Effects of habitat, season and flood on corvid scavenging dynamics in Central Australia
Abstract
Carrion is an ubiquitous resource that drives the dynamics of scavenger populations and shapes the structure and composition of their communities. Corvids (Family: Corvidae) are among the most common scavengers globally, facilitating carcass discovery by other species and contributing to carcass biomass removal. Here, we examine how environmental factors influence corvid scavenging dynamics in an arid region of Central Australia. Specifically, we investigate how habitat, season and a major flood event affect corvid discovery and visitation time, and group size around carcass sites. To do so, we used data collected from camera traps monitoring 80 experimentally positioned red kangaroo (Osphranter rufus) carcasses stratified across closed and open habitats, spring and winter seasons, and before and after a flood event. Corvids took longer to discover carcasses in closed compared to open habitats, but habitat did not affect how long corvids spent visiting carcasses, or corvid group size. Corvids discovered carcasses faster in winter compared to spring and in post-flood compared with the pre-flood periods, and season and flood interacted to influence both corvid visitation time and group size. Our results reflect a complex interplay between temperature, the extreme boom and bust cyclicity of the study region and changing corvid activity and abundance in the local study area. We identify environmental factors as key determinants of corvid scavenging dynamics and provide the first detailed description of scavenging by corvids in the arid zone of Australia.
INTRODUCTION
Carrion is a nutrient-rich resource that is exploited by multiple trophic groups within food webs (DeVault et al. 2011; Barton et al. 2013). In terrestrial environments, carcasses enter the landscape in a variety of ways, such as via animal mortality due to extreme weather, vehicular collision (i.e. roadkill) and lethal pest control (McDowell et al. 2017; Englefield et al. 2018). The dynamics of carcass use by scavengers are affected by many factors including habitat (Pardo-Barquín et al. 2019; Selva et al. 2005; Smith et al. 2017), season (O'Brien et al. 2010; Peers et al. 2020; Read and Wilson 2004), temperature (DeVault et al. 2004; Turner et al. 2017) and scavenger community composition (Cunningham et al. 2018; Sebastián-González et al. 2020). However, there is little consensus on the relative strength or influence of these factors on scavenging dynamics (Sebastián-González et al. 2019).
Birds are among the most common vertebrate consumers of carcasses. Vultures (Families Accipitridae and Cathartidae) dominate carcass use in many parts of the world as the only vertebrate obligate scavengers, and by removing carcass biomass may reduce the risk of disease transmission (Donázar et al. 2009; Markandya et al. 2008; Ogada et al. 2012; Sebastián-González et al. 2019). Corvids (Family Corvidae) are also frequent scavengers, although as omnivorous predators they are more flexible in their dietary habits (Heinrich 1988; O'Brien et al. 2010; Selva et al. 2003; Cardilini et al. 2015). Corvids can locate carcasses quickly, and their flight allows them to patrol large areas (Dermody et al. 2011; Pennycuick 2008; Whisson et al. 2015). They are often the first to arrive at carcasses, where vultures are not present, and they may use the resource for extended periods (Gomo et al. 2017; Heinrich 1994; Stahler et al. 2002; Lafferty et al. 2016; Read and Wilson 2004). As some corvid species can also form large groups to scavenge (Heinrich 1988; Selva et al. 2005; Rowley 1973a) they could contribute substantially to carcass removal. Further, the presence and vocalizations of corvids may be used by other larger species such as grey wolves (Canis lupus) to locate carcasses and in turn could hasten carcass depletion (Gomo et al. 2017; Heinrich and Marzluff 1991; Lafferty et al. 2016).
Spatial and temporal patterns of carcass use by corvids are strongly affected by factors such as habitat and season (Heinrich 1988; O'Brien et al. 2010; Read and Wilson 2004), although these patterns also likely differ between ecosystems. For example, in coastal Australia corvids do not frequent carrion on beaches (Brown et al. 2015; Schlacher et al. 2013), whereas in peri-urban and rural environments Australian corvids are among the most numerous scavengers (O'Brien et al. 2010; Read and Wilson 2004; Rowley and Vestjens 1973; Peisley et al. 2017).
Carcasses in open habitats are usually found more quickly by avian scavengers than those in closed habitats, such as dense forest (Carrete et al. 2010; Kirk and Houston 1994; Selva et al. 2005; Peisley et al. 2017). This may be due to avian scavengers relying primarily on their sight to locate carcasses, which could be less effective in dense or enclosed vegetation. However, there is evidence that certain avian scavengers, particularly turkey vultures (Cathartes aura), use scent to locate carcasses (Grigg et al. 2017; Potier et al. 2019). Corvids are known to use visual cues to locate food resources, for example, little ravens (C. mellori) are more effective at locating little penguin (Eudyptula minor) nests when the more visually conspicuous males are incubating the eggs (Ekanayake, Weston, et al. 2015a). Corvids are also known to respond to olfactory cues, with carrion crows (Corvus corone) avoiding areas marked with the scent of stressed conspecifics (Wascher et al. 2015). The importance of olfaction for corvids in locating food resources, and specifically carcasses, requires further attention as it is still unknown. Developing understanding on the patterns of carcass discovery by corvids across habitats may help to determine the relative importance of different senses in locating food resources. But there may also be differences in predation risk between habitats, which is likely to affect how corvids, as meso-scavengers, use carcasses. For example, corvids preferentially scavenge in habitat they perceive as low risk (Laundré et al. 2010; Mella et al. 2014). Aside from impacting carcass discovery and use, habitat can also influence the group size of scavenging corvids. For example, in the Bialowieza forest in Poland, territorial pairs of common ravens (C. corax) visited more carcasses in forested habitat whereas flocks appeared more frequently at carcasses in open clearings (Selva et al. 2005).
