Digit innervation of the thoracic limb of Criollo horses: Anatomical description and consequences to perineural blocks
Abstract
Criollo horse breeding is an important economic activity in South America. Because of their athletic performance, these animals tend to show great incidence of musculoskeletal disorders, many of them diagnosed by means of perineural blocks. However, incorrect interpretation of these blocks may be due to anatomical differences in nerve distribution. The objective of this study was to describe the innervation of the digit region of thoracic limbs in Criollo horses, in order to improve the interpretation of tests for claudication diagnosis based on nerve block. Thirty thoracic limbs from Criollo horses were dissected. It could be observed that in 90% of the limbs, dorsal branches of the palmar nerve originated proximally to the proximal sesamoid bone. In 93% of the cases, the palmar digital nerve and the dorsal branches communicated; in 87% of the cases, communication between branches of the dorsal branch was observed; and in 27% (8/30) of the limbs, the palmar metacarpal nerve and the dorsal branch presented communications. None of the specimens showed complete symmetry in the distribution of nerves in contralateral limbs. The high frequency of communication between the nerves may be a particularity of the Criollo breed that may interfere with the interpretation of perineural blocks. Based on the anatomical position, it may be inferred that divergent results in Criollo horses may occur when abaxial sesamoid nerve block is used. Palmar digital nerve block may be less influenced by these variations, provided it is performed as distal as possible from the ungular cartilage.
1 INTRODUCTION
Criollo horses are widely used in sports. Economic activities involving these animals generate more than 2.5 million dollars every year (ABCCC, 2017). There are several official sports modalities for Criollo horses, but they consist mainly of repetitive high-intensity movements. Together with other factors, these high-impact activities lead to the development of different problems in the locomotor system (Abreu et al., 2011).
Disorders of the musculoskeletal system are the main causes of poor performance in athletic horses (Morris & Seeherman, 1991; Rossdale, Hopes, & Digby, 1985). Most of the times, the thoracic limbs are more often affected (Abreu et al., 2011; Dabareiner, Cohen, Carter, Nunn, & Moyer, 2005) due to factors such as load distribution or the modality practiced (Ross & Dyson, 2010). It was estimated that, in Criollo horses, 68.6% of the problems in thoracic limbs occur distally to the metacarpophalangeal joint (Abreu et al., 2011). Among these distal changes, hoof disorders correspond to 44% of cases in the Criollo horse (Abreu et al., 2011), 33% in the Quarter horses (Dabareiner et al., 2005) and 20% in the Thoroughbred horses (Rossdale et al., 1985). Many times, these disorders are only diagnosed after perineural blocks.
Detailed anatomical knowledge, understanding of kinetics and a thorough physical examination are of extreme importance in the diagnosis of musculoskeletal disorders (Baxter, 2011). When flexion test and palpation do not provide consistent information, diagnostic blocks are important tools in the confirmation or identification of the sites of pain (Pilsworth & Dyson, 2015). Among diagnostic blocks, perineural block techniques are widely used because they are practical and low cost. However, these techniques should only be performed when the veterinarian has deep knowledge of neuroanatomy and the horse with consistent lameness (Dyson, 1984).
As the assessment of anaesthetic block is subjective, some interpretation errors may occur (Schumacher, Schumacher, Schramme, & Moyer, 2014). These errors are estimated to occur in up to 5% of the cases, and many times, reasons for these difficulties are little understood (Dyson, 1984). Some studies cite anatomical variations in limb innervation as one of the factors that may interfere with the interpretation of perineural blocks (Baxter, 2011; Dyson, 1986; Schumacher et al., 2014). Although other studies confirmed the occurrence of these variations, there is no estimate of the number of animals that show them (Borges, Souza, & de Paula, 1997; Getty, 1986; Sack, 1975).
The objective of this study was to understand the anatomical particularities of the palmar nerves and their branches in Criollo horses in order to improve the interpretation and diagnostic block techniques in this breed.
2 MATERIALS AND METHODS
Thirty thoracic limbs of 15 adult Criollo horses, nine males and six females, were analysed. Animals were either provided by a commercial abattoir, or died of natural causes, or were euthanized due to incurable diseases. Criteria for the exclusion of limbs were as follows: important deformities or occurrence of lesions prevented the identification of palmar nerves and their distal segments. When a limb was excluded, its contralateral part was excluded as well. Limbs were removed from the dead animals by a transversal section close to the mediocarpal joint.
