A novel QTL region for pH and meat color in Duroc pigs
Abstract
One of the most important processes that occur during the transformation of muscle to meat is the pH decline as a consequence of the post-mortem metabolism of muscle tissue. Abnormal pH declines lead to pork defects such as pale, soft, and exudative meat. There is genetic variance for ultimate pH and the role of some genes on this phenotype is well established. After conducting a genome-wide association study on ultimate pH using 526 purebred Duroc pigs, we identified associated regions on Sus scrofa chromosomes (SSC) 3, 8, and 15. Functional candidate genes in these regions included PRKAG3 and PHKG1. The SSC8 region, at 71.6 Mb, was novel and, although no candidate causative gene could be identified, it may have regulatory effects. Subsequent analysis on 828 pigs from the same population confirmed the impact of the three associated regions on pH and meat color. We detected no interaction between the three regions. Further investigations are necessary to unravel the functional significance of the novel genomic region at SSC8. These variants could be used as markers in marker-assisted selection for improving meat quality.
One of the most important processes that occur during the transformation of muscle to meat is the pH decline as a consequence of the post-mortem metabolism of muscle tissue. The pH decline rate is one of the paramount factors on determining meat quality; specifically in terms of color, water holding capacity and tenderness (England et al., 2013; Matarneh et al., 2021). Particularly in pork, but also in other livestock species, abnormal muscle pH declines can generate protein-denaturing conditions, which produce meat defects such as pale, soft, and exudative (PSE) meat (Barbut et al., 2008). The genetic component of ultimate pH has been well documented (Chung et al., 2015; Verardo et al., 2017; Xie et al., 2023), with low heritability estimates (0.14–0.27; De Vries et al., 1994; Gjerlaug-Enger et al., 2010; Lo et al., 1992; Tian et al., 2023). A noteworthy gene that has been associated with PSE phenotypes is the ryanodine receptor (RYR1) gene, commonly known as the halothane gene. A missense variant at exon 18 in the RYR1 gene (rs706913914) makes pig more susceptible to stress, which leads to a rapid muscle pH decline that results in PSE meat (Fujii et al., 1991; Klont et al., 1994; Murray & Johnson, 1998). Nowadays, a strict selection against this variant has purged the unfavorable allele from all the maternal commercial pork lines (Davoli & Braglia, 2007). Another gene that has been associated with PSE meat is the protein kinase AMP-activated non-catalytic subunit γ 3 (PRKAG3), commonly known as the Rendement Napole gene. A well-known mutation in the PRKAG3 gene in Hampshire pigs, the p.Arg200Gln substitution (rs1109104772) is responsible for eliminating the allosteric regulation by ATP/AMP, which increases the basal AMPK activity, resulting in PSE meat (Milan et al., 2000). Although this mutation is exclusive to Hampshire pigs, a nearby variant also affecting muscle glycogen content, glycolytic potential, pH, meat color, and drip loss (p.Ile199Val; rs1108399077) has been identified in other breeds (Ciobanu et al., 2001; Galve et al., 2013; Lindahl et al., 2004).
To identify other regions that affect pork ultimate pH, we used a total of 526 purebred Duroc barrows, from 199 sires and 400 dams, raised in 21 batches between 2002 and 2021 following a common protocol for data recording and tissue sampling (Ros-Freixedes et al., 2016). Pigs from each batch were raised from around 75 days until slaughter age (at 213 days, SD 7.8 days) under identical conditions. During this time, the pigs had ad libitum access to commercial feed (Esporc, Riudarenes, Spain). Ultimate pH of meat was measured in muscle gluteus medius after chilling for approximately 24 h at 2°C with a pH-meter equipped with a spear-tipped probe (Testo 205; Testo AG, Lenzkirch, Germany). On average, ultimate pH was 5.77 (SD 0.23).
DNA extraction was carried out following a conventional phenol: chloroform protocol (Green & Sambrook, 2017) and used for single nucleotide polymorphism (SNP) genotyping with either the PorcineSNP60 v2 Genotyping BeadChip (n = 138; Illumina, San Diego, CA, USA), the GGP Porcine HD Array (n = 256; Illumina) porcine arrays or whole-genome sequencing (n = 262, of which 130 were also genotyped using a SNP array) on a NovaSeq 6000 instrument (Illumina) in paired-end mode, as described by Molinero et al. (2022). SNPs from the SNP arrays that mapped to sex chromosomes or with minor allele frequency <0.05 were discarded from further analyses. Regarding the whole-genome sequence data, the average realized sequencing coverage depth was 7.3× (SD 2.0×) and a total of 7 093 644 variants were called and passed quality control criteria (minor allele frequency ≥0.20 and missing genotypes <0.10). We used such a stringent minor allele frequency threshold to ensure that the three possible genotypes were sufficiently represented in the dataset.
