Variant in the RFWD3 gene associated with PATN1, a modifier of leopard complex spotting
Summary
Leopard complex spotting (LP), the result of an incompletely dominant mutation in TRPM1, produces a collection of unique depigmentation patterns in the horse. Although the LP mutation allows for expression of the various patterns, other loci are responsible for modification of the extent of white. Pedigree analysis of families segregating for high levels of patterning indicated a single dominant gene, named Pattern-1 (PATN1), as a major modifier of LP. Linkage analysis in two half-sibling families segregating for PATN1 identified a 15-Mb region on ECA3p that warranted further investigation. Whole transcriptome sequencing of skin samples from horses with and without the PATN1 allele was performed to identify genic SNPs for fine mapping. Two Sequenom assays were utilized to genotype 192 individuals from five LP-carrying breeds. The initial panel highlighted a 1.6-Mb region without a clear candidate gene. In the second round of fine mapping, SNP ECA3:23 658 447T>G in the 3′-UTR of RING finger and WD repeat domain 3 (RFWD3) reached a significance level of P = 1.063 × 10−39. Sequencing of RFWD3 did not identify any coding polymorphisms specific to PATN1 horses. Genotyping of the RFWD3 3′-UTR SNP in 54 additional LP animals and 327 horses from nine breeds not segregating for LP further supported the association (P = 4.17 × 10−115). This variant is a strong candidate for PATN1 and may be particularly useful for LP breeders to select for high levels of white patterning.
Introduction
Coat color is a highly valued trait in domestic species. Studies of ancient DNA suggest that the wide variation of pigmentation seen in modern horses results partly from human artificial selection that began early in the process of domestication (Ludwig et al. 2009). The relative ease of obtaining phenotypes through photographs makes coat color an ideal model system to investigate the molecular basis of pigmentary changes. Genetic mapping studies have been successful in the horse, leading to the availability of a variety of molecular diagnostic tests that are routinely used by breeders (Rieder 2009).
Many coat colors variants have simple modes of inheritance, with a single autosomal mutant allele in each locus being responsible for a distinct alteration of phenotype. In contrast, leopard complex spotting (LP) is a collection of related patterns found in several breeds with a more complex inheritance akin to a polygenic trait (Sponenberg et al. 1990). An incompletely dominant allele (LP) allows for the expression of a range of depigmentation phenotypes, including variable symmetrical white patterning centered over the hips, striped hooves, white sclera, mottled skin and progressive depigmentation of the hair known as ‘varnish roaning’ (Bellone et al. 2013). Heterozygotes generally have many oval pigmented spots within regions of white coat, whereas homozygotes have few to no such spots. The wide range of white patterning variation is believed to be influenced by other modifying loci.
Previous research has linked LP to a retroviral insertion into an intron of the transient receptor potential cation channel, subfamily M, member 1 gene (TRPM1), resulting in premature polyadenylation (Bellone et al. 2013). TRPM1 is a calcium channel present in the brain, heart, melanocytes and retina. The function of TRPM1 in most tissues is not well understood, although studies in the retina have elucidated its function in low-light vision, as reviewed in Oancea & Wicks (2011). Horses homozygous for the LP allele are affected by congenital stationary night blindness, which points to a causal role of the TRPM1 mutation, although the molecular mechanism responsible for the depigmentation phenotype has yet to be determined (Sandmeyer et al. 2007, 2012; Bellone et al. 2013).
Variability in white pattern expression is not a unique characteristic of LP. Studies of white markings in the Arabian horse revealed that both genetic and stochastic factors influence pattern development that responds readily to selection (Rieder et al. 2008). Notably, the e allele of the MC1R gene (which leads to the inability of melanocytes to produce eumelanin) and the A allele of the ASIP gene (eumelanin production restricted to the extremities) are associated with increased size of white markings (Woolf 1992). Male horses were also shown to have larger markings than female horses (Woolf 1990). This observation has been made in other breeds and for other patterns, including LP (Sponenberg 2003). Recent work in the Franches–Montagnes breed has shown that an accumulation of mutations in the KIT and MITF genes are responsible for an increase in area of white markings (Haase et al. 2013).
Previous reports by breeders and preliminary pedigree investigation noted that horses with the more extreme white patterns (termed leopard and few-spot; Fig. 1) always had a parent with the same level of patterning, thus suggesting a dominant mode of inheritance for a modifier for LP patterns (Sponenberg 2009). This modifier has been termed PATN1 for pattern-1 or the first LP pattern modifier. To begin to investigate this modifying locus, we conducted pedigree and progeny record analysis of 17 LP stallions that produced 684 leopard complex-spotted offspring. We then used linkage analysis in two half-sibling families to map PATN1 to ECA3. Whole transcriptome sequencing was subsequently utilized to generate variants for fine-mapping analysis. Although the implicated region had no clear candidate genes or mutations, further genotyping showed a 3′-UTR variant of RFWD3 (RING finger and WD repeat domain 3) to be strongly associated with the PATN1 allele. Here, we present the results of the pedigree and progeny record analysis, mapping of PATN1 and the identification and analysis of an associated variant in RFWD3.