Season commonly affects the dynamics of scavenging animals (Bonnet et al. 1998; Read and Wilson 2004) and may also influence carcass use by corvids. For example, Australian ravens (C. coronoides) scavenge most intensively during their breeding season in the temperate semi-urban parts of their range (O'Brien et al. 2010), resulting in faster discovery of carcasses. Seasonal exploitation of resources has also been shown with little ravens flocking to little penguin breeding areas during breeding seasons (Ekanayake et al. 2016). Breeding corvids are likely to be in pairs rather than flocks, thus reducing average group size in the spring breeding season (Rowley et al. 1973). Season can also affect how long a carcass is used by scavengers; in extreme environments animals can be limited by temperature, or water availability (O'Brien et al. 2010; Peers et al. 2020; Turner et al. 2017; Schleucher 1993). In the desert large flocks of corvids are highly transient (Rowley 1973a) and may move out of these systems to avoid the high temperatures of the spring and summer, leading to a reduction in density in these seasons. Insect and microbial activity are also enhanced in warmer temperatures, often increasing the speed of putrefaction and reducing carcass persistence times (Farwig et al. 2014; Payne 1965; Putman 1978; Blandford et al. 2019). This may in turn enable microbes and insects to outcompete vertebrate scavengers (Devault et al. 2004) and could result in reduced corvid scavenging activity in warm periods. However, corvids are also likely to depredate insects that associate with animal carcasses (e.g. fly larvae) (Moreno-Opo and Margalida 2013) and so could alternatively be attracted to carcasses in warmer seasons when insects are more active.
In this study, we explore patterns of corvid scavenging in the Simpson Desert, Australia. The Simpson Desert is host to three corvid species, namely the Australian raven, the little crow (C. bennetti) and the Torresian crow (C. orru) (Campbell et al. 2015). These species are among the most abundant, but least studied, animals in the desert environment. Understanding how they use carcasses is therefore crucial for elucidating the role of carcasses in desert food webs. Carrion use by corvids likely varies among habitats which, in the Simpson Desert, comprise open sparsely vegetated dunes and closed but spatially limited stands of trees in the dune valleys (Greenville and Dickman 2009; Shephard 1992). The Simpson Desert also experiences large seasonal fluctuations in temperature (Purdie 1984), which likely affects the temporal use of carcasses by corvids.
The Simpson Desert is typically water-limited, but also experiences sporadic flooding rainfall events that lead to booms in primary (plant) productivity (Morton et al. 2011). Productivity booms have bottom-up effects on the whole ecosystem, leading to irruptions of small mammals and then movement of larger predators into the system (Letnic and Dickman 2006; Pavey et al. 2008; Poiani 2006). These rain events may therefore drive significant increases in scavenging activities, especially if scavenging animals that move into the area exploit the temporary pulses of high productivity (Cunningham et al. 2018; Huijbers et al. 2015; Morales-Reyes et al. 2017; Ogada et al. 2012). However, it is also possible that prior to the arrival of flood events, when fewer resources are available, corvid scavenging may be most intense; scavenging at high intensity during periods of low resource availability has been observed in other animals such as dingoes (Canis dingo) (Allen 2010; Doherty et al. 2019).
The distinct habitats, variable seasons and the extreme boom and bust cycle in the Simpson Desert allowed us to investigate how these factors influence carcass exploitation by corvids. We hypothesized that different habitats, seasonality and a flooding event would affect the discovery time, total visitation time and group size of corvids at carcasses. We predicted that: (1) carcasses would be found faster by corvids in open compared to closed habitats, in winter compared to spring and before flooding; (2) corvids would spend more time at carcasses in open than in closed habitats, in winter compared to spring and before flooding; and (3) carcasses would be exploited by larger groups of corvids in open than in closed habitats, in winter compared to spring, and after flooding.
METHODS
Study site
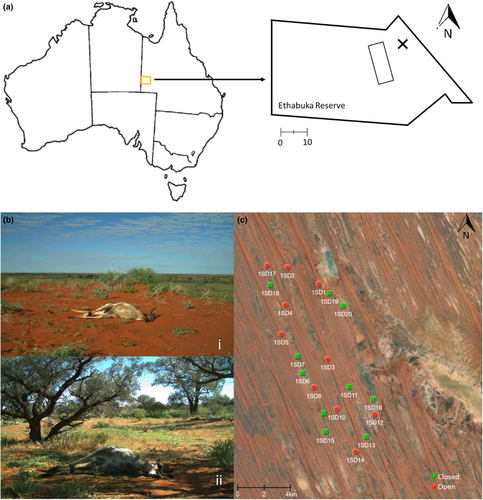
The study was conducted between June 2018 and September 2019 at Ethabuka Reserve (23°46′S, 138°28′E) on the edge of the Simpson Desert, Queensland, Australia (Fig. 1a). Ethabuka is a former cattle station that was converted to a conservation reserve by Bush Heritage Australia in 2004. The reserve is 2155 km2 and has been subject to ecological monitoring since 1990. The Simpson Desert covers 170 000 km2, with most of the region characterized by long, parallel sand dunes that run northwest to southeast (Greenville et al. 2016; Greenville and Dickman 2009; Shephard ). The dunes are 0.6–1 km apart and can reach elevations of 8–10 m (Dickman et al. 2010; Kwok et al. 2016; Purdie 1984). Spinifex (Triodia basedowii) grassland dominates the region, with stands of gidgee trees (Acacia georginae), mallee eucalypts, and other Acacia shrubs distributed on the heaver clay soils of interdunal swales (Wardle et al. 2015). Vegetation is usually sparse on the dune crests, with a patchy cover of ephemeral plants including Goodenia cycloptera, Euphorbia drummondii, Grevillea stenobotrya, Sida spp. and Acacia spp. (Greenville et al. 2009; Kwok et al. 2016). The two habitats of open dune crests and enclosed stands of gidgee trees provide different habitat structures and are likely to affect how carcasses are used by scavengers (Fig. 1b) (Morton et al. 2011; Pavey and Nano 2009; Turner et al. 2017).