A genome-wide association study (GWAS) using the pigs genotyped with SNP arrays (n = 394, with 52 267 SNPs after quality control) was performed by fitting a linear mixed model that included the effect of the batch (11 levels) and the genomic relationship matrix with GEMMA software (Zhou & Stephens, 2012). Then, we imputed genotypic data at the whole-genome sequence level for the pigs that were genotyped with the SNP arrays using Beagle (Browning & Browning, 2007). A second GWAS was performed on the imputed sequence data (n = 526) using the same model (21 levels for the batch effect). The association of each SNP was tested using the Wald test. We corrected for multiple testing with the method by Benjamini and Hochberg (1995) to control the false discovery rate at a level of 0.025 based on the distribution of nominal p-values resulting from the multiple tests.
When using the data from the SNP arrays, two independent regions were detected for the pH, one located at Sus scrofa chromosome (SSC) 3, and another one in SSC15 (Figure 1a). The region at SSC3 contained 339 associated variants and spanned from 15 280 660 to 17 098 903 bp; and the region at SSC15 contained 205 associated variants and spanned from 120 667 445 to 120 907 257 bp (Table S1). When using the imputed sequence data, an additional novel region was detected at SSC8 that contained 14 associated variants and spanned from 71 599 854 to 71 664 598 bp (Figure 1b; Table S1). The region at SSC8 was only detectable with the increased variant density because the associated variants were only present in the whole-genome sequencing data.
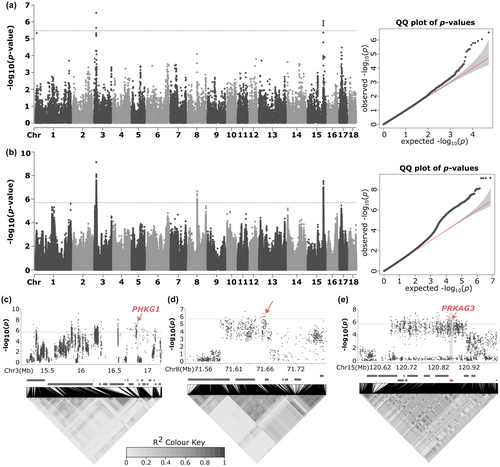
The two regions at SSC3 and SSC15 (Figure 1c,e) contained two clear functional candidate genes, the phosphorylase kinase catalytic subunit γ 1 (PHKG1), located at SSC3, and PRKAG3, at SSC15. Ma et al. (2014) identified a splicing mutation variant (rs330928088) at the PHKG1 gene that truncated the protein at the 5′ end of exon 10. The phosphorylase kinase activity of the truncated protein is reduced, causing an increase of glycolytic potential, a higher rate of pH decline and, consequently, higher drip loss. However, this variant seems to be exclusive to Duroc (Liu, Liu, et al., 2019) and Italian Large White pigs (Zappaterra et al., 2019). Finally, in the region at SSC8 there was a block of variants in high linkage disequilibrium (r2 > 0.70) that spanned from 71 591 592 to 71 669 932 (Figure 1d), which is much wider than the haplotype blocks around the most significantly associated variants in the SSC3 and SSC15 regions. However, although any of the genes within that linkage disequilibrium block might be responsible for the detected association, no clear functional candidate gene was present in that region.
To better comprehend the effect of the variants on the pH, a larger number of animals from the same population were raised and phenotyped for pH following the same protocol (n = 828 barrows, from 125 sires and 421 dams, of which 695 were not initially genotyped by whole-genome sequencing). Additionally, color measurements were performed in a subset of 497 pigs in gluteus medius muscle using a Konica Minolta CM-700d spectrophotometer (Konica Minolta Sensing Inc., Osaka, Japan) in the CIELAB space (Commission Internationale de l'Eclairage, 2018). For each sample, color-space values for lightness (L*), redness (a*), and yellowness (b*) were recorded as the average of three readings. All 828 pigs were genotyped for variants within the three regions using PCR coupled with restriction enzymes or real-time PCR (QuantStudio3; Applied Biosystems, Thermo Scientific) with High-Resolution Melt analysis (Meltdoctor HRM Master Mix; Thermo Scientific) (Table S2). The variants selected for genotyping were the causal mutations described in the bibliography for the PRKAG3 (rs1108399077) and PHKG1 (rs330928088) genes, while for SSC8 region we chose the tag variant rs326497155, since it was the most associated variant that fulfilled technical requirements for primer design and genotyping. We confirmed that the reference allele of PRKAG3 variant rs1109104772 is fixed in our population.