Materials and methods
Phenotyping and pedigree/progeny analysis
Seventeen stallions with a large number of offspring (>20) displaying a LP spotting pattern were utilized in the pedigree and progeny analysis. Photographic records of these stallions, 684 LP-spotted offspring, their dams (Appaloosa and outcrossed) and available grandparents and great grandparents were examined for the level of white spotting. A single experienced phenotyper (SA) used visual inspection of photographic records from the horses at birth to determine the percentage of the coat covered by white hair, assigned in increments of 10% (Fig. S1). Horses with 60% white or greater were classified as high white, whereas those with 40% or less were classified as low white. To control for small effect modifiers that could cause errors in classification, the horses with white levels in the middle range (horses between 40% and 60% white) were excluded from the study (Fig. S1). In addition, horses exhibiting obvious phenotypes associated with other white spotting patterns [sabino-1, tobiano and the various splashed white, as described in Brooks & Bailey (2005), Brooks et al. (2007) and Hauswirth et al. (2012)] were excluded, as these are known to affect leopard complex patterning (Hauswirth et al. 2013). Progeny records were grouped according to mare phenotype, specifically: LP with high-white phenotype (≥60% white), LP with low-white phenotype (≤40%), non-characteristic Appaloosa mares (lp/lp) and outcrossed mares (lp/lp). To investigate a dominant mode of inheritance, deviations from expected frequencies were tested using the chi-square goodness-of-fit test. The outcrossed mare group (73 Thoroughbreds, 45 Quarter Horses, 7 Arabians, 24 Warmbloods and 2 Drafts) was examined to determine whether other breeds may contribute alleles for high-white spotting. To investigate the connection of known coat color alleles with the amount of white in Appaloosas, the base coat color (chestnut, bay or black) and probable genotype for agouti and extension determined by breeding records were also recorded.
Sample collection
Two half-sibling families, consisting of two few-spot Appaloosa stallions (LP/LP PATN1/patn1) mated to Thoroughbred and American Quarter Horse mares (breeds not known to be carriers of LP or PATN1), were available for linkage mapping. Family A consisted of 17 offspring (nine PATN1 and eight patn1), and family B consisted of 30 offspring (13 PATN1 and 17 patn1). During our pedigree analysis, we identified four offspring that had 40% white spotting by stallions whose production records indicated homozygosity for PATN1 (Table S1). Thus, to eliminate potential errors in phenotyping, we decided to use a more stringent criterion for our initial mapping efforts and included only offspring with the extreme and easily distinguishable phenotypes at birth (>70% white for PATN1, <30% white for patn1).
In selecting a PATN1 animal for RNA-seq that was likely homozygous (PATN1/PATN1), we investigated the parental phenotypes and breeding records of several stallions. The stallion selected (06-92) was homozygous for the LP allele (as determined by genetic testing), his parents were both leopard-spotted and his breeding records supported homozygosity for PATN1 (as all 26 offspring were extensively patterned). For fine mapping, PATN1 phenotyping was performed on 246 horses with LP [118 Appaloosas (ApHC), 13 British Spotted Ponies (BSP), 87 Knabstruppers (KB), 25 Miniature Horses (MINI) and 2 Pony of Americas (PoA)]. Of these, 192 were used for fine mapping (92 ApHC, 10 BSP, 69 KB, 19 MINI and 2 PoA) and the remaining 54 were used to validate the associated SNP as a marker for DNA testing. An additional 327 horses were available from banked DNA of nine diverse breeds that were not believed to carry PATN1 (22 Andalusians, 57 Arabians, 25 Colombian Creole Horses, 11 Lusitanos, 14 Morgan Horses, 13 Oldenburgs, 101 Quarter Horses, 30 Standardbreds, 32 Thoroughbreds and 2 Arabian/Quarter Horse crosses). DNA from blood samples was extracted using either the Puregene whole-blood extraction kit (Qiagen Inc.) or Nucleon Bacc2 kit (GE Healthcare Bio-Sciences Corp.). High-quality DNA from hair was prepared for Sequenom genotyping by a modified Puregene protocol (Cook et al. 2010). Hair lysates were prepared for restriction digest genotyping as described in Locke et al. (2002). To maximize the number of samples utilized, for fine mapping and polymorphism association testing, horses with greater than 60% white patterning were designated with the phenotype PATN1, whereas horses with less than 40% white hair were designated as the phenotype patn1.