Animals were sent to the Animal Anatomy Laboratory at UNIPAMPA at refrigeration temperatures, and limbs were kept frozen (−20°C) until the time of dissection. After thawing at room temperature, trichotomy was carried out for microdissection, which consisted of removal of the skin and cleaning of the fascia until lateral and medial palmar nerves were recognized. Then, the distal part of these nerves and their branches were dissected to the coronal region.
After the isolation of the dorsal branch of the palmar digital nerve, the origin of this branch was classified as proximal, over or distal to the proximal sesamoid bones. This classification took into account the ease of palpation of the proximal sesamoid bones and was adapted from the work of Borges et al. (1997).
When the communication between the digital palmar nerve and the dorsal branch was distal to the metacarpophalangeal joint, the distance between the base of the proximal sesamoid bone and the point of communication was measured. Nomenclature used for the description of the nerves was based on the Nomina Anatomica Veterinaria (ICVGAN, 2017).
Nerve branches were described and drawn in Paint 3D®. A Sony® Cyber-Shot DSC-HX400V (21.1 Megapixels) camera was used to photograph the nerves at the end of the dissection process. Photomacrographs were edited in Adobe Photoshop® (CC 2019). The skeletal structure of the distal region of the thoracic limb was photographed and used as a reference to illustrate the anatomical findings, after treatment in Adobe Illustrator® (CC 2019).
3 RESULTS
Lateral and medial palmar nerves ran parallel to the third metacarpal bone, between the deep digital flexor tendon and the interosseus muscle (suspensory ligament). In the proximal and medium third of the diaphysis of the third metacarpal bone, a communicating branch appeared, uniting the palmar nerves. It may have originated either from the median or the lateral palmar nerve.
Four to five branches emitted from the palmar nerves were identified. On average, a greater number of branches originated from the medial palmar nerve (Table 1), concentrated at the distal half of the third metacarpal bone region. These branches ran parallel to this bone and ended in the skin or directed to the palmar aspect in the region of the third metacarpal bone, inserting superficially in the flexor tendons and digital sheath.
| Horse | Right thoracic limb | Left thoracic limb | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lateral | Medial | Lateral | Medial | |||||||||||||
| PDN | DB | PN | PDN | DB | PN | PDN | DB | PN | PDN | DB | PN | |||||
| S | D | S | D | S | D | S | D | |||||||||
| 1 | 2 | 1 | 2 | 3 | 2 | 1 | 5 | 4 | 1 | 1 | 5 | 5 | 2 | 2 | 6 | 3 |
| 2 | 2 | 1 | 5 | 5 | 1 | 2 | 3 | 2 | 3 | 2 | 4 | 5 | 2 | 2 | 8 | 4 |
