Hold your breath: Observations of the endangered pygmy bluetongue (Tiliqua adelaidensis) submerged in flooded burrows
Abstract
Understanding adaptations to extreme weather events by endangered species is critical to inform conservation decisions, particularly when their adaptations relate to artificial habitat supplementation at translocation sites. Apnoea, temporary suspension of breathing, has been observed as an anti-predator adaptation by semi-aquatic reptiles that dive underwater for periods of time to avoid detection. This study reports on the observations of an endangered grassland skink, the pygmy bluetongue (Tiliqua adelaidensis), remaining submerged in rain-induced flooded artificial burrows at an experimental translocation site.
Terrestrial lizards inhabiting riparian environments may have a greater ability to avoid predator detection by submerging in water in aquatic environments due to their physiology, including a lower metabolic rate and larger lung capacity than mammals (Bhaisare & Pelling, 2015; Heatwole, 1977). Some lizards such as the Anolis genus have adapted to aquatic environments by rebreathing air bubbles trapped to their heads allowing them to stay submerged for up to 18 min (Boccia et al., 2021). While apnoea and diving behaviour of semi-aquatic reptiles have been extensively studied (Boccia et al., 2021; Hare & Miller, 2009; Heatwole et al., 1962; Heatwole & Torres, 1963), our study provides a novel insight into the adaptation exhibited by a terrestrial grassland skink.
The endangered pygmy bluetongue (Tiliqua adelaidensis) is the smallest member of the Tiliqua genus (average snout–vent length of 95 mm) that occurs in fragmented patches of agricultural grassland in mid-north South Australia (Armstrong & Reid, 1992; Hutchinson et al., 1994). Pygmy bluetongues are closely associated with mygalomorph and lycosid spider burrows, occupying a vacant burrow with a narrow entrance to fit the lizard's head but not wide enough for an intruder to enter the burrow, and a preferred depth of at least 30 cm (Milne & Bull, 2000). The lizards have not been observed altering the burrow or digging another and so an abundance of spider burrows is integral to the maintenance of a pygmy bluetongue population. Burrows provide defence from predators, shelter from extreme temperatures, and a means to ambush invertebrate prey outside the burrow entrance (Milne et al., 2003; Souter et al., 2007). The species will also use artificial burrows (Souter et al., 2004) constructed of a wooden dowel hollowed to 19 mm in diameter and 30 cm in length hammered vertically into the ground. These artificial burrows are used to provide home sites in translocations of the species.
During heavy rainfall events, both the artificial and naturally occurring spider burrows can fill with water. Ebrahimi et al. (2012) reported in warmer spring and summer months that pygmy bluetongues tolerate a flooded burrow, partially emerging to bask and submerging themselves when disturbed; however, one lizard was observed stuck in the clay within a burrow and was rescued, and another vacated the flooded burrow. Furthermore, it was suggested during winter months when ectothermic reptiles have a reduced ability to respond to heavy rainfall, flooded burrows may be a disadvantage to the species (Ebrahimi et al., 2012). Here, we report on the submersion ability of the pygmy bluetongue during multiple heavy rainfall events in cooler winter and spring months.
Our initial observation of submerged pygmy bluetongues occurred in June 2022, and the duration of their submersion was recorded in October 2022. Observations took place at an experimental translocation site near Tarlee, South Australia (34.2723° S, 138.7699° E) where, in 2020 and 2021, pygmy bluetongues from three source populations (Burra, Kapunda and Jamestown) were translocated into ten 25 × 25 m2 enclosures with metal walls (30 cm high) dug underground and a lipped top to prevent escape. Within each enclosure, 16 clusters of three artificial burrows were positioned evenly. Pygmy bluetongues (n = 16 per enclosure) were initially released into one of three clustered artificial burrows, with each lizard released in a separate cluster, within each enclosure. Spider burrows naturally occurred within each enclosure.
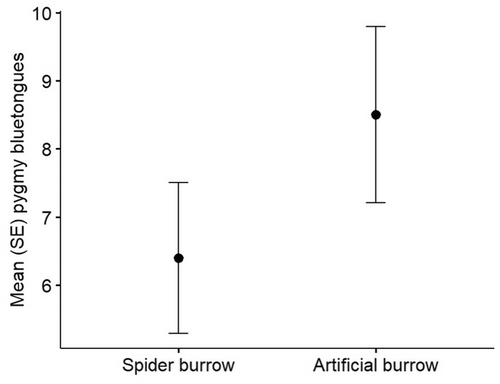
The initial observation was made on 5 June, with 25.6 mm rainfall within the preceding 24 h. Pygmy bluetongues in multiple translocation enclosures were found fully submerged at the bottom of artificial burrows, but the total found submerged was not recorded. One pygmy bluetongue was found within a spider burrow up to its neck in water and once disturbed it retreated to the bottom of the burrow fully submerged in water, consistent with previous observations (Ebrahimi et al., 2012). The next day, the spider burrow containing the pygmy bluetongue was no longer flooded but other pygmy bluetongues submerged at the bottom of flooded artificial burrows continued to be observed until 10 June. During this time, annual burrow surveys were being conducted where every natural spider burrow (n = 1593) and artificial burrow (n = 519) were checked for occupancy. We hypothesized pygmy bluetongues would preferentially occupy spider burrows due to the greater drainage ability than artificial burrows. We found there was no significant difference that pygmy bluetongues preferentially occupied a spider burrow rather than an artificial burrow within the 10 translocation enclosures (Student's t-test: = −1.233, df = 18, p = 0.233; Figure 1). However, there was a low number of available spider burrows for pygmy bluetongues to occupy, that is suitably deep enough and vacant from other fauna (3.3% naturally occurring burrows were a minimum of 15 cm deep and vacant of >1500 burrows surveyed), so the result could be an artefact of burrow availability rather than preference.