Temperatures during summer in the study site exceed 40°C and fall below 0°C in winter (Appendix S1) (Purdie 1984). Over the study period, the temperature difference between winter (average = 13°C) and spring (average = 27.6°C) provided a climatic contrast to assess seasonal effects on carcass use by corvids.
Annual rainfall on the study site is between 100 and 150 mm and is summer dominant (Purdie 1984), but large rainfall events can occur at any time. One such event occurred in March 2019, when there was 149 mm of rainfall in 1 day, leading to widespread flooding across the region (Appendix S1). This event provided an opportunity to assess corvid scavenging dynamics before and after the flood.
Carcass drops
Four trips were conducted to the study site over 2 years, in June and October 2018, and in June and September 2019. Thus, two trips were conducted during spring (October and September) and two during winter (June). Similarly, as the flood event occurred in March 2019, two trips (in 2018) occurred before the flood event and two trips (in 2019) occurred post-flood. The flood was widespread throughout the study area and it was not possible to have ‘control’ sites where there was no effect from the flooding, however important conclusions about the effect of the flood can still be made by comparisons of the pre- and post-flood periods. During each trip, 20 unique carcass monitoring sites were established, with 10 in open habitat (defined as having no trees present within 50 m of the carcass), and 10 in closed habitat (defined as having canopy over the carcass with more than 20% projective cover). Attempts were made to ensure that monitoring sites in closed habitats were at least 50 m away from open habitat, but due to the scarcity of trees in the interdune swales this was not always possible. The monitoring sites were spaced at least 1 km apart to reduce overlap of odours from each carcass (Fig. 1C) (Forsyth et al. 2014; Peisley et al. 2017; Turner et al. 2017). Given the large home range of corvids (Ekanayake et al. 2018; Whisson et al. 2015; Rowley 1973a) it is probable that the same birds visited multiple carcasses, the distance between carcasses was to ensure the independence of visitations. Human activity was very limited and consistent at all sites, and all sites were located within 100–500 m of a private unsealed vehicle track that had very low traffic.
A single dead adult red kangaroo (Osphranter rufus) weighing 20–36 kg (mean ± SD = 25.0 ± 3.5 kg) was used at each unique carcass monitoring site. The carcasses were acquired from a commercial kangaroo harvester working on nearby agricultural properties and were placed at monitoring sites generally within 24 h of death. The exception was June 2018, when the carcasses were set out over a period of 48 h. Red kangaroos are the largest native Australian land mammal (weighing up to 90 kg) and occur across arid Australia. In many areas kangaroos are managed through commercial and non-commercial culling to control their numbers, with the latter potentially leading to the deposition of large numbers of carcasses in the landscape (Lunney et al. 2018; Wilson and Edwards 2019).
A metal stake was hammered into the ground 3 m away from each carcass and a Reconyx PC800 Hyperfire camera trap (Professional Reconyx Inc., Holmen, WI, USA) was secured to the stake 1 m above the ground (Peisley et al. 2017). These cameras take photos by day and night using infrared flash triggered by a heat and movement signature (rapid fire, with 10 consecutive triggers and no period) and then store information in each image (EXIF data; temperature, date and time). To secure each carcass and prevent animals taking it out of range of the camera, two 0.45 m metal stakes were hammered into the ground on either side of each carcass, and a wire was passed through the stakes and the Achilles tendon and around the neck of the kangaroo. The carcasses and cameras were monitored for 30 days, ensuring an equal sampling effort across all carcasses, and encompassing peak corvid activity (Fig. 2).
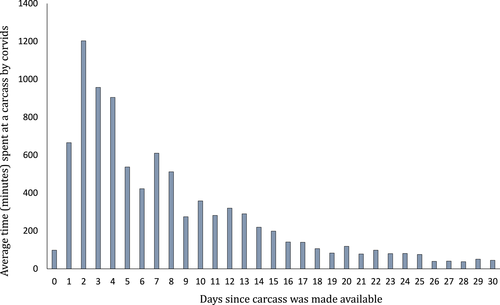
Photo analysis
The photos were downloaded from SD cards in the field and the data processed and tagged at a later date using the program Digikam (Version 6.4.0). This program alters the EXIF data stored within each image using tags that reflect information on the content of each photo. We first tagged and sorted photos according to the identity of all species present. Due to the similarities in appearance of Australian ravens, little crows and Torresian crows, it is very difficult to confidently identify corvids to species level using only photographs (these species are best distinguished by their calls) (Campbell et al. 2015). We therefore grouped these species as ‘Corvus spp.’ in our tagging, whilst acknowledging that different species may have varied scavenging behaviours.
