Mouse Incisor Stem Cell Niche and Myb Transcription Factors
Summary
Dental hard tissues are formed particularly by odontoblasts (dentin) and ameloblasts (enamel). Whereas the reparation of dentin is often observed, enamel does not regenerate in most species. However, in mouse incisor, a population of somatic stem cells in the cervical loop is responsible for the incisor regeneration. Understanding of the specificities of these cells is therefore of an interest in basic research as well as regenerative therapies. The Myb transcription factors are involved in essential cellular processes. B-Myb is often linked to the stem cell phenotype, and c-Myb expression marks undifferentiated and proliferating cells such as the stem cells. In the presented study, temporo-spatial expression of B-Myb and c-Myb proteins was correlated with localisation of putative somatic stem cells in the mouse incisor cervical loop by immunohistochemistry. B-Myb expression was localised mostly in the zone of transit-amplifying cells, and c-Myb was found in the inner enamel epithelium, the surrounding mesenchyme and in differentiated cells. Taken together, neither B-Myb nor c-Myb was exclusively present or abundant in the area of the incisor stem cell niche. Their distribution, however, supports recently reported novel functions of c-Myb in differentiation of hard tissue cells.
Introduction
Stem cell niches are highly specific microenvironments that prevent somatic stem cells (SSCs) from differentiation and apoptosis-inducing stimuli, they also provide developmental signals and nurture. The niches are supposed to protect SSCs from abnormal division and carcinogenesis (Moore and Lemischka, 2006). The molecular profile of stem cell maintenance, cell division and differentiation has not been completely understood.
Enamel regeneration and reparation in most mammals is generally limited due to the loss of ameloblasts upon tooth eruption and the absence of ameloblast stem cell population, whereas there are ever-growing incisors in rodents having capacity for continuous ameloblast production. Recently, SSCs isolated from different parts of tooth, such as human dental pulp, periodontal ligament or dental follicle were suggested as a potential source of mesenchymal cells for tooth tissues engineering. However, a suitable source of human epithelial cells for the purpose was not resolved (Volponi et al., 2010).
Labial part (la) of the cervical loop (CL) in the mouse incisor was suggested to be the niche of epithelial SSCs (Harada et al., 1999). However, subsequent experiments proposed the presence of some stem cells also in smaller lingual (li) CL. These SSCs do not differentiate into ameloblasts due to different pattern of gene expression in the lingual part (Wang et al., 2004, 2007; Klein et al., 2008). These asymmetric distributions of molecular factors result in asymmetric incisor structure; the enamel is located only on the labial side of the incisor to ensure sharpness and correct function of the tooth.
A large network of signalling molecules related to the incisor stem cell niche was recently reviewed by Kuang-Hsien Hu et al. (2014). Briefly, family of fibroblast growth factors (FGF) is responsible for the continuous growth of the incisors (Harada et al., 2002). The FGF effect is neutralised by sprouty genes that play a role in prevention of lingual ameloblasts formation (Klein et al., 2008). Other molecules involved in the incisor development include activin expressed exclusively in the mesenchyme with stronger signal in the labial region. Activin is known as a positive regulator of SSCs (Thesleff et al., 2007) and is negatively regulated by follistatin (as well as BMPs or TGFβ). Follistatin is expressed in the lingual part of the epithelium inhibiting ameloblast differentiation in the lingual cervical loop (Wang et al., 2004, 2007). Undifferentiated state of stem cells is likely to be connected with Notch expression (Fortini and Artavanis-Tsakonas, 1993). Notch genes are completely absent from ameloblasts. Moreover, positive correlation between stem cell niche and Notch1 expressing area was reported (Harada et al., 1999). Notch signalling is regulated by Jagged1, Jagged2 and Serrate1 ligands expressed in inner enamel epithelium and ameloblasts and modulated by lunatic fringe expressed mostly in basal epithelium (Fortini and Artavanis-Tsakonas, 1993; Harada et al., 1999). Recently, Bmi1 (encoded by gene from polycomb group) has been described as an important factor in maintenance of CL SSCs (Biehs et al., 2013). On the other hand, the expression of bone morphogenetic protein 4 (BMP4), responsible for ameloblast differentiation and negative regulation of stem cells, is situated in the mesenchyme adjacent to la and li area of incisor similarly (Plikus et al., 2005). Another molecule involved in ameloblast differentiation is Shh located in the zone of TA cells, pre-ameloblasts and immature ameloblasts (Seidel et al., 2010).