Linkage mapping of ECA3
Stallions, offspring and available dams were typed for nine microsatellites spanning 80 cM surrounding MC1R on ECA3 (Penedo et al. 2005). Microsatellite markers were amplified in 10-μl PCRs containing 25 ng of genomic DNA, 1X PCR buffer with 2.0 mm of MgCl2 (Roche Diagnostics Corp.), 100 μm of each dNTP, 0.1U of FastStart Taq DNA Polymerase (Roche Diagnostics Corp.) and either 0.1 or 1.0 μm of each primer. An ABI 310 genetic analyzer was used to detect fluorescently labeled primers (Applied Biosystems Inc.). strand microsatellite analysis software (available at http://www.vgl.ucdavis.edu/informatics/strand.php, accessed March 19, 2015) was used to call alleles (Toonen & Hughes 2001). Linkage analysis was performed using the crimap program version 2.4; the two-point option and the build options were utilized to calculate the maximum-likelihood estimates of recombination fraction (Ɵ) and localize PATN1 (Green et al. 1990). Primer sequences, concentrations and annealing temperatures can be found in Table S2.
RNA extraction and sequencing
Full-thickness skin biopsies were obtained from two Appaloosas (06-92 LP/LP PATN1/PATN1, 07-49 LP/lp patn1/patn1) and one Thoroughbred (D-052 lp/lp, assumed to be patn1/patn1). Sample information is provided in Table S3. Punch biopsies were performed as previously described (Bellone et al. 2008). Total RNA was isolated either using the RNeasy Lipid Tissue mini kit (Qiagen Inc.), according to the manufacturer's protocol, or by acid guanidinium thiocyanate–phenol–chloroform extraction (Chomczynski & Sacchi 1987; MacLeod et al. 1996). Quantification was performed using a NanoDrop spectrophotometer, and quality was assessed using a 2100 Bioanalyzer (Agilent Technologies).
Library preparation and sequencing was performed by Cornell University's Life Sciences Core Laboratory Center. Single-end libraries for horses 06-92 and D-052 were constructed using the manufacturer's protocols for poly-T selection and sequenced on an Illumina HiSeq 2000. A paired-end library from an unpigmented skin sample from a single horse (07-49) was constructed using the manufacturer's protocols for poly-T selection and sequenced on an Illumina GAIIx. Five additional RNA-seq samples from a previous study were included, as the PATN1 genotypes of the sampled horses were known and thus could be used to screen variants for further testing (LP/LP patn1/patn1 pigmented skin; LP/lp patn1/patn1 pigmented skin; lp/lp patn1/patn1 pigmented skin; LP/LP patn1/patn1 retina; lp/lp patn1/patn1 retina; see Table S3 and Bellone et al. 2013). The resulting reads were aligned to the EquCab2 reference genome using the bwa software package under default parameters (Wade et al. 2009; Li & Durbin 2009; Table S3). samtools was used to convert alignments to BAM format for visualization and to call variants (Li et al. 2009). Variants were called using the samtools ‘mpileup’ option and were filtered to retain those with a Phred-scaled quality over 30 and at least four observations. Visualization was performed using the UCSC Genome Browser and the Integrated Genome Viewer (Kent et al. 2002; Robinson et al. 2011). Raw reads were submitted to the European Nucleotide Archive (accession number PRJEB6101).
Sequenom SNP genotyping
Identified variants from six horses [two from this study (D-052, 06-92), three from the previous study (07-45, 07-46, 07-54) and one from both studies (07-49)] were compared to create two pools of SNPs for fine mapping. The first set comprised homozygous SNPs that were observed only in the single PATN1/PATN1 sample (06-92). The second set comprised SNPs that were observed in at least two, but not all, sequenced horses, corresponding to polymorphic loci in the Appaloosa breed. Publicly available SNPs discovered in other breeds were selected to obtain variants from areas without RNA-seq coverage (Wade et al. 2009).
Two fine-mapping sets of 80 SNPs were generated for genotyping with the Sequenom MassArray Spectrophotometry platform using the iPlex system (Gabriel et al. 2009). Multiplex design and assays were carried out by GeneSeek, Inc. An initial panel spanned the 15-Mb linked region, with preference given to include the PATN1-specific variants in the assay. A second panel spanning the refined 1.6-Mb region contained the top five markers from the first panel and additional SNPs representing almost all 34 genes in the region (Table 1).
| Set | Total | Panel 1 | Panel 2 |
|---|---|---|---|
| PATN1-specific | 100 | 25 | 11 |
| LP-polymorphic | 288 | 19 | 34 |
| EquCab2 | 330 | 36 | 35 |
| Total | 718 | 80 | 80 |
- PATN1-specific SNPs were detected only in PATN1 sequences (derived from a single individual), whereas LP-polymorphic SNPs were found in multiple LP sample sequences. Available samples were of genotypes LP/LP PATN1/_, LP/LP patn1/patn1 and LP/lp patn1/patn1.