| 3 | 4 | 2 | 3 | 3 | 2 | 1 | 6 | 5 | 3 | 3 | 4 | 4 | 3 | 3 | 3 | 5 |
| 4 | 1 | 3 | 4 | 6 | 2 | 2 | 6 | 4 | 2 | 3 | 3 | 6 | 2 | 2 | 3 | 3 |
| 5 | 1 | 2 | 4 | 3 | 1 | 1 | 4 | 4 | 2 | 3 | 5 | 6 | 2 | 2 | 4 | 3 |
| 6 | 2 | 2 | 5 | 4 | 2 | 2 | 8 | 5 | 1 | 3 | 6 | 5 | 2 | 2 | 4 | 5 |
| 7 | 1 | 3 | 5 | 3 | 2 | 3 | 5 | 3 | 2 | 4 | 5 | 5 | 1 | 3 | 6 | 4 |
| 8 | 0 | 2 | 8 | 9 | 1 | 3 | 4 | 4 | 0 | 2 | 5 | 4 | 2 | 2 | 4 | 4 |
| 9 | 1 | 3 | 4 | 4 | 2 | 3 | 4 | 3 | 3 | 3 | 4 | 4 | 1 | 3 | 3 | 3 |
| 10 | 2 | 2 | 5 | 5 | 3 | 2 | 5 | 5 | 1 | 3 | 6 | 4 | 3 | 3 | 7 | 4 |
| 11 | 3 | 4 | 5 | 5 | 2 | 4 | 7 | 4 | 2 | 2 | 6 | 5 | 2 | 3 | 8 | 5 |
| 12 | 2 | 3 | 5 | 4 | 3 | 2 | 4 | 3 | 1 | 2 | 5 | 3 | 1 | 3 | 4 | 4 |
| 13 | 3 | 2 | 6 | 5 | 2 | 2 | 5 | 3 | 1 | 2 | 4 | 4 | 1 | 2 | 7 | 3 |
| 14 | 0 | 3 | 5 | 5 | 2 | 2 | 3 | 3 | 2 | 3 | 5 | 5 | 3 | 4 | 4 | 5 |
| 15 | 3 | 4 | 5 | 4 | 3 | 2 | 4 | 5 | 1 | 3 | 7 | 6 | 4 | 3 | 3 | 4 |
| Mean | 1.8 | 2.5 | 4.7 | 4.5 | 2.0 | 2.1 | 4.9 | 3.8 | 1.7 | 2.6 | 4.9 | 4.7 | 2.1 | 2.6 | 4.9 | 3.9 |
- Abbreviations: D, deep; DB, dorsal branch; PDN, palmar digital nerve; PN, palmar nerve; S, superficial.
Distally to the metacarpophalangeal joint, the palmar nerve became the digital palmar nerve. It continued close to the plexus, running palmarly to the digital palmar artery, in the palmarolateral and palmaromedial aspects of the proximal phalanx, until they reach the hoof, passing deeply into the ungular cartilages. In the region of the proximal phalanx, it emitted four to five branches, on average, two superficial, to supplying the skin, and two deep to the proximal phalanx and proximal interphalangeal joint.
The dorsal branch originated from the palmar digital nerve, close to the metacarpophalangeal joint and followed distally, between the corresponding artery and vein. It emitted on average five terminal branches, which were distributed to the dorsolateral and dorsomedial aspects of the proximal phalanx, up to the coronal region of the hoof. It was observed that 90% (27/30) of the dorsal branches originated proximal to the proximal sesamoid bones (Figure 1), whereas only 7% (2/30) originated over the sesamoid bones and 3% (1/30) distal to the sesamoid bones.
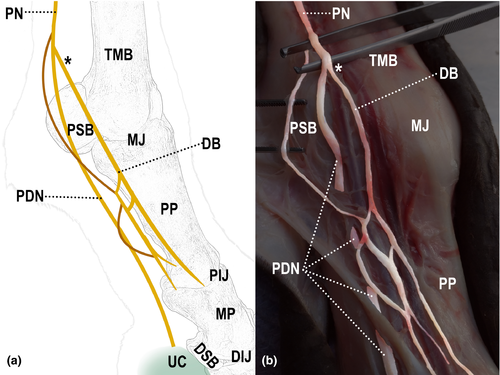
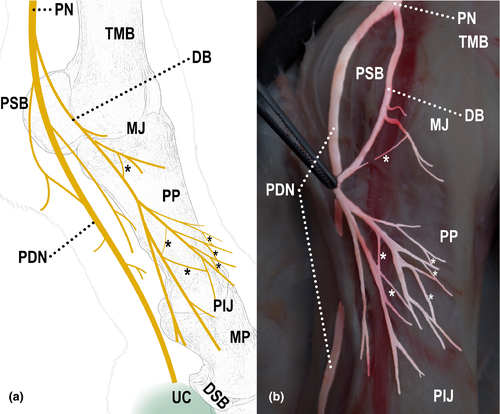
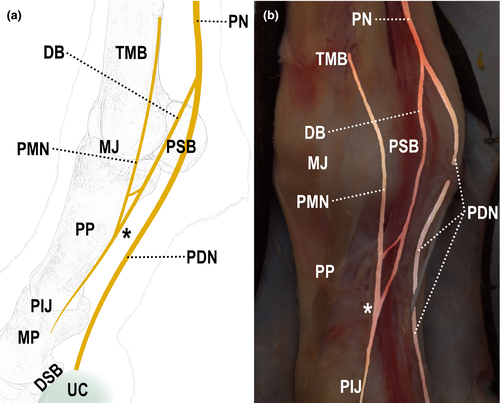
All limbs showed different forms of communication between the nerves, both in the lateral and medial sides (Table 2). In 93% (28/30) of the limbs, communication between the digital palmar nerve and its dorsal branch was observed (Figure 2); in 87% (26/30) of them, communication was between branches of the dorsal branch of the digital palmar nerve (Figure 3); and in 27% (8/30) of them, communication was between the palmar metacarpal nerve and the dorsal branch of the digital palmar nerve (Figure 4).