Our recorded durations of pygmy bluetongues underwater within burrows occurred after rainfall had ceased on 14 October when 11.6 mm rain fell in the preceding 24 h, and 24 October when 51.2 mm rain fell in the preceding 24 h. Observations were made by recording a video using a mounted mobile phone (Samsung Galaxy S20 FE) or iPad situated on a rock near the burrow. The area was vacated by the observer for a minimum of 30 min, so the burrow area was undisturbed. One observation was made by KHM sitting near the burrow but out of view of the inhabiting lizard and recording the duration with a stopwatch. Observations began when the pygmy bluetongue was observed fully submerging itself within the burrow and stopped when the nose was observed breaking the water surface. All observations were made on artificial burrows. The artificial burrows were constructed from wood with no areas within the burrow that air could be trapped in pockets (Figure 2).
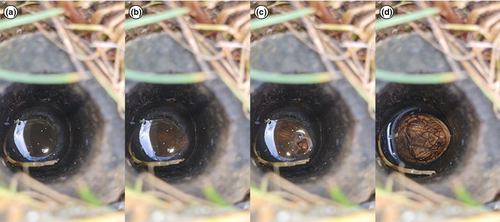
Six observations of submerged duration were made, with two lizards being observed for two consecutive submersions. The mean submersion time was 28:32 min ± 2:43 min. The longest observed submersion was 38:21 min, and one pygmy bluetongue was observed submerged for 35:20 and 24:06 min consecutively with an approximate 3-s period above the water line between these recordings. Bubbles were observed at the water surface in the preceding 30 s before a pygmy bluetongue emerged. Three lizards were also observed to only submerge themselves when disturbed by the observer, in which the lizard would stay submerged for approximately 10 min before emerging their head above the water until disturbed again.
Our observations of lizards not coming up for air in burrows may indicate pygmy bluetongues have adapted to a landscape that experiences heavy rainfall and flooding seasonally, and the lizards can remain submerged for almost 40 min. Due to an increased predation risk associated with finding an alternative dry burrow, and reduced mobility in cooler climates being ectothermic, the lizards may be adapted to tolerate flooded burrows (Ebrahimi et al., 2012). Furthermore, ectotherms in hypoxic environments have been shown to have a lower thermal set-point; therefore, submerged pygmy bluetongues may not emerge from their burrow to thermoregulate (Cadena & Tattersall, 2009; Hicks & Wood, 1985; Wood & Gonzales, 1996). No apparent detrimental effects were observed, such as deceased or stuck lizards; however, a risk remains a lizard may become stuck within the burrow (Ebrahimi et al., 2012) and submersion tolerance of juvenile lizards is unknown.
Although there was no significant difference between the number of pygmy bluetongues occupying a spider burrow that drains more readily than an artificial burrow, these results may be impeded due to the lack of available suitable spider burrows within the restricted area of the enclosures. It is unlikely pygmy bluetongues occurring in natural spider burrow populations would encounter flooded burrows for several days in a row, but given the translocated lizards were founded from wild populations, wild pygmy bluetongue populations would likely share the same submersion tolerance.
Pygmy bluetongues face a predicted extinction without translocation intervention due to climate change (Delean et al., 2013; Fordham et al., 2012). Potential translocation sites may not harbour a high abundance of suitable spider burrows; therefore, supplementing the habitat with artificial burrows is a useful tool as the lizards readily accept them (Milne et al., 2003; Milne & Bull, 2000; Souter et al., 2004). We found in the one to 2 years post-translocation lizards did not preferentially occupy spider burrows that drain more readily and accepted artificial burrows despite rain-induced flooding. Although no apparent detrimental effects on lizards were observed within flooded artificial burrows, it may provide benefit to construct and install artificial burrows that drain more readily, such as drilling holes into the side of the wooden dowel and investigate natural burrow draining speed. Further studies on submersion tolerance of other reptiles that utilize arthropod burrows, such as the grassland earless dragon (Tympanocryptis spp.), would shed further light on apnoea as an adaptation in seasonally flooded habitats that may experience more frequent heavy rainfall events in the face of climate change (Debortoli et al., 2017), and the potential detrimental effects on endangered populations.
AUTHOR CONTRIBUTIONS
Kimberley Michael: Conceptualization (equal); data curation (lead); formal analysis (lead); investigation (lead); writing – original draft (lead); writing – review and editing (equal). Michael Gardner: Conceptualization (equal); supervision (lead); writing – review and editing (equal).
ACKNOWLEDGEMENTS
We thank the Koch family for allowing access to the study site. We are grateful to volunteers for their assistance in the field. This research was supported by the Australian Government RTP Scholarship to KHM and Australian Research Council to MGG. This research was conducted under the Flinders Animal Welfare Committee (AEC BIOL5017) and South Australian Department for Environment and Water Permit (G25011). Open access publishing facilitated by Flinders University, as part of the Wiley - Flinders University agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
This study was supported by the Australian Research Council Linkage grant (LP190100071) and Australian Government Research Training Program Scholarship.
CONFLICT OF INTEREST STATEMENT
The authors declare there are no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data available on request from the authors.