Genotyping files were analyzed using plink 1.07 (Purcell et al. 2007). Filtering parameters were 85% individual call rate, 90% genotyping rate and 5% minor allele frequency. After filtering, there were 186 individuals and 74 SNPs in the first panel and 192 individuals and 68 SNPs in the second panel. Association was performed using the genotypic model with the Fisher's exact test. To identify the strongest associations and investigate PATN1-specific haplotypes, analyses were performed on all breeds, only Appaloosas and only Knabstruppers. haploview was used to assess haplotypes and linkage disequilibrium (Barrett et al. 2005).
RT-PCR and sequencing of candidate gene RFWD3
To obtain equine-specific gene annotation, transcripts assembled from hoof RNA-seq were aligned to EquCab2 using blat and visualized with the UCSC Genome Browser (Holl et al., 2015), transcripts available from the NRSP-8 Bioinformatics Data Repository at http://www.animalgenome.org/repository/horse/; Kent 2002). As de novo assembly is computationally intensive, we chose to evaluate the available hoof dataset as a model for our skin sequencing. The most associated fine-mapping SNP fell within a transcript corresponding to the 3′-UTR of the RFWD3 gene. However, as a portion of the 5′-UTR appeared to be missing from the assembled transcript, coverage of mapped skin RNA-seq reads was used to incorporate additional sequence from the genomic reference. The incorporated genomic sequence comprised a series of highly similar 51-bp repeats, which likely interfered with the de novo assembly process. RNA-seq reads from skin samples displaying sufficient RFWD3 coverage as viewed on the UCSC Genome Browser (06-92, 07-49, D-052) were then aligned to the RFWD3 transcript using bwa and loaded into the Integrated Genome Viewer (Kent et al. 2002; Li & Durbin 2009; Robinson et al. 2011). The remaining two skin samples had too few overall reads and thus lacked the read depth required for further analysis. Alignments were visually inspected to confirm full coverage of all exons in the model, as well as to screen for any additional polymorphisms.
Due to the inconsistent coverage of RNA-seq reads observed across the RFWD3 transcript model, Sanger sequencing in five horses [06-92 and D-052 from this project and three additional horses banked in the Bellone Laboratory (LP/lp PATN1/patn1; LP/LP patn1/patn1; lp/lp)] was performed to more closely examine the regions of RFWD3 with lower coverage. Primers spanning these regions, described in Table S4, were designed using primer 3 software (Rozen & Skaletsky 1999), based on the hoof transcript alignment on EquCab2 (Wade et al. 2009). Two-step RT-PCR was performed using the SuperScript VILO MasterMix kit (Life Technologies) followed by standard PCR. All DNA was amplified using 20-μl volume PCR with FastStart Taq DNA polymerase (Roche Applied Science) and included all reagents as per the manufacturers recommended conditions.
PCR products were submitted to the Cornell Core Life Sciences Laboratories Center for sequencing using standard cycle-sequencing chemistry on a 3730 DNA Analyzer (Applied Biosystems Inc.). Amplicons were aligned and screened for mutations using Consed (Gordon et al. 1998).
PCR-RFLP SNP Genotyping
During RNA-seq analysis, two SNPs unique to the single PATN1 horse were detected in the 3′-UTR of RFWD3, one of which was the most associated SNP from our fine-mapping analysis. Both of these SNPs were investigated further for association with PATN1 in a second sample set (Table S4). For each SNP, RFLP genotyping was performed by 10-μl PCRs followed by restriction digestion and visualization on 4% agarose gels. SNP ECA3:23 658 447T>G (termed RFWD3-3U1) was digested for 20 min at 37 °C using EcoRV (New England BioLabs Inc.), resulting in either a single 455-bp band representing the G allele or 276-bp + 170-bp bands for the T allele. As SNP ECA3:23 659 162T>C (termed RFWD3-3U2) does not normally alter a restriction site, dcaps finder 2.0 was used to design PCR primers to generate a TaqI site specific to the C allele, resulting in 281-bp + 30-bp bands, vs. a single 311-bp band for the T allele (Neff et al. 2002). SNP genotypes were tested for association with PATN1 by a Fisher's exact test using r 3.0.1 (R Core Team 2013).