| Horse | Right thoracic limb | Left thoracic limb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lateral | Medial | Lateral | Medial | |||||||||
| PDN × DB | DB × DB | DB × PMN | PDN × DB | DB × DB | DB × PMN | PDN × DB | DB × DB | DB × PMN | PDN × DB | DB × DB | DB × PMN | |
| 1 | 2 | 2 | 1 | 1 | 2 | |||||||
| 2 | 2 | 1 | 1 | 1 | 2 | 1 | ||||||
| 3 | 3 | 1 | 2 | 2 | 1 | 2 | 1 | |||||
| 4 | 2 | 1 | 1 | 1 | 1 | 1 | ||||||
| 5 | 1 | 1 | 1 | 3 | 2 | 2 | ||||||
| 6 | 1 | 2 | 2 | 1 | 3 | 1 | 2 | |||||
| 7 | 1 | 8 | 1 | 1 | 1 | 1 | ||||||
| 8 | 1 | 2 | 1 | 3 | 1 | 1 | ||||||
| 9 | 3 | 1 | 1 | 2 | 1 | |||||||
| 10 | 1 | 2 | 2 | 3 | 1 | 2 | 2 | 2 | ||||
| 11 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||
| 12 | 1 | 1 | 2 | 2 | 1 | 1 | ||||||
| 13 | 2 | 1 | 1 | |||||||||
| 14 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | |||||
| 15 | 1 | 1 | 2 | 1 | ||||||||
- Abbreviations: DB, dorsal branch; PDN, palmar digital nerve; PMN, palmar metacarpal nerve; PN, palmar nerve.
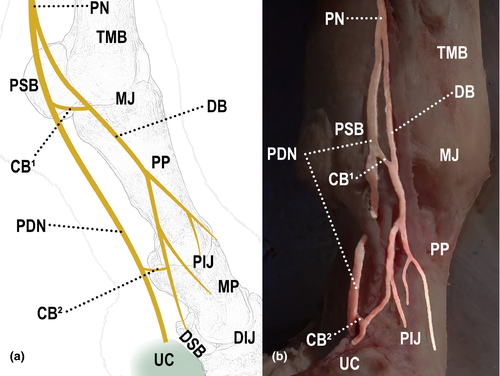
Communication between the digital palmar nerve and its dorsal branch occurred in 100% of the animals (15/15), 93% of the limbs (28/30). In these cases of communication, it occurred distally to the proximal sesamoid bone in 89% (25/28) of the limbs (Figure 5) and over the surface of the sesamoid bone in only 11% (3/28) of the limbs. When communication was distal to the sesamoid bone (83% of the limbs, 25/30), the communicating branch and the base of the bone were 3.3 cm apart, in average.
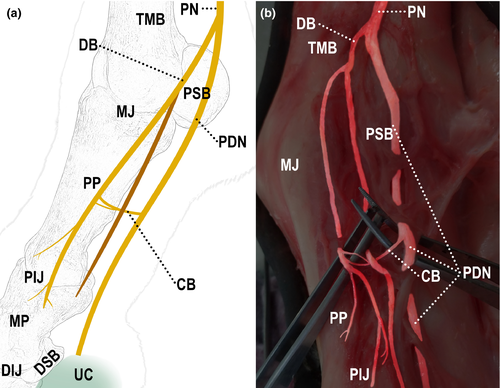
Communication between the palmar metacarpal nerve and the dorsal branch of the digital palmar nerve occurred in 27% of the dissected limbs. Most of the times, it occurred both in the medial and lateral sides of the same limb.