Photos where corvids were present were tagged with the maximum number of individuals observed across carcass visitation ‘events’. An event started when a corvid first appeared in a photo and ended when corvids were absent from photos for more than 10 minutes (O'Brien et al. 2010). This approach allowed us to account for animals that may have been near the carcass but not in frame and to ensure independence between events. We chose 10-minute time intervals as most photos that included corvids were generally separated by approximately 5 min or less (images likely representing the same animal moving in and out of frame) or greater than 30 min (Appendix S2). Only corvids that were in the immediate vicinity of the carcass were recorded. In some cases, corvids that were at the carcass could be seen flying away, and these images were also recorded as having an animal present. There were instances when birds flew past the carcass in the distant background; these animals were not recorded. We also assessed whether corvids were scavenging on the carcasses for 65 out of 80 sites, with data included from all four carcass drops. A corvid was recorded as scavenging if its head was seen in contact with the carcass, or the area around the carcass, or if carrion or other food was visible in its beak (Appendix S3).
Statistical analysis
To assess corvid scavenging patterns we explored three different metrics including: discovery time, visitation time and group size. Discovery time was the time (to the nearest minute) between when a carcass was first positioned in the field, and when the first corvid appeared but did not necessarily feed on that carcass. Visitation time was the total amount of time in minutes (to the nearest minute) that corvids spent at a carcass site over the 30-day study period. Visitation time was the additive time of each visitation event by corvids. Group size was the maximum number of corvids present at a carcass across each event. Group size was not averaged across each carcass site (as visitation time was), rather it was determined and analysed for each separate event. Before conducting analyses on each metric, spatial autocorrelation was assessed by calculating Global Moran's Index in ArcGIS Pro 2.6.0 (Scott and Janikas 2010) to ensure independence between carcass sites for each trip (Appendix S4). All data were analysed using the R statistics package, Version 4.0.2 (R Core Team 2020).
Discovery time
Corvids are intelligent birds and it is possible that they can learn to follow researchers in their cars, thus resulting in decreasing discovery times over the course of the study (Ekanayake, Whisson, et al. 2015b; Emery and Clayton 2004). We therefore assessed whether discovery time decreased with each subsequent carcass deployment, to test for the possibility of corvids learning to follow researchers in their cars (Appendix S5). Corvids are also diurnal scavengers, and it is therefore possible that carcasses deployed later in the evening or at night would have had longer discovery times, as corvids may not have been active until the next day (Brown et al. 2015; Ekanayake, Weston, et al. 2015a). To ensure this was not the case we tested if carcass discovery time was correlated with the time of day the carcass was deployed (Appendix S6). There was no significant effect of either the sequence of carcass deployment (t = −1.743, P = 0.085) or time of day deployed (t = 0.429, P = 0.795), so it is likely that any effects on discovery time are due to the tested factors rather than human presence and the carcass deployment method. To test whether the time it took for corvids to discover carcasses varied with habitat (closed, open), season (winter, spring), or flood (before, after), we fitted generalized linear models (GLMs) using combinations of the factors to address multiple hypotheses (Appendix S7). We only considered carcasses discovered by corvids (n = 79). A dispersion test on the global model fitted with Poisson distribution indicated overdispersion (φ (dispersion parameter) = 3351.1; Hilbe 2011), and so we used quasi-Poisson GLMs where the variance is given by φ × μ, where μ is the mean and φ the dispersion parameter (Hoef and Boveng 2007; Wedderburn 1974). We ranked our models using Quasi Akaike Information Criteria, correcting for small sample size (QAICc), and selected the best model by comparing QAICc differences (Δi). Models with Δi < 2 units relative to the model with the lowest QAIC were considered (Burnham and Anderson 2002). We then visually assessed the best supported model's predicted values against their residual values to confirm the residuals were randomly dispersed (Zuur et al. 2009).
Visitation time
To test whether habitat (closed and open), season (winter and spring) and the flood (before and after) affected the total visitation time of corvids at carcasses we first fitted a global GLM. Again, due to overdispersion (φ = 294) we used quasi-Poisson GLMs and we fitted multiple models to explore different hypotheses on the effects of habitat, season and flood on visitation time (Appendix S8). These models were ranked using Quasi Akaike Information Criteria (QAICc) and the best model was selected by comparing QAICc differences (Δi). We then visually assessed the best supported model's predicted values against their residual values to confirm the residuals were randomly dispersed (Zuur et al., 2009).
The best supported model contained interacting terms so we also calculated the estimated marginal means of each group using the package ‘emmeans’ (Lenth et al. 2020). Contrasts between groups were calculated to ascertain the influence of the different factors on visitation time. The P-values for these tests were subjected to sequential Bonferroni adjustment (Abdi 2010).
Group size
To analyse the effect of habitat (closed and open), season (winter and spring) and the flood (before and after) on the group size of corvids we fitted GLMMs with Poisson distribution. We also included site as a random factor to account for repeated measures of group sizes across multiple events at single carcasses. Each model tested a specific hypothesis and was ranked using Akaike Information Criteria (AIC) (Appendix S9) and the best model (Δi < 2) was selected.
Again, we visually assessed the best model's predicted values against its residual values to confirm the residuals were randomly dispersed against the predicted. As the best supported model contained an interaction term, we estimated the marginal means and contrasts between these means under each treatment (adjusting P-values for these significance tests using sequential Bonferroni correction) to determine the effect of each treatment group on the average group size of corvids.