Myb transcription factors were investigated in several stem cell systems, but nothing is known about their relation to the dental stem cell niches (Ess et al., 1999). Moreover, c-Myb was recently reported as one of the factors influencing differentiation of dental hard tissues (Matalova et al., 2011). The Myb protein family includes the sequence-specific DNA-binding proteins with strong impact on cellular proliferation (Introna et al., 1994). Three members of the Myb family are known in mammals: A-Myb, B-Myb and c-Myb. A-Myb has been observed in B and T lymphocytes, male reproductive systems, mammary gland and central nervous system (Trauth et al., 1994; Toscani et al., 1997). B-Myb was linked with cell differentiation, cell cycle progression and apoptosis (Sala and Watson, 1999). Roles of B-Myb in pluripotency maintenance, chromatin stability and cell cycle progression have been also described (Tarasov et al., 2008). c-Myb is a transcription factor involved in cell proliferation, differentiation, survival and cell death (Ramsay and Gonda, 2008). c-Myb has been connected with undifferentiated state and recently supposed to be relevant to stem or progenitor cells re-entry in to the cell cycle (Cheasley et al., 2011).
This investigation aims to contribute to incomplete signalling pathways of somatic stem cells of the incisors by investigation of the B-Myb and c-Myb expression in the area.
Materials and Methods
Samples
Mouse heads at embryonic days (ED) 11.5, 12.5, 15.5, 17.5 and postnatal (P) days 2 and 10 were used to investigate incisor stem cell niches. Collected samples were fixed in 4% buffered paraformaldehyde for 24 h, then dehydrated in ethanol gradient, treated with xylene and embedded in paraffin. Postnatal and ED17.5 mouse heads were decalcified in buffered EDTA before dehydration. Mice were sacrificed according to the experimental protocol approved by the Laboratory Animal Science Committee of the IAPG CAS, v.v.i., Brno, Czech Republic.
Immunohistochemistry
Sagittal serial sections (5 μm) of mouse heads were prepared. Sections were stained by specific antibodies: proliferating cell nuclear antigen (PCNA), B-Myb, c-Myb. Morphology was visualised by haematoxylin–eosin staining. Sections were deparaffinized, rehydrated and for B-Myb antigen retrieval was performed in citrate buffer (pH = 6.0/5 min). Endogenous peroxidase activity was eliminated in all sections by 3% hydrogen peroxide/5 min. The primary antibodies were used as follows: B-Myb (ab76009) 1:100, c-Myb (ab59233) 1:200 and PCNA (sc7907) 1:50. The c-Myb antibody was applied overnight/4°C. B-Myb and PCNA were applied for 1 h/room temperature. Peroxidase-conjugated streptavidin-biotin system (Vectastain; Vector Laboratories, Burlingame, CA, USA) and chromogen substrate diaminobenzidine (DAB, K3466; Dako, Carpinteria, CA, USA) were used for primary antibody detection and visualisation. Sections were counterstained in haematoxylin. As positive controls, mouse duodenum was used, and primary antibody was omitted in negative controls.
Results
At the initiation of development (ED11.5), the incisor formed an epithelial thickening (Fig. 1a). Intensive proliferation was observed in both the epithelial and the mesenchymal parts (Fig. 1b). Scattered B-Myb positive cells were detected in the epithelium and the surrounding mesenchyme (Fig. 1c). c-Myb was abundant in both parts of the developing incisor area (Fig. 1d).
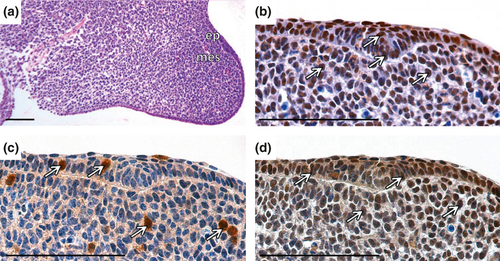
At the bud stage (Fig. 2a) of the incisor development (ED12.5), high proliferation was detected predominantly in the epithelial part of the developing incisor. In the surrounding mesenchyme, several proliferating cells were also observed (Fig. 2b). Few B-Myb positive cells were located scattered in the epithelial and the mesenchymal parts of the incisor (Fig. 2c). c-Myb-positive cells were observed particularly in the epithelial part (Fig. 2d).