When the limbs of the same animal were compared, there never was perfect symmetry in the distribution of the nerves and their communications between the antimeres.
4 DISCUSSION
The occurrence of a communicating branch between the lateral and medial palmar nerves was observed in 100% of the dissected limbs. These findings have been described in animal anatomy textbooks for a long time (Budras, Sack, Rock, Horowitz, & Berg, 2012; Getty, 1986; Ghoshal, 1966; König & Liebich, 2016; Liautard, 1879; Orsini & Sack, 2003). Al-Bagdadi, Schumacher, Carter, Tóth, and Henry (2018) suggested, by means of histological and ultrastructural techniques, that communicating branch sends sensory fibres from the lateral plantar nerve of the pelvic limbs to the medial plantar and vice versa. Furthermore, not all fibres passed through the ramus communicans. Thus, it can be imagined that the same would happen between the communicating branches of the palmar nerves.
Branches originated from the palmar nerves confer adjacent cutaneous and palmaromedial and palmarolateral skin innervation in the region of the third metacarpal bone, up to the proximal aspect of the fetlock (Getty, 1986; Liautard, 1879; Sack, 1975), as observed in the Criollo horses of the present study. As described by Sack (1975), three deep branches, coming from the medial palmar nerve, were responsible for supplying the end of the carpal sheath and proximal aspect of the digital sheath. Flexor tendons proximal to the metacarpophalangeal joint and the palmar recess of the metacarpophalangeal joint were innervated by the palmar nerves. The superficial structures of the lateral aspect of the proximal half of the metacarpal region were supplied by the dorsal branch of the ulnar nerve.
The digital palmar nerve runs through the region of the proximal phalanx, palmarly to the digital palmar artery and plexus, parallel to the deep digital flexor tendon (Barone & Simoens, 2010; Budras et al., 2012; Getty, 1986; König & Liebich, 2016; Orsini & Sack, 2003; Sack, 1975). The digital palmar nerve and its branches are responsible for sensitivity in the deepest structures of the region of the proximal phalanx (pastern) and the internal region of the foot (Sack, 1975). After emerging from the digital palmar nerve, the dorsal branch runs distally between the digital palmar artery and vein, as previously described (Barone & Simoens, 2010; Getty, 1986; König & Liebich, 2016; Liautard, 1879; Orsini & Sack, 2003). The superficial tissues of the region of the proximal phalanx (fetlock) are innervated mainly by the dorsal branch of the digital palmar nerve, distributing branches to the lateral, medial and dorsal surfaces, up to the coronal region of the hoof (Getty, 1986; Ghoshal, 1966; Orsini & Sack, 2003), forming an interconnected network of nerves, as described by Sack (1975).
In horses of the present study, 90% of the cases showed that the dorsal branch originated proximally to the proximal sesamoid bones. This site of origin was also the most frequent one in thoracic limbs of 20 cross-bred horses (Borges et al., 1997).
Some studies mention possible communications between the nerves in the distal aspect of thoracic limbs of horses (Borges et al., 1997; Getty, 1986; Sack, 1975). However, these communicating branches were not quantified in most of these studies. There is the possibility of small nervous fascicles of the digital palmar nerve that, passing over the homonymous artery, can establish discreet communications with the dorsal branch (Getty, 1986). Sack (1975) mentioned the possibility of one or more branches being emitted from the digital palmar nerve to the dorsal branch. A study carried out from the dissection of thoracic limbs of cross-bred horses found the presence of communication between the palmar digital nerve and the dorsal branch of the palmar digital nerve in 40% of the animals and 27% of the forelimbs (Borges et al., 1997). These values are different from those found in the present study, 100% and 93%, respectively. It is known that different anatomical presentations may interfere with the results of diagnostic blocks (Baxter, 2011; Dyson, 1984; Schumacher et al., 2014); thus, our results can suggest more caution when interpreting anaesthetic blocks for diagnostic purposes in the Criollo horses.