Results
Pedigree analysis
Pedigree analysis and progeny records conclusively supported a dominant mode of inheritance for PATN1 (Fig. 2, Table S1). Eleven of the 17 stallions investigated had a high-white phenotype (60% white or more) and at least one parent with a similar phenotype. In addition, 357 high-white offspring had at least one parent with a high-white phenotype. Further support for a dominant mode of inheritance comes from investigating the breeding records of stallions with a low-white coat phenotype bred to low-white mares (≤40% white), as all 85 LP progeny had a low-white phenotype. Five stallions (stallions 13–17, Table S1) had production records consistent with homozygosity for PATN1: 181 of 185 offspring had a high-white phenotype regardless of the mare's phenotype. The four exceptions in this group all had phenotypes at the cutoff threshold value of 40% white; thus, it is possible that some other factor (genetic and/or environmental) contributed to increasing pigment production in these four exceptions. The other six high-white stallions had production records consistent with heterozygosity for a dominant gene, as the observed frequencies did not deviate from the expected 3:1 ratios (P = 0.12) in the high-white mare group and the 1:1 ratio (P = 0.07) in the low-white mare group. Investigating progeny records of these 17 stallions bred to 151 outcrossed mares suggested that PATN1 is not found in non-LP breeds. Records of the six stallions that appeared to be heterozygous for PATN1 (stallion 1-6, Table S1) bred to 72 non-Appaloosa mares produced 55 high-white and 51 low-white offspring consistent with the expected 1:1 ratio (P = 0.70) of PATN1/patn1 × patn1/patn1 matings. Furthermore, of the 63 crosses of low-white stallions (assumed patn1/patn1) to 37 outcrossed mares, only one offspring of a Thoroughbred mare had a high-white phenotype (70%), indicating that PATN1 is likely absent in other breeds.
Two stallions whose progeny records demonstrated they were heterozygous for PATN1 (stallion 1 and 2 in Table S1) and were also heterozygous for the chestnut allele at MC1R (E/e, bay coat color) were examined more closely. These two stallions were crossed to a total of 48 patn1/patn1 chestnut (e/e) appaloosas (LP) or outcrossed mares, allowing for the investigation of the linkage of PATN1 and MC1R. In family one, 11 of 12 offspring inherited the chestnut (e) and the PATN1 alleles from the sire. However, in family two the reverse was true, where 9 of 10 PATN1 offspring inherited the bay (E) allele from the stallion, suggesting linkage and thus localization to ECA3 (Table 2).
| Black or Bay (E) | Chestnut (e) | |
|---|---|---|
| Stallion 1 Bay few-spot (genotype LP/LP, PATN1/patn1, E/e) | ||
| PATN1 | 1 | 11 |
| patn1 | 15 | 1 |
| Stallion 2 Bay few-spot (genotype LP/LP, PATN1/patn1, E/e) | ||
| PATN1 | 9 | 2 |
| patn1 | 1 | 8 |
Linkage mapping
To investigate the hypothesis that PATN1 maps to ECA3, DNA from two available half-sibling families segregating for PATN1 were examined for linkage with nine microsatellite markers flanking MC1R. Five of the nine microsatellite markers were strongly linked to PATN1 (Z ≥ 3.0), thus mapping PATN1 to ECA3 (Table 3). Multipoint linkage analysis mapped PATN1 closest to TKY215 and AHT022 with no recombination detected between PATN1 and AHT022 in the 14 available informative meioses from stallion 2 (stallion 1 was homozygous for this marker). This region corresponds to 18–21 Mb in EquCab2. Marker order differed for these two microsatellite markers (as well as that for AHT036) between the published linkage map (Penedo et al. 2005) and the horse reference genome (Wade et al. 2009; Table 3). In our family data, the order of AHT022 and TKY215 agreed with that of the reference genome. Because marker order differed between the two reference resources for fine mapping, we extended our candidate region to ECA3:11–26 Mb, encompassing and extending beyond the three closest significantly linked loci.
| Marker | Published position (cM) | EquCab2 position (Mb) | Linkage | |
|---|---|---|---|---|
| Ɵ | Z | |||
| AHT036 | 0 | 29.4 | 0.49 | 0.00 |
| COR028 | 12.5 | 11.1 | 0.08 | 6.81 |
| AHT022 | 20.4 | 21.1 | 0.00 | 3.31 |
| TKY215 | 28.3 | 18.2 | 0.04 | 4.84 |
| UCDEQ437 | 30.7 | 31.3 | 0.16a | 2.45 |
| TKY651 | 39.9 | 37.9 | 0.10 | 3.15 |
| TKY528 | 39.9 | 39.8 | 0.13 | 3.79 |
| TKY447 | 55.0 | 70.0 | 0.24 | 1.24 |
| ASB023 | 59.1 | 79.2 | 0.30 | 0.83 |
- a Stallion 2 was homozygous for marker UCDEQ437; thus, this recombination frequency is based on the informative meioses in only one family.