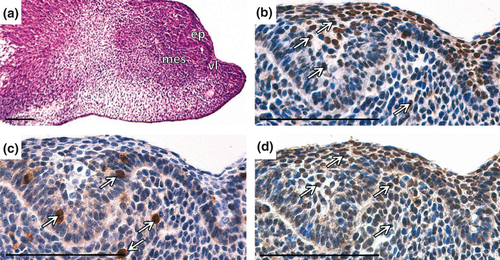
At the early bell stage (ED15.5), when the cervical loop was clearly morphologically distinct (Fig. 3a), proliferation was apparent in most of the epithelial and mesenchymal cells (Fig. 3b) of the incisor. B-Myb expression occurred mainly in the inner enamel epithelium (iee), particularly in the future zone of transit-amplifying (TA) cells (Fig. 3c). c-Myb positive cells were found in both intensively proliferating parts, the epithelium and the mesenchyme (Fig. 3d).
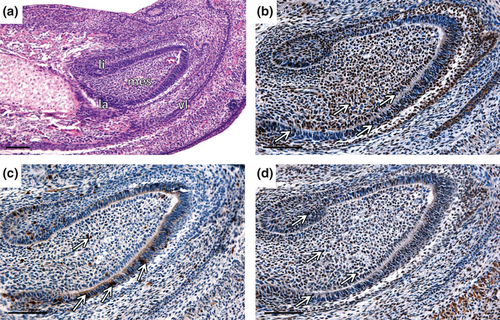
At the bell stage (ED17.5; Fig. 4a), the epithelial and the mesenchymal proliferation were still evident (Fig. 4b,c). The B-Myb positivity was clearly apparent in the labial part of CL, particularly in the TA zone (Fig. 4e). However, slight positive signal was detected in proposed SSCs niche. Few B-Myb positive cells were observed also in lingual part of CL (Fig. 4d). In la CL, c-Myb was localised mostly in the outer enamel epithelium (oee), stellate reticulum (sr), iee and adjacent mesenchyme (Fig. 4g). High number of c-Myb positive cells was detected in li CL (Fig. 4f). PCNA-negative mesenchymal cells adjacent to the epithelial tip of the CL were negative for both Mybs.
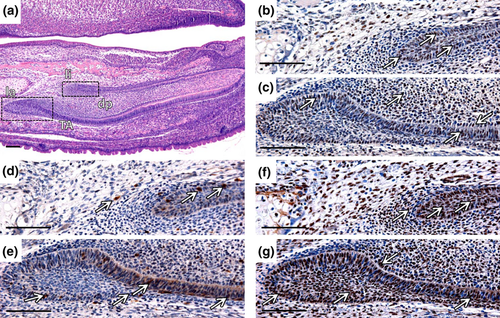
At the stage P2 (Fig. 5a), proliferation occurred particularly in the iee of la CL (Fig. 5b) and the adjacent mesenchyme. Few B-Myb positive cells were observed in the mesenchyme, and the majority of positive cells were situated in the iee, particularly in the TA zone (Fig. 5c). c-Myb expression was distributed throughout the iee, oee and the epithelium surrounding mesenchyme (Fig. 5d). c-Myb signal was observed also in differentiated ameloblasts and odontoblasts (data not shown). The expression of all molecules under study was randomly present also in li CL or/and mesenchymal cells adjacent to li CL (Fig. 5e–g).
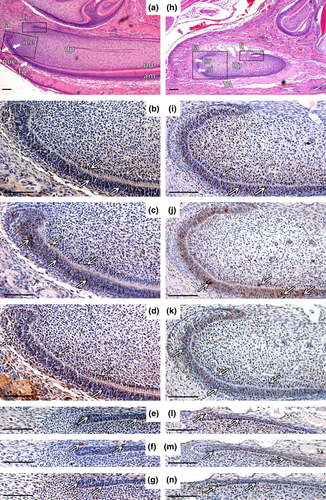
At the stage P10 (Fig. 5h), proliferation persisted in the TA zone and in the adjacent mesenchyme, contrary to zones of differentiation (Fig. 5i). Expression of Myb molecules in la CL predominantly resembled the stage P2 (Fig. 5j,k). As at the P2, c-Myb signal was observed also in differentiated ameloblasts and odontoblasts (data not shown), and all examined molecules were present in the lingual part of cervical loop (Fig. 5l–n).
