partly removed. The nerve branches´ location was recorded as in the previous layers (Figure 2c).
To expose the nerve origins at the intervertebral foramina in Layer 4, the long epaxial muscles of the back were removed. Furthermore, branches innervating musculature were severed and cutaneous branches were further dissected. The related intervertebral foramina and the dorsal branches´ exit points were determined as in previous layers (Figure 2d).
2.2 Determination of the Caudal Shift Index (CSI)
According to Bernigau (2013), the Caudal Shift Index (CSI) provides information about the distance covered by a nerve branch between its exit from the intervertebral foramen via its craniocaudal course through all tissue layers until skin entry.
The following formulas were used for calculating each CSI (Bernigau, 2013):
CSI (x)
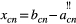
CSI of a particular nerve branch (CSIn)
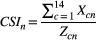
The CSI for each dorsal branch was determined for 14 cats on each layer and both body sides. To obtain the mean CSIn of a specific nerve, the CSIs were added and divided by the number of involved nerve branches. Therefore, the CSIn was determined for each nerve branch between Th9 and L7 for the Layer 1 (skin, CSIn-S), Layer 2 (fascia, CSIn-F) and Layer 3 (musculature, CSIn-M).
CSIn-S was used to describe the cutaneous innervation areas in the thoracolumbar region.
2.3 Statistical analysis
Statistical analysis was conducted with IBM SPSS Statistics 25 Software (IBM Corporation 1989, 2017). The Shapiro–Wilk test was applied to determine normal distribution. Due to non-parametric data frames, statistical significances between the body sides for the CSIn-S, CSIn-F and CSIn-M were tested using the Mann–Whitney U test. CSIn of the Layers 1, 2 and 3 for both sides were compared with the Kruskal–Wallis H test. The post hoc correction was carried out with the Bonferroni procedure. Differences were significant at p < .05. The correlation coefficient was evaluated according to Cohen (2013).
3 RESULTS
To demonstrate characteristics and symmetry of nerve branches in the thoracolumbar region, the dissection was carried out in four layers. The total number of symmetrical and bilaterally present dorsal branches varied between six and eleven (Table 1).
| Th9 | Th10 | Th11 | Th12 | Th13 | L1 | L2 | L3 | L4 | L5 | L6 | L7 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cat 1 | both | both | both | both | both | both | both | both | both | both | – | both |
| Cat 2 | both | both | both | both | both | both | both VR r | both VR r | both | both | – | right |
| Cat 3 | both | both | both | both | both | both | both | both | both | – | – | – |
| Cat 4 | both | left | both | both | both | both | both | both | both | both | – | left |
| Cat 5 | both | both | both | both | both | both | both | both | both | right | – | right |
| Cat 6 | both | both | both | both | both | both | both | both | – | – | – | – |
| Cat 8 | both | both | both | both | both | both | both | both | both | – | – | – |
| Cat 9 | right | both | left | both | both | both | both | both | both | – | – | both |
| Cat 10 | both | both | both | both VR b | right | both | both | both | both | both | – | both |
| Cat 11 | both | both | both | both | both | both | both | both | both | both | – | – |
| Cat 12 | both | both | both | both | both | both | both | both | both | left | – | – |
| Cat 13 | left | both | right | both | both | both | both | both | both | – | – | – |
| Cat 14 | both | both | both | both | both | both | both | both | both | – | – | right |
| Cat 15 | both | both | both | left | left VR r | both | both | both | left | – | – | – |
Note
- right = right side, left = left side, both = bilateral, – = missing, VR r = ventral branch on the right side, VR l = ventral branch on the left side, VR b = ventral branches on both sides.
3.1 Dorsal branches Th9–Th13
In nine cats, all the dorsal branches of Th9 to Th13 were found on both sides, while some of the dorsal branches could not be identified in the remaining five cats. The left dorsal branches of Th10 and Th12 were present in all 14 cats.
The right dorsal branch of Th9 was untraceable in Cat 13, while the left dorsal branch of Th9 was not identified in Cat 9. The right dorsal branch of Th10 was undetectable in Cat 4. Furthermore, the dorsal branch of Th11 was unilaterally undetected in Cats 9 (right) and 13 (left). The right dorsal branch of Th12 was not identified in Cat 15. Finally, the dorsal branches of Th13 were unilaterally untraceable in Cats 10 (left) and 15 (right).