- Bold values represent statistically significant LOD scores and corresponding recombination frequencies.
Illumina RNA-seq analysis
Whole transcriptome sequencing of the four skin samples from this study produced a total of 114 728 413 reads/pairs (Table S3). Visualization of the alignments in UCSC did not reveal any extreme differences in gene expression within the PATN1-associated region. As no replicates were available, differential expression analysis could not be attempted. Putative functional variants found only in the PATN1/PATN1 horse (06-92) are presented in Table S5.
Sequenom fine mapping
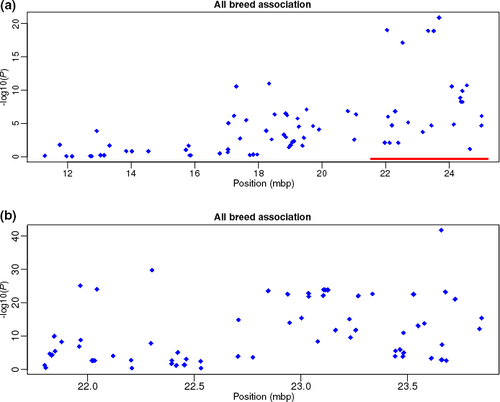
The initial fine-mapping panel showed a strong association (P < 2.4 × 10−18) with region ECA3:22 043 005–23 677 469 (Fig. 3a; the strongest association occurred for SNP ECA3:23 677 469G>A, P = 3.539 × 10−23). None of the 34 genes in this region have a reported association with white spotting phenotypes, although mutations in Vac14 are associated with coat color dilution in mice (Jin et al. 2008). Thus, to refine the position and identify a potential candidate gene for further investigation, we genotyped 80 SNPs in the 1.6-Mb associated region. The association signal strengthened for the second set of markers (Fig. 3b, Table S6), with the strongest association at ECA3:23 658 447T>G (P = 1.063 × 10−39). This SNP (RFWD3-3U1) lies in the 3′-UTR of RFWD3. There were no differences in the position of the primary association signal when analyzing the dataset by breed (Appaloosa P = 2.288 × 10−25, Knabstrupper P = 4.359 × 10−16).
Haplotype analysis identified a 365-kb interval displaying strong linkage disequilibrium. When considering only SNPs that were found in the sequenced PATN1 horse, there were two significantly associated haplotype blocks (P = 2.61 × 10−25, P = 1.11 × 10−33). However, this analysis did not provide any additional mapping information, as the significant haplotypes in each block were defined solely by the allele of the top two associated single markers (P = 1.12 × 10−25, P = 7.06 × 10−34). Additionally, phased data showed recombination between the highest associated SNP and its closest genotyped markers (located 79 863 and 3980 bp on either side).
Analysis of RFWD3
The gene RFWD3 was selected for further mutational screening on the basis of the strong association detected between PATN1 and SNP RFWD3-3U1. Alignments of RNA-seq reads to a RFWD3 transcript annotated in another tissue type (hoof; H. Holl et al., in review) showed full coverage with no reads indicative of missing exons or alternative splicing (Fig. S2). However, there was a dip in read coverage over exon 10 apparent in the PATN1 horse (06-92, from 70 read depth to 15 read depth) that was not observed in two non-PATN1 individuals (07-49 and D-052; Fig. S2).
Coverage over the 5′-UTR was significantly lower than over the rest of the transcript model in all animals sequenced, and closer examination of the reference sequence showed highly similar 51-bp repeats present in both the EquCab2 reference and assembled hoof data. Analyzing variants in this gene using the available RNA-seq data identified one additional variant in the 3′-UTR and two in the 5′-UTR uniquely observed in the PATN1 horse. However, Sanger sequencing of genomic DNA and partial cDNA did not reveal any additional polymorphisms, with all amplicons producing high-quality sequences of the expected length. The abnormal coverage over exon 10 in the RNA-seq data was likely due to computational artifacts.
Genotyping of additional animals
An additional 381 horses were genotyped for SNP RFWD3-3U1 using PCR-RFLP. Fifty-four of these horses were from LP-exhibiting breeds and thus had been phenotyped as having the PATN1 allele. A total of 327 horses were from non-LP breeds and thus were not expected to carry PATN1. Data from the Sequenom panel and PCR-RFLP genotyping were pooled for statistical analysis (Table 4). SNP RFWD3-3U1 was strongly associated with PATN1 in the LP breeds tested (P = 4.68e-56) and was not found in any of the nine non-LP breed samples.