Discussion
Mouse incisor development starts around ED11.5 by the epithelial thickening and continues by the bud stage (ED12.0–13.0), the cap stage (ED13.5–15.0) and the bell stage is observed at ED16.0 (Kieffer et al., 1999). The cervical loop of the incisor was described to host epithelial SSCs (Harada et al., 1999; Wang et al., 2004, 2007; Klein et al., 2008). The progenitors that give rise to enamel-producing ameloblasts were suggested to reside in sr (Harada et al., 1999; Suomalainen and Thesleff, 2010) and also in oee (Chang et al., 2013). The current concept of the incisor niche was based on the morphology, ablation experiments, the presence of long-term label-retaining cells, Lgr5 positive cells, and knock-out models (Harada et al., 1999; Wang et al., 2004, 2007; Klein et al., 2008; Chang et al., 2013). The investigation showed that the incisor epithelial stem cells of la CL develop into TA cells that divide rapidly and give rise to the epithelial incisor part: iee, oee, stratum inter-medium (si) and sr (Harada et al., 2002). Newly formed ameloblasts residing in the iee migrate towards incisal end and secrete enamel (Harada et al., 1999).
In our study, B-Myb and c-Myb expression was correlated with the incisor stem cell niche, the cervical loop and cell proliferation. Based on their reported functions, both Mybs could be potentially associated with stem cell phenotype and therefore might be considered as markers of the stem state or novel members in the SSCs signalling pathway.
B-Myb was suggested to play a role in pluripotency maintenance. The proposed mechanism includes B-Myb as a regulator of Oct-4 or Sox-2, the pluripotency associated markers in embryonic stem cells (ESCs; Tarasov et al., 2008). However, there are also contradictory data published (Lorvellec et al., 2010). In the presented study, B-Myb expression in sr and oee of the cervical loop was not conspicuous in comparison with other incisor parts. At ED17.5, B-Myb was almost absent from the sr. Furthermore, B-Myb expression in the iee was much more prominent than in the stem cell area at all investigated stages. From ED17.5 onwards, B-Myb positive cells were concentrated in extremely proliferating TA zone, thus confirming important role of B-Myb in control of cell proliferation (Tarasov et al., 2008), whereas B-Myb expression pattern in the iee resembled the lunatic fringe expression (Harada et al., 1999). Lunatic fringe is a modulator of the Notch receptor which is connected with undifferentiated state (Mitsiadis et al., 1995). Connection between B-Myb and Notch signalling was not examined so far but might represent one of possible engagements of B-Myb in incisor stem cell signalling networks.
c-Myb was described as one of the markers of undifferentiated state (Ramsay and Gonda, 2008). In our investigation, c-Myb was plentifully present in the epithelial and mesenchymal parts of the incisor at all examined stages as shown also in molar teeth (Matalova et al., 2011). At prenatal stages, c-Myb positive signal correlated with proliferating zones, but later, at postnatal stages c-Myb appeared in differentiated odontoblasts and ameloblasts. This may indicate a dual stage-dependent function of c-Myb in dental hard tissue formation (Lungova et al., 2012), supported by recently published functional experiments in the bone tissue (Bhattarai et al., 2013).
Nevertheless, none of both investigated Myb proteins was restricted to stem cell niche. Expression of B- and c-Myb was detected in the posterior part of the superior cervical loop (li CL) where molecular signalling does not allow for ameloblast differentiation. These observations might point to role of Mybs in cell proliferation or to multiple roles in the mouse incisor.
Several signalling pathways in the mouse incisor were described in previous studies (reviewed in Kuang-Hsien Hu et al., 2014). However, detailed molecular interactions have not been completely resolved. A complication in the incisor stem cell investigation is caused by the lack of specific markers of SSCs in the incisor. Some markers of pluripotency/multipotency such as Sox-2, Lgr5, CD49f, Oct3/4 and Bmi1 were studied in CL (Suomalainen and Thesleff, 2010; Li et al., 2011; Juuri et al., 2012; Chang et al., 2013). Many of them, however, do not seem to be strictly specific for epithelial incisor stem cells (Li et al., 2011). Recently, Lgr5 was reported in the intestinal tissue, where c-Myb was nominated as its regulator (Cheasley et al., 2011). Similar regulation might be performed by c-Myb in the incisor, which might be supported by proliferating status of Lgr5 positive cells (Chang et al., 2013).
In conclusion, many questions in SSCs signalling of incisors remain unanswered. The examination of the signalling pathways in SSCs niches is important also for the comprehension of reparative mechanism of organs and may contribute to the progress in tissue engineering. The mouse incisor appears as a valuable model to study not only SSCs populations but also proposed dual role of c-Myb in proliferation and differentiation of cells forming hard tissues.
Acknowledgements
The stem cell work was supported by the Grant Agency of the Czech Republic, project P304/11/1418. Related odontogenic research at the IAPG is granted by the Bilateral Project GACR P302/12/J059. The laboratories at the FS MU are supported by Center of Experimental Biomedicine (CZ.1.07/2.3.00/20.0183) and the IAPG CAS, v.v.i. runs under RVO: 67985904.