RESULTS
Successful camera runs were obtained on all 80 kangaroo carcasses, yielding 2400 camera trap days. Corvids were the most numerous scavengers recorded with 725 663 images of corvids analysed, comprising 6135 independent carcass visitation events. A subset of these events (2422) were analysed for corvid scavenging, with scavenging occurring in 87% (2082) of the events assessed (Appendix S3). We found no spatial autocorrelation between sites in terms of discovery time, visitation time or corvid group size (Appendix S4). The activity of larger scavengers was significantly lower, with dingoes visiting carcasses only 204 times over the study period, wedge-tailed eagles (Aquila audax) 610 times, red foxes (Vulpes vulpes) 523 times and feral cats (Felis catus) 63 times. Compared with other scavengers, corvids were the first species to arrive in 68% of cases.
Discovery time
Corvids discovered 79 of the 80 carcasses during the 30-day monitoring period and arrived before any other vertebrate species at 54 of the 80 carcasses (68%). It took corvids on average (±SE) 58 ± 11 h to discover the carcasses, with the fastest discovery time 15 min and the longest 29 days and 19 h.
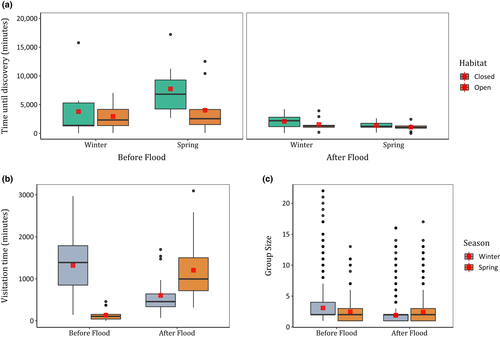
Only one model of carcass discovery time was competitive (Δi < 2). This model included habitat, season and flood (Appendix S7), and showed that all three factors significantly affected how long corvids took to discover carcasses (Table 1). Corvids took 1.9 times longer to discover carcasses in closed compared with open habitats, 1.7 times longer to discover carcasses in spring than in winter and 3.6 times longer to discover carcasses before compared with after the flood (Fig. 3a).
| Variables | Estimate | SE | t-Value | P |
|---|---|---|---|---|
| Intercept | 8.591 | 0.217 | 39.668 | <0.001 |
| Habitat | −0.663 | 0.246 | −2.701 | 0.0085 |
| Season | 0.543 | 0.242 | 2.247 | 0.028 |
| Flood | −1.293 | 0.282 | −4.590 | <0.001 |
- Bold P values indicate significance at α = 0.05.
Visitation time
Overall, corvids spent an average (±SE) of 816 ± 82 min at each carcass over the 30-day study period. They engaged in scavenging behaviour at every carcass they visited, with only one carcass remaining undiscovered throughout the study period. Only one model of carcass visitation time was competitive (Δi < 2). This contained the main effects of season and flood, as well as their interaction (Appendix S8) and showed that each factor, and the interaction, significantly affected the total amount of time corvids spent visiting carcasses (Table 2). Before the flood, corvids spent 9.7-fold more time at carcasses in winter compared with spring (Fig. 3b; Table 3). However, this pattern was reversed after the flood, with corvids spending twice the amount of time at carcasses in spring compared to winter (Fig. 3b; Table 3).
| Variables | Estimate | SE | t-value | P |
|---|---|---|---|---|
| Intercept | 7.184 | 0.112 | 64.044 | <0.001 |
| Season | −2.276 | −0.368 | −6.192 | <0.001 |
| Flood | −0.778 | 0.200 | −3.889 | <0.001 |
| Season X Flood | 2.963 | 0.420 | 7.057 | <0.001 |
- Bold P values indicate significance at α = 0.05.
| Variable | Contrast | Estimate | SE | z-ratio | P |
|---|---|---|---|---|---|
| Before flood | Winter–Spring | 2.276 | 0.368 | 6.192 | <0.001 |
| After flood | Winter–Spring | −0.687 | 0.203 | −3.386 | 0.0028 |
| Winter | Before flood–After flood | 0.778 | 0.200 | 3.889 | <0.001 |
| Spring | Before flood–After flood | −2.185 | 0.369 | −5.919 | <0.001 |
- Bold P values indicate significance at α = 0.05.
Corvids also spent 2.2-fold more time at carcasses in the winter before the flood than after the flood (Fig. 3b; Table 3). This pattern was different for spring, with 8.9-fold more time spent scavenging by corvids after compared with before the flood (Fig. 3b; Table 3).
Group size
Corvid group size at carcasses ranged from one to 22 individuals. The mean group size (±SE) across sites was 2.5 ± 0.03. The best (Δi < 2) supported model for corvid group size contained season and flood and their interaction (Appendix S9). Both factors and their interactions were significant (Table 4). Before the flood, corvid groups were 1.3 times larger in winter than in spring, but after the flood, group size was 1.2 times larger in spring compared to winter (Fig. 3C; Table 5). Groups were 1.6 times larger in the winter before the flood than in the winter post-flood (Fig. 3C; Table 5). There was no significant change in group size in spring before and after the flood (Fig. 3C; Table 5).
| Variables | Estimate | SE | z-value | P |
|---|---|---|---|---|
| Random effects | ||||
| Site | 0.046 | 0.214 | ||
| Fixed effects | ||||
| Intercept | 1.104 | 0.050 | 22.099 | <0.001 |
| Season | −0.246 | 0.078 | −3.164 | 0.002 |
| Flood | −0.465 | 0.071 | −6.510 | <0.001 |
| Season X flood | 0.443 | 0.106 | 4.173 | <0.001 |
- Bold P values indicate significance at α = 0.05.
| Variable | Contrast | Estimate | SE | z-ratio | P |
|---|---|---|---|---|---|
| Before flood | Winter–Spring | 0.248 | 0.078 | 3.164 | 0.006 |
| After flood | Winter–Spring | −0.195 | 0.072 | −2.723 | 0.026 |
| Winter | Before flood–After flood | 0.465 | 0.071 | 6.510 | <0.001 |
| Spring | Before flood–After flood | 0.022 | 0.079 | 0.276 | 1.000 |
- Bold P values indicate significance at α = 0.05.