The effect of each SNP on meat quality traits (pH and color-space values L*, a*, and b*) was estimated using a linear model with the batch (12 levels) and the genotype for all of the three variants (three levels each) as fixed effects and the sire as a random effect. The effect of the genotyped variant was tested using the F-statistic. Multiple pairwise comparisons among genotypes were tested with the Tukey HSD test. We tested the interaction between the genotyped SNPs by adding the interaction terms into the previous model. The additive and dominant effects were also estimated by replacing each genotype factor with two covariates coded as (−1, 0, 1) and (0, 1, 0) for the homozygous reference, and heterozygous and homozygous alternatives, respectively. All the analyses were performed using the statistical package JMP PRO 16 (SAS Institute Inc., Cary, NC, USA).
Our results showed that all variants had an effect on pH and meat color, specifically on L* and b* (Table 1). For the variant located at PHKG1 gene, we observed that the alternative allele has a negative effect on ultimate pH with an additive effect of −0.07 ± 0.01 (p < 0.001), but increasing the L* and b* (+0.67 ± 0.21, p = 0.001, and +0.27 ± 0.11, p = 0.02; respectively). For the variant at PRKAG3 gene, with a positive additive effect on ultimate pH of +0.08 ± 0.01 (p < 0.001), but reducing the L* and b* (−0.62 ± 0.22, p = 0.005, and −0.28 ± 0.12, p = 0.01; respectively). A similar scenario is observed for the variant located at SSC8, we observed that the alternative allele has a positive additive effect on ultimate pH of +0.04 ± 0.01 (p < 0.001), but reducing the L* and b* (−0.62 ± 0.20, p = 0.003, and −0.33 ± 0.11, p = 0.002, respectively). The effect of the three variants is consistent with the negative correlation between pH and the color parameters (Jankowiak et al., 2021; Ros-Freixedes et al., 2014; Schwab et al., 2006). In our population, this correlation was more noticeable for L* and b*, with correlations around −0.43 (p < 0.001) and −0.41 (p < 0.001), respectively. Since both PHKG1 and PRKAG3 affect glycolytic potential (Liu, Zhou, et al., 2019), we tested the interaction between these two genes, as well as with the variant in SSC8. As far as we are aware, the interaction between the PHKG1 and PRKAG3 variants had not been studied before. Our results showed no interactions and that the effect of the three individual variants is conserved when the other two are included in the model. This indicates that each variant affects glycolytic potential and ultimate pH through different pathways or mechanisms, resulting in small but cumulative effects on pH.
| Variant and trait | Genotype | Additive effect1 | Dominant effect | ||||
|---|---|---|---|---|---|---|---|
| PHKG1 (rs330928088) | CC (n = 374) | CA (n = 373) | AA (n = 90) | a | p-value | d | p-value |
| pH | 5.84 ± 0.01a | 5.79 ± 0.01b | 5.70 ± 0.02c | +0.07 ± 0.01 | <0.001 | +0.02 ± 0.01 | 0.28 |
| Color L* | 42.70 ± 0.22a | 43.42 ± 0.25b | 44.05 ± 0.40b | +0.67 ± 0.21 | 0.001 | +0.10 ± 0.31 | 0.87 |
| Color a* | 3.08 ± 0.11 | 3.06 ± 0.12 | 2.91 ± 0.19 | −0.08 ± 0.09 | 0.39 | +0.07 ± 0.12 | 0.57 |
| Color b* | 5.88 ± 0.13a | 6.17 ± 0.14ab | 6.42 ± 0.22b | +0.27 ± 0.11 | 0.02 | +0.02 ± 0.14 | 0.87 |
| SSC8 (rs326497155) | CC (n = 310) | CT (n = 401) | TT (n = 126) | a | p-value | d | p-value |
| pH | 5.74 ± 0.01a | 5.76 ± 0.01a | 5.83 ± 0.02b | +0.04 ± 0.01 | <0.001 | −0.03 ± 0.01 | 0.05 |
| Color L* | 43.87 ± 0.26a | 43.67 ± 0.24b | 42.62 ± 0.37b | −0.62 ± 0.20 | 0.003 | +0.42 ± 0.26 | 0.11 |
| Color a* | 3.04 ± 0.13 | 2.87 ± 0.12 | 3.13 ± 0.17 | +0.04 ± 0.09 | 0.66 | −0.22 ± 0.12 | 0.06 |
| Color b* | 6.40 ± 0.15a | 6.35 ± 0.14a | 5.74 ± 0.20b | −0.33 ± 0.11 | 0.002 | +0.28 ± 0.14 | 0.04 |
| PRKAG3 (rs1108399077) | CC (n = 490) | CT (n = 270) | TT (n = 77) | a | p-value | d | p-value |