3.2 Dorsal branches L1–L7
The bilateral dorsal branches of L1 to L3 were found in all 14 cats. Furthermore, the presence of the dorsal branches of L4 showed only two exceptions. In Cat 6, the nerve branch was missing completely, and in Cat 15, it could not be found on the right side.
A strong irregularity in the dorsal branches could be detected caudal to the fourth lumbar vertebra. Bilateral dorsal branches of L5 were only traceable in five of fourteen cats, while unilateral presence was detected in two additional cats (Cat 5 and 12) and the dorsal branches appeared to be undetectable in the remaining cats. Dorsal branches of L6 were lacking in all cats. Moreover, branches for the musculature were unverifiable, too. Bilateral dorsal branches of L7 were found in three cats (Cats 1, 9 and 10). In addition, unilateral branches were identified in four cats (Cats 2, 4, 5 and 14; Table 1).
3.3 Ventral branches
Three cats had ventral branches, which entered the skin at the same level as the dorsal branches. In Cat 15, the ventral branch took over as the dorsal branch was untraceable. In the other two cats, ventral branches were found arising on the same side and reaching the skin at the same level as the corresponding dorsal branch. This observation was made in the right lumbar region of Cat 2 and bilateral thoracic regions of Cat 10 (Table 1).
3.4 Nerve courses
By tracing the nerves’ passage through each layer, it was possible to measure the distance between the intervertebral foramina and skin entry points. The Caudal Shift Index (CSI) was used to describe the covered distance for each dorsal branch, each layer and each body side. The mean values of the CSIn for the individual dorsal branches of Th9 to L7 are presented in Table 2. The observed ventral branches followed the same course through the layers as the dorsal branches of the same origin.
| left | right | |||||||
|---|---|---|---|---|---|---|---|---|
| n | CSIn-S | CSIn-F | CSIn-M | n | CSIn-S | CSIn-F | CSIn-M | |
| Th9 | 13 | 2.61 ± 1.27 | 3.00 ± 0.96 | 1.30 ± 1.26 | 13 | 1.84 ± 1.70 | 2.76 ± 0.89 | 0.92 ± 0.82 |
| Th10 | 14 | 2.78 ± 1.14 | 2.92 ± 0.88 | 1.28 ± 1.03 | 13 | 2.07 ± 1.32 | 2.69 ± 0.82 | 0.92 ± 0.91 |
| Th11 | 13 | 2.61 ± 1.14 | 2.46 ± 1.00 | 1.15 ± 1.09 | 13 | 2.46 ± 1.49 | 2.53 ± 1.21 | 1.00 ± 0.96 |
| Th12 | 14 | 2.92 ± 0.96 | 2.35 ± 0.81 | 1.07 ± 0.88 | 13 | 2.46 ± 1.21 | 2.15 ± 0.66 | 0.84 ± 0.66 |
| Th13 | 13 | 3.23 ± 0.97 | 2.00 ± 0.39 | 0.92 ± 0.47 | 13 | 3.15 ± 1.16 | 2.15 ± 0.66 | 1.00 ± 0.55 |
| L1 | 14 | 3.07 ± 0.88 | 1.92 ± 0.45 | 0.92 ± 0.45 | 14 | 3.07 ± 1.09 | 2.14 ± 0.91 | 0.85 ± 0.51 |
| L2 | 14 | 2.64 ± 1.23 | 1.71 ± 0.79 | 1.00 ± 0.37 | 14 | 2.85 ± 1.12 | 1.92 ± 0.88 | 0.92 ± 0.45 |
| L3 | 14 | 2.85 ± 0.91 | 1.78 ± 0.41 | 1.00 ± 0.37 | 14 | 3.07 ± 1.98 | 2.21 ± 1.42 | 0.92 ± 0.45 |
| L4 | 13 | 2.92 ± 1.14 | 1.76 ± 0.42 | 1.07 ± 0.47 | 12 | 3.16 ± 1.28 | 1.83 ± 0.55 | 1.08 ± 0.64 |
| L5 | 6 | 2.83 ± 1.34 | 1.66 ± 1.10 | 1.00 ± 0.57 | 6 | 2.66 ± 1.49 | 1.16 ± 0.37 | 0.83 ± 0.37 |
| L7 | 4 | 1.25 ± 0.82 | 0.50 ± 1.50 | 0.75 ± 1.29 | 6 | 0.83 ± 1.21 | 1.00 ± 1.41 | 1.00 ± 1.15 |
Note
- n = number of nerve branches used to compute the average.