DISCUSSION
This study shows that corvids are common carcass visitors and scavengers in the Simpson Desert. Corvids successfully located all but one experimentally positioned carcass and were the first species to discover most carcasses (68% of cases). They usually scavenged (Appendix S3) when they visited carcasses, although they may have additionally been depredating on the insects (e.g. fly larvae) that associate with the resource (Moreno-Opo and Margalida 2013). Corvids were also observed removing fur from the kangaroo carcasses. This suggests that they could use carcasses for nesting material here, as they have been observed doing in other systems (Margalida and Bertean 2000, Riney 1951). Our results further highlighted variability in carcass use by corvid species across different habitats, seasons and following a major flood event. Supporting our first prediction, carcass discovery was faster in open compared to closed habitats. Faster discovery of carcasses by corvids was also influenced by season and the flood event. In contrast, our second prediction received only partial support; while corvid visitation did vary across seasons, the birds only spent longer visiting carcasses in winter compared to spring before the flood. In the post-flood period, the opposite was observed, with longer visitation by corvids occurring in spring compared to winter. There was also mixed support for our third prediction, with corvids visiting carcasses in larger groups in winter before the flood compared to after the flood. On the other hand, there were no significant changes in group size in spring before and after the flood. We expand upon our findings below and explore the potential processes driving patterns in discovery time, visitation time and group size of corvids at carcasses in arid environments.
Carcass detection by corvids
Corvids took longer to discover carcasses in closed compared with open habitat. Similar findings have been reported in other systems, with increased habitat complexity or canopy cover generally equating to longer carcass discovery times by scavengers (e.g. Turner et al. 2017; Peisley et al. 2017; Smith et al. 2017). In contrast to these studies, however, which used temperate or boreal forests as their ‘closed’ habitats, the gidgee trees in our ‘closed’ habitat were sparse and typically provided low canopy cover (sometimes only 20% canopy cover above carcasses). This suggests that habitat complexity, or the extent of canopy cover, may still be important in influencing carcass discovery time by avian scavengers, even when the ‘closed’ habitat is relatively open, and sparsely vegetated.
There is also evidence that some avian scavengers use smell to locate carcasses (Potier et al. 2019; Grigg et al. 2017). The importance of olfaction for carcass discovery by the corvids studied here is unknown, but closely related little ravens seem to rely heavily on visual, rather than olfactory cues, to locate food (Ekanayake, Weston, et al. 2015a). The results herein indicate that sight may be the primary method used by corvids to locate food in this system, given they are faster at locating carcasses in open habitat. However, corvids might also prefer to forage in open habitat in these systems, as they may perceive these areas to have reduced predation risk or increased food availability (Wirsing et al. 2010; Peers et al. 2020; Whisson et al. 2015). Studies that tease apart how predation risk, habitat preference and carcass detection methods (e.g. scent vs. visual detection) influence carcass discovery by corvids should be a focus of future research.
Carcass discovery times by corvids in our study were also influenced by season and the flood event. Corvids may have taken longer to discover carcasses in spring compared to winter because in the hotter months high temperatures may limit their ability to disperse great distances in search of spatially patchy resources such as animal carcasses (Schleucher 1993). In other areas of Australia, ravens scavenge more intensely in spring than any other season due to increased energetic requirements during breeding (O'Brien et al. 2010). However, in arid Australia the breeding season of corvids is influenced more by rainfall than by seasonality (Rowley et al. 1973). The shorter (3.6-fold) carcass discovery time post-flood was probably due to an increase in corvid abundance during this time. Indeed, scavenger density is an important determinant of carrion discovery and use (Gil et al. 2018; Huijbers et al. 2015; Morales-Reyes et al. 2017). We did not specifically measure corvid abundance, but flooding events in arid Australia typically lead to rapid increases in primary productivity and food resources (e.g. seeds and small mammals). These events attract a range of species into the area, including corvids, which exploit this boom in resources (Pavey and Nano 2009; Poiani 2006).
Corvids were the first vertebrate species to locate the majority of carcasses. While travelling around our field site, we also often spotted corvids circling carcasses in the air, even when we were at distances of up to several kilometres away from the carcass site. Based on these observations, we feel that corvid activity at carcass sites would have probably recruited additional vertebrate scavengers to the carcasses, as seen in other systems (Heinrich and Marzluff 1991; Gomo et al. 2017). Investigating faciliatory relationships between corvids and other scavengers was outside the scope of this study, however such research would help to provide evidence to confirm our theory that other animals use corvids to find carcasses in this system. It would also help to demonstrate the importance of corvids in shaping scavenging food webs, especially if they attract larger scavengers (e.g. dingoes or eagles) that rapidly consume carcass biomass or open carcasses for other species to gain access (Rowley 1973a; Peisley et al. 2017).