| Breeda | Phenotype | G/_ | T/T | Total | P-value |
|---|---|---|---|---|---|
| ApHC | PATN1 | 55 | 2 | 57 | |
| patn1 | 0 | 60 | 60 | ||
| Total | 55 | 62 | 117 | 1.61e-31 | |
| KB | PATN1 | 58 | 1 | 59 | |
| patn1 | 1 | 27 | 28 | ||
| Total | 59 | 28 | 87 | 3.32e-20 | |
| MINI | PATN1 | 6 | 0 | 6 | |
| patn1 | 5 | 14 | 19 | ||
| Total | 11 | 14 | 25 | n/a | |
| BSP | PATN1 | 9 | 0 | 9 | |
| patn1 | 1 | 3 | 4 | ||
| Total | 10 | 3 | 13 | n/a | |
| PoA | PATN1 | 1 | 0 | 1 | |
| patn1 | 0 | 1 | 1 | ||
| Total | 1 | 1 | 2 | n/a | |
| Total | PATN1 | 129 | 3 | 132 | |
| patn1 | 7 | 105 | 112 | ||
| Total | 136 | 108 | 244 | 4.68e-56 | |
| Non-LPb | PATN1 | 0 | 0 | 0 | |
| patn1 | 0 | 327 | 327 | ||
| All breeds | P-value | 4.17e-115 |
- a LP-carrying breeds are Appaloosa (ApHC), British Spotted Pony (BSP), Knabstrupper (KB), Miniature Horse (MINI) and Pony of Americas (PoA).
- b The non-LP-carrying set consisted of Andalusian (n = 22), Arabian (n = 57), Colombian Creole Horse (n = 25), Lusitano (n = 11), Morgan Horse (n = 14), Oldenburg (n = 13), Quarter Horse (n = 101), Standardbred (n = 30), Thoroughbred (n = 32) and Arabian/Quarter Horse crosses (n = 2).
An additional SNP (ECA3:23 659 162T>C, named SNP RFWD3-3U2) was identified by RNA-seq within the 3′-UTR of RFWD3 that was not part of the genotyping panel. Because RFWD3-3U1 was not perfectly associated with the PATN1-allele phenotype, SNP RFWD3-3U2 was investigated for use as a DNA test. Initially, 39 horses were tested, and this SNP was not as tightly associated with the trait as was RFWD3-3U1 (P = 3.66e-4). Although the PATN1-associated haplotype T-T (3U1-3U2) was observed in most PATN1 animals, four PATN1 horses exhibited a T-C haplotype (Table S7). Thus, additional horses were not genotyped for RFWD3-3U2.
Discussion
Leopard complex spotting is a collection of depigmentation patterns resulting from the incompletely dominant LP allele of TRPM1 and its interactions with other modifying loci. One such genetic factor, PATN1, is responsible for large differences in pattern levels observed segregating in families. Linkage between PATN1 and MC1R alleles as well as with microsatellite markers supports PATN1 being mapped to ECA3. Whole transcriptome sequencing and two rounds of fine mapping by targeted association identified a polymorphism in the 3′-UTR of RFWD3 with strong association with PATN1. This variant was not detected in any of the nine non-LP breeds tested, three of which (Arabian, Thoroughbred and Quarter Horse) were utilized in the formation of the Appaloosa breed. Although the variant was not in complete concordance with the PATN1 phenotype, there was no other detected variant with a stronger association.
Although clear differences in siblings segregating for the PATN1 allele can be easily observed, the complex nature of white patterning can make phenotyping of unrelated individuals difficult. Beyond known factors, such as base coat color and sex, there are likely many unknown genes involved in the enhancement or repression of white patterning. Variation in the degree of depigmentation in white patterning was previously documented in both the frame overo and tobiano coat colors (Santschi et al. 2001; Lightbody 2002; Stamatelakys 2011). Despite strong functional evidence and similarity to homologous phenotypes tying these patterns to their respective mutations, individuals with extremely low levels of white patterning were described. The most extreme examples have been reported in Miniature Horses; animals genotyped positive for a dominantly inherited white spotting allele, but lacked even a single discernible white hair. For example, Santschi et al. (2001) observed two such Miniature Horses that had been genotyped as heterozygous for the frame overo pattern. Five of the Miniature Horses in this study were typed as heterozygous for RFWD3-3U1 despite having below 40% white patterning and may be additional examples of incomplete penetrance of the white pattern phenotype. The modifying effects of known coat color loci in LP breeds warrant further investigation.
RING finger and WD repeat domain 3 (RFWD3) is an E3 ubiquitin ligase involved in DNA damage response. Initial studies indicated that, in response to DNA damage, RFWD3 forms a complex with Mdm2, another E3 ubiquitin ligase that normally degrades p53 (Fu et al. 2010). However, the RFWD3–Mdm2 complex instead stabilizes p53, which activates the G1 checkpoint. Once this checkpoint is passed, RFWD3 becomes inactive and Mdm2 is able to return p53 to its usual low level of expression. RFWD3 also associates with replication protein A and is recruited to sites of DNA damage (Gong & Chen 2011; Liu et al. 2011). The expression pattern of RFWD3 in vivo has not been described.