Abaxial sesamoid block involves blocking the digital palmar nerve on the abaxial face of the proximal sesamoid bone. Biaxial block leads to desensitization of the hoof, middle phalanx, distal interphalangeal joint, palmarodistal aspect of the proximal phalanx, distal portion of the superficial and deep digital flexor tendons, and distal portion of the sesamoid and annular ligaments. This block should be performed near the base of the proximal sesamoid bone, with the needle directed distally to it (Baxter, 2011).
These anatomical variations between animals, or even between left and right thoracic limbs of the same individual, may lead to different interpretations in the abaxial sesamoid block in Criollo horses. Anaesthetics may not block the stimuli from the dorsal branch, and consequently, the transmission of impulses may not be completely interrupted. Alternatively, more proximal sites may be blocked in order to reach the origin of the painful stimuli in the dorsal branch. However, this approach may desensitize other structures, which may also affect interpretation (Dyson & Murray, 2006).
One alternative in Criollo horses is a two-step block: abaxial sesamoid block and basisesamoid block. This process may decrease the uncertainty of the assessment (Baxter, 2011; Ross & Dyson, 2010). This two-step process may be especially useful when clinical suspect is related to the metacarpophalangeal joint or to the point of attachment of the interosseous muscle (suspensory ligament). Performing a basisesamoid block firstly might reduce the likelihood of partial or total desensitization of the metacarpophalangeal joint and branches of the interosseous muscle, while a positive response after the abaxial sesamoid block may be related to injuries to the metacarpophalangeal joint. This is true especially if time was long enough to allow a proximal diffusion of the anaesthetic to the neurovascular band and the anaesthetic volume is >2 or 2.5 ml per site (Schumacher et al., 2014).
There are studies that report potential proximal diffusion of anaesthetics, considering the length of time and the strides of the animal, which support the hypothesis of desensitization of proximal structures (Nagy, Bodó, Dyson, Szabo, & Barr, 2009). The proximal diffusion of anaesthetics and the correct interpretation of the blocks can also be affected by the volume of anaesthetic and the time used for evaluation. According to Nagy et al. (2009), 10 min after the perineural injection of the palmar nerves with radiocontrast is enough to observe an important proximal migration of radiocontrast. Likewise, Contino, Werpy, Morton, and McIlwraith (2012) demonstrated a significative proximal diffusion after digital palmar block technique. In this study, fifteen horses diagnosed, by magnetic resonance, with primary metacarpophalangeal injuries, showed an improvement >90% after digital palmar block with 2.5 ml of anaesthetic when evaluated 10 min after the injection.
Communications between the digital palmar nerve and the dorsal branch may interfere with the technique used in digital palmar block (Baxter, 2011). The objective of this block is to inhibit the transmission, by the digital palmar nerve, of painful stimuli coming from deep structures of the hoof (Baxter, 2011; Ross & Dyson, 2010). When this communication exists, stimuli coming from these regions may follow an alternative route, if the block is performed proximally to the communication, interfering with the assessment and interpretation of the results. According to Schumacher et al. (2004), the more distal the block, the lesser the probability of error. It is important to emphasize that most of the cases of thoracic limb claudication in Criollo horses are caused by problems distal to the metacarpophalangeal joint, with 44% of them related to the hooves (Abreu et al., 2011).
In the analysis of this nerve communication in fetuses, Sack (1975) reported possible occurrence of communication between the palmar metacarpal nerve and the dorsal branch of the digital palmar nerve. These communications occurred without fibre interchange. However, as the metacarpal nerve is wide, and it joins the dorsal branch, there may be regions where sensory innervation is coincident. Further investigation with histology and/or ultrastructural techniques could help to clarify the functional role of this communication. Methods such as ultrasound could eventually help to identify some of these communications. However, limitations in routine distal blocks, such as the unavailability of more sophisticated devices and/or well-trained ultrasonographers at the time of the procedure, can be impeditive.
5 CONCLUSION
The differences in the innervation of the distal region of the thoracic limb were observed in Criollo horses, mainly numerous communications between the nerves. From an anatomical standpoint, erroneous interpretations may be more common when the abaxial sesamoid block technique is used. Digital palmar block may lead to less erroneous interpretations, provided it is carried out as close as possible to the ungular cartilage.
ACKNOWLEDGEMENTS
The authors acknowledge Frigorífico Foresta in São Gabriel, RS, Brazil.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