Carcass visitation time by corvids
Carcass visitation by corvids in our study was predominantly affected by an interaction between season and flood, with no support for habitat influencing the time corvids spent at carcasses. One potential driver of differences in foraging time between habitats is varied predation risk (Brown 1988; Latty and Beekman 2010; Whittingham and Evans 2004). The lack of differentiation between open and closed habitat use by corvids suggests that they could have perceived predation risk as being similar in both open and closed habitats in the Simpson Desert. It is possible that although corvids may be able to escape from potential predators more easily in open habitat, the gidgee trees in the closed habitat offer perches that improve the effectiveness of vigilance and act as refuges from terrestrial predators (Dear et al. 2015). Actual visitations to carcass sites for larger scavengers that may be perceived as a threat to corvids (e.g. dingoes and eagles), were however, relatively low and variable throughout the study. For example, dingoes did not visit any carcasses in the second carcass drop (Spencer and Newsome 2021) and most of the time (79%) eagles spent at carcass occurred during the first carcass drop. So, the overall levels of predation risk at carcass sites may have been low irrespective of habitat type. Further investigations into the vigilance behaviours and direct predation risk experienced by corvids would help to improve our knowledge of habitat selection by corvids.
Before the flood event, corvids probably spent more time at carcasses in winter compared to spring due to increased competition with insects and microbes. Warmer weather increases insect and microbial activity, which accelerates carcass biomass loss and reduces the time that carcasses are available to vertebrate scavengers (Putman 1978; Farwig et al. 2014; Forsyth et al. 2014). Corvid activity was also potentially limited by extremely high temperatures in October 2018 (Appendix S1). Compared to the cooler September 2019 spring study period, corvid activity was much reduced, probably due to the marked difference in temperature between these two spring study seasons.
The flood also likely resulted in an increase in the number of corvids in the area (Pavey and Nano 2013; Poiani 2006), which could have elevated the time they spent scavenging on carcasses (Gil et al. 2018; Huijbers et al. 2015; Morales-Reyes et al. 2017). This may not, however, have affected scavenging in the winter (post-flood) due to a lag effect in the flood. Indeed, it can take months for plants and animals to respond to large rainfall events (Dickman et al. 2010; Letnic et al. 2005). Quantifying the responses of plants and small mammals in association with corvid dietary studies before and after a flood may help to determine the effect of flooding on food choices by corvids, including relative rates of scavenging versus predation. A further possibility is that access to free water reduced potential water loss associated with prolonged visits at carcasses; corvids in the study area have been shown to forage for longer on dry food when water is available than when it is not (Kotler et al. 1998). Exploring the response of corvid scavenging dynamics to flood events that occur at different times within the seasonal cycle could also help to improve understanding of the general effects of floods.
Corvid group size at carcasses
Habitat did not appear to influence corvid group sizes at carcass sites in our study. This is contrary to previous work, such as the carcass surveys carried out in the Bialowieza forest, Poland (Selva et al. 2005). As noted above, this may be due to the sparser canopy cover and foliage density of closed habitat in the Simpson Desert compared to the temperate and boreal Bialowieza forest (Purdie 1984; Selva et al. 2003). Additionally, the trend described in the Bialowieza forest was associated with variable foraging techniques used by different life stages of ravens, with juveniles forming large transient flocks that foraged over great distances and adult pairs concentrating foraging within smaller territories. On the other hand, little crows (probably the most abundant corvid in the Simpson Desert), form pairs for only a short time during the breeding season (Rowley et al. 1973), which may explain the lack of support for habitat effects on group size in our system.
Like carcass visitation times by corvids, there were significant and interactive effects of both flood and season on corvid group size (Table 5). Group size was greater in the winter before the flood compared to the winter after the flood, possibly due to an increase in breeding in response to the rainfall (Rowley et al. 1973). The group forming behaviours of corvid species differ in the Simpson Desert; Little Crows and Torresian Crows normally form large transient groups whereas Australian Ravens usually form territorial pairs (Rowley 1973a). It is therefore possible that group size is limited due to social factors related to the specific corvid species. The differences in group size before and after the flood could also reflect a flood-driven boom in resource availability which may have led corvids to exploit food resources other than carcasses (Letnic et al. 2005; Morton et al. 2011). In the spring after the flood these resources were likely no longer available, explaining the consistent group size of corvids in the spring before and after the flood. These results corroborate the possible effects of flood on visitation time and provide further evidence of reduced scavenging immediately after the flood. However, these results do not support a lag in flood effects, as previously proposed, as there was no difference in the group size of corvids between the spring before the flood and the spring after the flood.
Although the effects of season and flood on group size were significant, they may have limited biological meaning. The effect sizes were related to changes in group size of 1.2–1.6, representing small changes considering that average group size was 2.5. A change in group size of two to three corvids is likely to have little effect on the consumption of carrion. The large sample size (6137 events), which may contribute to the statistically significant results (Quinn and Keough 2002), also leads to the conclusion that season had little meaningful biological effect on corvid group size around carcasses. But despite these considerations corvid group size did vary and thus other unmeasured factors such as species identity may have influenced this. As mentioned previously, Australian ravens primarily form territorial pairs whereas little crows and Torresian crows are more transient birds that often form large flocks (Rowley 1973a). It is also likely that flocks of mixed species occur, especially around carrion as it is likely an important resource for all species of corvid in the desert. The scavenging behaviour of these species may be different and could further drive the variation in group size. The transient nature of corvids (primarily little crows and Torresian crows) in arid Australia also means that high population turnover is likely.