In detail, the CSIn values in Layer 1 (skin, CSIn-S) show a mean expansion of three back regions for nerves branches originating from Th10 to L5. CSIn-S values of the dorsal branches of Th9 differed between the body sides and had a mean value of two back regions on the right and three on the left side.
In Layer 2 (fascia, CSIn-F), mean CSIn-F of the dorsal branches of Th9 and Th10 were three back regions. The mean CSIn-F for the dorsal branches of Th11 to L4 was two back regions, while the mean CSIn-F for the right dorsal branch of L5 was one back region.
In Layer 3 (musculature, CSIn-M), mean CSIn-M was one back region for each dorsal branch of Th9 to L5.
Finally, the dorsal branches of L7 had an average CSIn of one in all layers on both sides.
3.5 Lumbosacral cutaneous innervation areas
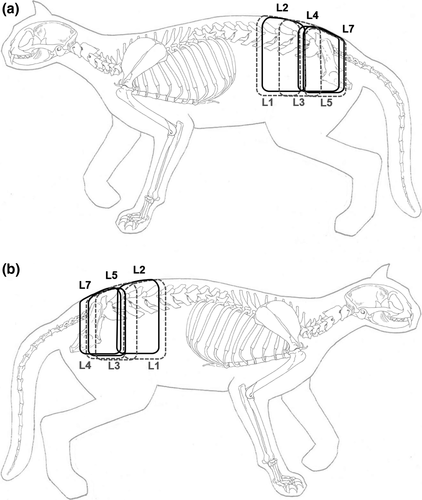
Correlations between nerve branches and innervated skin regions for each dorsal branch were determined based on mean CSIn-S values. The highest CSIn-S (CSIn-S max) and smallest CSIn-S (CSIn-S min) values for each dorsal branch provided a general idea of the extent of the innervated cutaneous area and was used to map innervated lumbar cutaneous areas for the dorsal branches of L1 to L7 (Figure 3). These CSIn-S based areas show only the entry points of the nerve branches into the subcutaneous tissue and should not be considered as dermatomes.
The map illustrates the overlap between innervation areas that covers up to two-thirds of an innervation area (Figure 3). In addition, it was observed that the CSIn-S min values were located in the anterior third of the preceding innervation area, whereas the CSIn-S max were located in the posterior third of the following innervation area. In most cases, the mean CSIn-S of each dorsal branch was found in the posterior third of the corresponding innervation area (Figure 3).
The extent of innervation areas decreased towards the caudal spinal column, while overlap increased correspondingly (Figure 3). Hence, innervation of cutaneous areas between L1 and L7 was continuously given, despite the absence of a dorsal branch originating from L6.
3.6 Statistical evaluation
No statistically significant differences were found between the left and right body side for the CSIn-S (p = .652), CSIn-F (p = .652) and CSIn-M (p = .065) values.
However, significant differences within the left (χ2 = 20.160, df = 2, p < .001) and the right (χ2 = 18.068, df = 2, p < .001) body side were found. A strong correlation was observed between CSIn-M and CSIn-F (left: r = 0.57, p = .021; right: r = 0.65, p = .006) as well as between CSIn-M and CSIn-S (left: r = 0.95, p < .001; right: r = 0.86, p < .001).
4 DISCUSSION
The number of bilaterally identified symmetrical nerve branches among the fourteen dissected cats varied between six and eleven. The most constant and symmetrical pattern was present in the dorsal branches of Th9 to L3 and found in nine cats. The more caudal nerve branches from L4 to L7 showed higher variations in the pattern of their course through the different layers towards the skin.
A cutaneous branch of the dorsal branch of L6 could not be detected in any of the dissected cats. The following dorsal branch of L7 was also absent in half of the cats. The absence of the cutaneous branch of the dorsal branch of L6 and L7 is a physiological phenomenon that has been reported in previous studies by Bogduk (1976) and Salomon (2015) for cats and dogs. Due to the chosen dissection technique, the evaluation of existence and course of the corresponding muscular nerve branches originating from L6 and L7 were beyond the scope of this study. As shown in Figure 3, the overlaps of cutaneous areas of the dorsal branches of L3 to L5 guarantee nerve supply of the caudal lumbar and sacral region. Hence, cutaneous branches of dorsal branches of L6 and L7 do not appear to be essential.