| pH | 5.70 ± 0.01a | 5.77 ± 0.01b | 5.86 ± 0.02c | +0.08 ± 0.01 | <0.001 | −0.02 ± 0.02 | 0.31 |
| Color L* | 43.97 ± 0.22a | 43.46 ± 0.25ab | 42.74 ± 0.43b | −0.62 ± 0.22 | 0.005 | +0.10 ± 0.31 | 0.73 |
| Color a* | 3.15 ± 0.11 | 3.16 ± 0.12 | 2.75 ± 0.20 | −0.20 ± 0.10 | 0.04 | +0.21 ± 0.14 | 0.13 |
| Color b* | 6.44 ± 0.13a | 6.15 ± 0.14ab | 5.88 ± 0.23b | −0.28 ± 0.12 | 0.01 | −0.01 ± 0.16 | 0.96 |
- Note: a–c Within trait, means with different superscripts indicate statistically significant differences (p ≤ 0.05). Sample size for color traits was 241, 201, and 55; 189, 244, and 64; 303, 152, and 42 for the three genotypes of the PHKG1, SSC8, and PRKAG3 variants, respectively.
- 1 Additive allele substitution of the alternative allele.
Since no clear functional candidate gene was identified for the association of the SSC8 region, we cannot discard a trans-regulatory effect. Preliminary results on the differential gene expression analysis using RNA-Seq data of 40 pigs of the same population (Solé et al., 2022) with the deseq2 software (Love et al., 2014) revealed that the SSC8 rs326497155 genotype was associated with differential expression of seven genes, among which there was the thyrotropin-releasing hormone (TRH) gene (log2 fold change = +1.42 ± 0.35; q = 0.07; Table S3). TRH inhibits the expression of glycogen synthesize kinase-3β (Luo et al., 2002), an enzyme that in its active form inhibits glycogen synthesis (Rayasam et al., 2009) and, therefore, could affect the glycolytic potential of the muscle. However, in this dataset, the expression of TRH and pH was positive (+0.45; p = 0.003), which is counterintuitive. These results provide a plausible mechanism by which the region at SSC8 could affect pH through the trans-regulation of TRH expression, but since this relationship is likely to be a complex one, this hypothesis should be properly tested through a more suitable experimental design.
In conclusion, our research has unveiled a previously undiscovered genomic region influencing ultimate pH in Duroc pigs. Despite the absence of functional candidate genes within this region, we used the variant rs326497155 as a tag variant for the entire QTL, extending from SSC8 71 591 592 to 71 669 932 bp. Further research would be needed in order to ascertain the causality of any of the genes located at that region. Furthermore, our GWAS analysis confirmed the effect of two regions previously linked to the studied trait. Notably, our investigation explored potential interactions between the causal variants in the PHKG1 and PRKAG3 genes, revealing that their effects on ultimate pH are independent of each other.
AUTHOR CONTRIBUTIONS
Eduard Molinero: Conceptualization; data curation; investigation; methodology; validation; writing – original draft. Ramona N. Pena: Methodology; resources; writing – review and editing. Joan Estany: Funding acquisition; methodology; writing – review and editing. Roger Ros-Freixedes: Funding acquisition; methodology; resources; supervision; writing – original draft.
ACKNOWLEDGMENTS
We acknowledge the personnel at Selección Batallé for their cooperation for the recording of on-farm data and sample collection. We gratefully acknowledge Pilar Sopeña from the Animal Breeding group, University of Lleida, for laboratory assistance. This research was part of grant PID2021-125689OB-I00 funded by MCIN, AEI, doi: 10.13039/501100011033, and by ERDF “A way of making Europe”. E.M. is recipient of a UdL-Santander Predoc scholarship.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The dataset for the replication of the main results of this study is available from CORA Research Data Repository with doi 10.34810/data1084. Sequence variants are available from the European Variation Archive of EMBL with the accession number PRJEB43318.