There is limited data on the relative abundance of corvid species in this area. Australian ravens and little crows have been observed in similar abundances at monitoring stations in the area, however, Torresian crows are only rarely observed (A. Tulloch, unpublished data, 2019). There may also be differences in the abundance of these species following rain (particularly C. coronoides), however, further monitoring is necessary to determine any trends in their abundance. In future, using videos of corvids at carcasses to identify them to species level or concurrently measuring their abundance will help to resolve species-specific effects on the scavenging dynamics of these corvids.
Finally, the study design of 20 carcasses set in reasonably close proximity (1 km) to each other also may have presented a relatively high carrion load in relation to likely background rates of carrion. This could have allowed larger groups to split up and exploit carrion across the study area. Further investigations into the population dynamics of corvids in relation to different carcass loads would help to further determine the influence of carcasses on corvid group sizes.
Conclusions and study implications
Our study provides the first detailed insight into the use of carrion in arid Australia by corvids and demonstrates that corvids are important scavengers in this desert ecosystem. Our work also established the effects that different factors can have on corvid scavenging patterns. Habitat affected the discovery time of carcasses by corvids, corroborating other studies (Selva et al. 2003; Turner et al. 2017). The lack of habitat effect on corvid visitation time and group size, however, is likely due to the less distinct habitat types monitored here compared with other studies (Houston 1974; Peers et al. 2020; Turner et al. 2017). The interaction between season and flood, and the factors they represent, also influenced corvid scavenging in our study. The most likely explanation for these results lies in the large temperature differences (DeVault et al. 2004; Peers et al. 2020; Turner et al. 2017), combined with differing levels of resources (Allen 2010; Doherty et al. 2019) and numbers of corvids (Gil et al. 2018; Huijbers et al. 2015; Morales-Reyes et al. 2017) that together contributed to the scavenging dynamics we observed.
Our results suggest that the importance of carrion in the diet of corvids may fluctuate with the availability of other resources, affecting how carrion is processed in the ecosystem. Indeed, we found that although discovery time was shortest after the flood the amount of time corvids spent at carcasses did not follow this pattern. This indicates that, particularly in the winter, corvids likely preferred to exploit other food resources. The high level of exploitation of carrion by corvids also has implications for the wider ecosystem. Corvids also act as predators and their effect on ground nesting birds and small passerines in Australia has been documented (Rees et al. 2020, Rees et al. 2015; Spencer et al. 2021). It is possible that in the spring following the flood, carrion was an important food subsidy for corvids that maintained their population in the area, as seen in other scavengers (Newsome et al. 2015; Gompper and Vanak 2008), potentially increasing this negative effect. Therefore, it is important to consider the impact of hyper-abundant corvid populations on other species in the vicinity of carrion, especially when carcasses enter the landscape as a result of culling or other anthropogenic means. Further investigation is also needed to quantify the amount of carrion in different ecosystems (Barton et al. 2019) and the possible effects of current carcass management practices. However, increasing our understanding of the factors that influence the scavenging dynamics of corvids, as we have done, helps to expand our knowledge of how food webs function under different environmental conditions.
ACKNOWLEDGEMENTS
We are indebted to Bush Heritage Australia for providing access and accommodation during field studies, the reserve managers Helene Aubault and Kyle Barton for their advice and support throughout this study, and Wangkamadla Traditional Owners for permission to work on country. We acknowledge the Wangkamadla people as the Traditional Owners of Ethabuka Reserve. We recognize and respect the enduring relationship they have with their lands and waters, and we pay our respects to Elders past, present and future. Invaluable assistance was provided in and out of the field by members of the Desert Ecology Research Group, including Glenda Wardle, Bobby Tamayo, and members of the Global Ecology Lab including James Vandersteen and Chris Fust. Thanks to landholders in Boulia Shire, western Queensland, who provided materials for this project. We are also very thankful to the many co-workers and volunteers who assisted, particularly Guillaume Tutton, Joon Kim, James MacDiarmid and Hayden Griffith.
AUTHOR CONTRIBUTIONS
Patrick Bragato: Conceptualization (lead); formal analysis (lead); investigation (supporting); methodology (equal); software (lead); visualization (lead); writing – original draft (lead). Emma Spencer: Conceptualization (supporting); investigation (lead); methodology (equal); software (supporting); writing – review and editing (supporting). Christopher Dickman: Conceptualization (supporting); methodology (supporting); supervision (supporting); writing – review and editing (supporting). Mathew Samuel Crowther: Formal analysis (supporting); methodology (supporting); writing – review and editing (supporting). Ayesha Tulloch: Investigation (supporting); methodology (supporting); writing – review and editing (supporting). Thomas Newsome: Conceptualization (supporting); investigation (supporting); methodology (supporting); supervision (supporting); writing – review and editing (supporting).
FUNDING
This work was supported by the Australian Government's National Environmental Science Program through the Threatened Species Recovery Hub [Theme 1, Subproject 1.1.11 Cat suppression to conserve the night parrot]; and the Margaret Middleton Fund for Endangered Species.
CONFLICTS OF INTEREST
The authors have determined that there are no conflicts of interests.
ETHICAL APPROVAL
Scientific licences were obtained to relocate and monitor the carcasses (SL WA0006737), and all research was approved by the University of Sydney Animal Ethics Committee (Project number 2017/1173).
Open Research
DATA AVAILABILITY STATEMENT
The data and code used in the analyses for this paper are available in a 30 GitHub repository at: https://github.com/PatBragato/Corvid_scavenging_paper.