Our results indicate that the course of the ventral branches is subject to some variation and, therefore, may differ from general descriptions as found in anatomical textbooks such as Budras (2004) or Nickel et al. (2003a). In one cat, the right ventral branch of Th13 extended so far dorsally that it perforated the skin region, while the dorsal branch was missing. In contrast, the remaining ventral branches emerged in areas where the dorsal branches were present. All of these ventral branches perforated the thoracolumbar fascia on the same level as the dorsal branches did and innervated areas which correspond to those from the dorsal branches. Similar observations have been reported in dogs (Bernigau, 2013). The study found that 50 per cent of the examined dogs also exhibited ventral branches supplying cutaneous areas usually being innervated by dorsal branches.
A study of the nervous and vascular growth during embryonic development by Cui and Yuan (2007) showed that nerve branches and blood vessels have varying individual patterns in their approach to the body surface. This is in line with the fact that nerve branches are associated with blood vessels as a neurovascular bundle and provides a rationale for the variability in nerve courses and their innervation regions.
The CSIn provides information on the distance nerve branches cover on their way through the layers from the intervertebral foramen to the skin which equals an average area of three back regions for CSIn-S. This finding is supported by previous reports. A study on dogs found the same caudal shift ratio between the layers (Bernigau, 2013). Moreover, Bailey et al. (1984) described a caudal shift of lumbar dermatomes corresponding to the length of about three vertebrae. In addition, Kuhn (1953) described a general dermatome caudal shift in cats, although the study did not provide dimensions. There is a good body of evidence that the CSI is a physiological morphological finding in dogs and cats, which needs to be considered in the conduction of different manual therapy methods.
- Spatial limitations towards the trunk end in combination with the reported caudal shift of nerve branches on their course to the skin.
- Spatial proximity to the lumbosacral plexus which provides its own cutaneous nerves for the hind limb and sacral/gluteal region.
During embryonic and fetal development, the initial growth of axons is not determined by defined segments (Wang & Scott, 1999). Hereby, the ectoderm seems to play a key role for the directed sprouting of nerves. The development of the skin and sensory nerves takes place simultaneously (O’Brien et al., 2012). Growing nerve branches release inhibitory messenger substances which affect other axons (Tessier-Lavigne & Goodman, 1996). Furthermore, it is possible that some nerve branches respond to growth stimuli faster than others.
In the current investigation, the dorsal branches of L6 were undetectable, while the branches of L7 were only inconsistently present. In addition, the dorsal branches L3 to L5 showed a large coverage to the caudal lumbar and sacral region. Therefore, it can be hypothesised that the skin in this region mainly receives its innervation from nerve branches originating between L3 and L5. These branches have a larger CSIn and thus are much longer than the more caudal branches. Consequently, the subsequent nerve branches are limited in their caudal extension. Because of the close proximity to the trunk and the innervation coverage provided by the preceding nerve branches, there is no need for cutaneous branches in this area, which is why they show decreased length or total absence. This explanation is also applicable to the involvement of ventral branches, for example from the lumbosacral plexus. Additional investigations should be carried out to obtain more information.
In general, the CSIn of the dorsal branches only showed small differences between both sides. The CSIn-F showed the most distinct differences when considering the values from cranial to caudal. This layer is the only one, in which the CSIn continuously decreased from 2.76 and 3.00 (Th9, right/left) to 1.16 and 1.66 (L5 right/left). However, since the most dorsal branches had an average CSIn-F of two, this value can be accepted as an average for the CSIn-F in this layer. There is no obvious anatomical explanation for this.
Based on the mean values of three back regions for the CSIn-S, of two for the CSIn-F and of one for the CSIn-M, one can derive a ratio of 3:2:1 for all dorsal branches between the individual layers.
The statistical analysis showed no significant differences for the CSIn between the body sides. This result is reducible to the general bilateral symmetry of nerves. The comparison of CSIn values of the same side among each other show significant differences between CSIn-M and CSIn-F as well as CSIn-M and CSIn-S, but no significant differences between the CSIn-F and CSIn-S. Two facts must be considered with these findings: On the one hand, the space between Layers 1 and 2 (skin and fascia) as well as Layers 3 and 4 (musculature and intervertebral foramen) is smaller than between Layer 1 and 3 (skin and musculature) as well as between Layer 2 and 3 (fascia and musculature). On the other hand, dorsal branches show a higher variability in their caudal shift towards the body surface. Both aspects can explain the significant differences and high correlation between the respective layers and their CSIn of the same body side.