There are no RFWD3 variants present in the literature. However, phenotypic similarities exist between PATN1 and harlequin, a depigmentation phenotype in the domestic dog. Harlequin Great Danes are the result of an interaction of Merle (in SILV) and Harlequin (in PSMB7) alleles (Clark et al. 2006, 2011). Merle is an incompletely dominant allele that produces a coat color with variable patches of dilute and fully pigmented fur. A lethal-dominant Harlequin allele selectively removes the dilute color, leading to fully pigmented patches on a completely depigmented background in merle dogs, but with no visible phenotype in non-merle individuals. It was hypothesized that Merle may generate abnormal proteins targeted for degradation by the ubiquitin proteasome pathway that cannot be cleared by the Harlequin variant, resulting in either an accumulation of cytotoxic components leading to melanocyte death or abnormal SILVM–SILVm protein complexes that alter cell morphology. The resulting patches containing the abnormal protein thus lack normal melanocytes and are unable to produce pigment, leading to the shift from merle coloration to white in harlequin dogs.
The depigmentation phenotype seen in horses with both LP and PATN1 alleles could result from an interaction similar to that in harlequin dogs. One hypothesis is that abnormal melanosome morphology produced by the insertion of TRPM1 long terminal repeats, as observed in LP melanocyte cultures, causes death of melanocytes and leads to regions of the skin lacking pigmentation at birth and progressive roaning of hair through the life of LP horses (Bellone et al. 2013). A depigmentation phenotype in zebrafish, caused by a mutation in trpm7, was shown to be the result of abnormal melanosome morphology leading to ruptured cellular membranes and melanophore death (McNeill et al. 2007). Inhibition of melanin synthesis was shown to rescue the phenotype, implicating a mechanism of accumulation of cytotoxic intermediates as a part of melanogenesis. If RFWD3 is involved in the removal of deformed melanosomes seen in LP horses, either directly or through downstream targets, alterations of its expression could lead to a much earlier melanocyte death and thus a larger reduction in pigmentation. Differential expression of RFWD3 could not be investigated as part of our RNA-seq analysis as only one PATN1/PATN1 sample was sequenced. Therefore, to further investigate this hypothesis, expression of RFWD3 and melanosome morphology should be examined in additional samples.
The RFWD3-3U1 SNP was strongly but not perfectly associated with the PATN1-related phenotype. Specifically, three horses with high levels of white patterning genotyped homozygous for the unassociated allele (T), whereas seven horses had at least one copy of the PATN1-associated allele (G) but displayed <40% white patterning. It is possible that this is the causal mutation and that other modifying genes could be affecting the expression of the white patterning as described above. Thus, the polymorphism in the 3′-UTR could be responsible for abnormal expression of RFWD3 during development or for disruption of normal translational control through an alteration of RNA secondary structures (Jia et al. 2013). Structures in the 3′-UTR are often targeted by proteins and miRNAs to moderate translation into proteins.
However, other possibilities are also likely, and this variant could be in close linkage to the causal mutation. The next gene closest to RFWD3-3U1 is golgi glycoprotein 1 (GLG1). This gene was briefly examined during the RNA-seq data analysis but was discarded as no variants specific to the PATN1 horse were observed. Targeted genomic resequencing could be utilized to interrogate this region for additional polymorphisms.
Given the strong association signal and that the G allele was not observed in nine non-LP breeds, the RFWD3-3U1 SNP could be utilized as a DNA test for selective breeding, as this color is a highly desirable trait in LP breeds. Future investigations of the functional consequences of this polymorphism may help elucidate the role of RFWD3 and TRPM1 in melanocyte function and pigmentation development.
Acknowledgements
The authors would like to thank all of the breeders and owners who provided samples from their horses for this study. We thank Taryn Cranford for her technical assistance as part of her Independent Study courses at the University of Tampa as well as Dr. Ernest Bailey, Dr. Domenico Bernoco and Lee Millon for their technical assistance. The study was supported in part by grants from the Appaloosa Horse Club of Canada and by generous donations by Appaloosa breeders who belong to the Appaloosa Project's Electronic Classroom. Karla Brown was supported by a University of Tampa Department of Biology Summer research fellowship.
Conflict of interest
Rebecca R. Bellone, Julia Malvick and M. Cecilia T. Penedo are affiliated with the Veterinary Genetics Laboratory, a genetic testing laboratory offering coat color tests in horses. Heather M. Holl and Samantha A. Brooks are scientific advisers for a company offering genetic testing in horses.