The findings concerning CSIn indicate the presence of innervation areas of the dorsal branches for the thoracolumbar region, which correspond with so-called ‘subcutaneous dermatomes’ (Elze, 1961; Wancura-Kampik & Fanghänel, 2009). The CSIn values calculated in this study only refer to the macroscopically detected entry points of the dorsal branches into the skin and do not consider the expansion of intracutaneous branching.
Hekmatpanah (1961) found evidence of an increased electrical activity at the centre of a dermatome. This leads to the hypothesis that this is the entry point of dorsal branches into the skin, which is supported by findings of Bailey et al. (1984) who described a central innervation zone of the dermatome.
Furthermore, the size of a dermatome in dogs amounts to the length one and a half vertebral bodies (Bailey et al., 1984), whereas the expansion of a dermatome in the cat amounts to about the length of three vertebral bodies (Hekmatpanah, 1961). When comparing the cranial and caudal borders of dermatomes from the studies of Bailey et al. (1984) and Hekmatpanah (1961) with the findings presented here, it can be assumed that there are correlations between the caudal border of the dermatome and the CSIn-S max but there are differences between the cranial border of the dermatome and the CSIn-S min. This indicates variations in the nerve supply between cats and dogs (Table 3).
| L1 | L2 | L3 | L4 | |||
|---|---|---|---|---|---|---|
| cranial border | dog | Bailey et al. (1984) | LV 4–5 | LV 5–6 | LV 6–7 | LV 7-SV 1 |
| cat | Hekmatpanah (1961) | LV 2 | LV 2–3 | LV 4 | ― | |
| CSIn-S min left | LV 2 | LV 3 | LV 4 | LV 5 | ||
| CSIn-S min right | LV 2 | LV 3 | LV 4 | LV 5 | ||
| caudal border | dog | Bailey et al. (1984) | LV 5–7 | LV 6–7 | LV 7-SV 3 | SV 1–3 |
| cat | Hekmatpanah (1961) | LV 4–5 | LV 4 | LV 6–7 | ― | |
| CSIn-S max left | LV 5 | LV 6 | LV 7 | SV 2 | ||
| CSIn-S max right | LV 6 | LV 7 | CV 2 | SV 3 | ||
- Abbreviations: Cv, coccygeal vertebra; Lv, lumbar vertebra; Sv, sacral vertebra.
Although the determination of dermatomes is usually conducted by electrophysiological studies, we propose to delineate dermatomes by combining the mean entry points (CSIn-S) of our study with the findings of Hekmatpanah (1961), who reported a dermatome to extend over the length of three vertebrae. For this, one and a half vertebral body length in cranial and caudal direction was added to the CSIn-S to determine the dermatome borders. The dermatomes of the cats examined in this study can be deduced like as shown in Figure 4. Also, the overlapping of dermatomes and thus the complete innervation of the lumbar region could be demonstrated despite the absence of the dorsal branch of L6. Furthermore, it was obvious that the overlapping regions are larger than described in dogs (Bailey et al., 1984).
In conclusion, the high variability of the variable existence of nerve branches, especially of the caudal dorsal branches and the variations between dogs and cats, need to be considered when applying different kinds of segmental therapy methods. These methods include therapies commonly used in complementary veterinary medicine such as massages, acupuncture, physiotherapy and transcutaneous electric nerve stimulation (TENS). These therapeutic techniques work mainly through the skin with a direct effect on cutaneous nerve branches. Studies about applying surface electrodes, like TENS, to dermatomes (Garrison & Foreman, 2002; Gessler, 1991; Mayor, 2008) show that a sound knowledge about nerve paths, cutaneous areas and differences between species is essential for those therapeutic methods. In cats with a neurologic injury including pathological contractions of their external urethral sphincter, a decreasing urethral spasm could be achieved through therapeutic superficial dermatome stimulation (McCoin et al., 2013).
Furthermore, studies about comparing dermatomal maps show the importance of knowledge and research on dermatomes to harmonise them for clinical practice and medical education (Challoumas, Ferro, Walker, & Brassett, 2018; Lee, McPhee, & Stringer, 2008).
The individual differences presented in this study, the mean CSIn-S and the findings of Hekmatpanah (1961) led us to conclude that any complementary therapeutic approach to the skin should preferably target two to three consecutive segments. This ensures a sufficient stimulation of nerve branches leading to the intended effect.
ACKNOWLEDGEMENTS
The authors thank Lea Strobel, Nadia Anantama and Luise Grace Klass for critical revision of the manuscript and Martin Pfeffer for his statistical support.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.




