Using monitors to monitor ecological restoration: Presence may not indicate persistence
Abstract
Habitat loss is a leading cause of biodiversity declines globally, and there has been increasing recognition in recent years of the importance of restoring degraded habitats to functional ecosystems to ameliorate this loss. Despite the critical roles animals play in ecosystems, animals are often overlooked in assessments of ecological restoration success, particularly beyond their presence or absence in these habitats. Apex predators are critical to ecosystems, regulating predator–prey dynamics, and in arid Australia, monitor lizards (Reptilia: Varanidae) often fill high-order predatory roles. Varanids are highly diverse in size and occupy a variety of ecological niches, providing an ideal group for assessing habitat change over multiple spatial scales. Here, we assess the responses of varanids to early-stage habitat restoration following the discontinuation of mining activities, by mapping behavioural signs of habitat usage including burrows, tracks and diggings. We recorded burrow size and track measurements to gauge the size of varanids utilising reference and restored habitats, and mapped tortuosity of tracks to assess their movement through habitats. Restored areas had significantly fewer signs of varanid presence than the reference bushland and largely appeared to be just traversed or used only by larger individuals. Restored landscapes, particularly those in early successional stages, often lack established vegetation cover and present increased metabolic costs and predation risks. Providing fauna refuges (e.g. hollow logs) to mitigate the metabolic costs and predation risks in areas undergoing restoration may aid in facilitating the return of varanids and of other animal populations, particularly during the early stages of vegetation establishment. Understanding the behavioural responses and movement ecology of animals within landscapes undergoing restoration is key to facilitating the conservation of self-sustaining and functional ecosystems.
Introduction
Habitat loss and degradation through anthropogenic activities such as agriculture, urbanisation and mining, is a leading driver of species extinctions worldwide (Lande 1998; Tilman et al. 2001; Fahrig 2007; Cristescu et al. 2012). Consequently, there has been growing recognition of the importance of returning degraded habitats to pre-disturbance conditions (Standards Reference Group SERA 2017; Miller et al.2017; Gann et al. 2019; Cross et al. 2020a). Historically, there has been an emphasis placed upon assessing vegetation structure and community dynamics in post-restoration monitoring (Ruiz-Jaen & Mitchell Aide 2005; Koch et al. 2010). Consequently, animal taxa are often overlooked in assessments of restoration success (Lindell 2008; Cross et al. 2019; 2020a) despite their role in providing critical ecological services, such as soil decomposition (Jouquet et al. 2006; Lavelle et al. 2006), pollination (Phillips et al. 2010; Menz et al. 2011; Frick et al. 2014) and regulation of predator–prey dynamics (Mace et al. 2012).
The ‘Field of Dreams’ hypothesis, or ‘build it and they will come’ (Palmer et al. 1997), assumes that animal taxa will return to habitats following the restoration of vegetation (Block et al. 2001; Cristescu et al. 2012; Cross et al. 2020a). Few studies, however, have demonstrated that the return of vegetation and habitat structure promote the unassisted return of fauna, or their re-integration into ecological processes, to a level comparable to that of the pre-disturbance habitats (Hilderbrand et al. 2005; Cross et al. 2020a). Among existing studies of fauna responses to restoration, there is a strong focus towards assessments of species’ presence, absence or abundance within restored habitats (Lindell 2008; Cross et al. 2019). While providing important tools for assessing population dynamics and habitat quality (Mackenzie 2005), such studies risk failing to provide sufficiently detailed information on the complexities of ecological interactions within habitats (Aldridge & Boyce 2007; Lindell 2008; Cross et al. 2020a) and have a high chance of missing rare or cryptic species due to capturing only a ‘snapshot’ of biodiversity (Chiarucci et al. 2011). Successful restoration of degraded sites may hinge on both presence and abundance of key resources in restoration (Lindell 2008). Presence/absence studies are unlikely to identify the fundamental resource and habitat requirements that support reproductive populations, and hence be able to show whether habitat restoration is facilitating the return of self-sustaining, functional populations (Maron et al. 2005; Lindell 2008; Cross et al. 2019).
Understanding the behavioural responses of animals to habitat change and restoration, and the complex environmental factors facilitating their persistence and viability in habitats, is fundamental to achieving successful restoration outcomes (Sutherland 1998; Lindell 2008; Hale & Swearer 2017; Cross et al. 2019, 2020a). Monitoring visible signs of the presence of animals in habitats, such as tracks, burrows and diggings, provides an effective method for indirectly assessing behavioural and movement ecology (Gese 2001; Jewell et al. 2001; Stephens et al. 2006), particularly for shy or cryptic species where visual observations can be challenging (Silveira et al. 2003; Balme et al. 2009). Monitoring habitat use can provide important insights into habitat quality, resource availability and predator–prey dynamics (Lindell 2008; Salo et al. 2008; Van Beest et al. 2013). Habitats with few signs of animal activity often lack fundamental resources and may impose high metabolic costs and predation risks, subsequently impacting foraging efficiency (e.g. Tomlinson et al.2017). Areas of higher foraging and burrow activity are more likely to contain an abundance of food and thermal refuges, and may have decreased predation risk and competition pressures (Lindell 2008; Bruton et al. 2016).
Movement and habitat use by animals is influenced by the perceived risks associated with their surrounding landscape (Fahrig 2007). Within habitats perceived to be of lower quality, such as fragmented, or spatially and structurally homogenous landscapes, movement tends to be direct and without deviation to minimise time spent within these areas (Haynes & Cronin 2006; Fahrig 2007). Ectothermic species, such as reptiles, rely on an availability of suitable microclimates and thermal refuges for thermoregulation (Lindell 2008; Basson et al. 2017). Tracks crossing directly through a habitat with minimal tortuosity may indicate that these areas are lacking key resources (e.g. thermal refugia and less diverse microhabitats; Bruton et al. 2016) and are consequently thermally unsuitable or expose individuals to high predation risk. The identification of key resources and microhabitats required to support populations with diverse demography is vital to understanding the suitability of restoration to support self-sustaining faunal populations (Craig 2002; Craig et al. 2007; Fahrig 2007).
High-order predators play critical roles in ecosystems, maintaining top-down control through predator–prey dynamics (Post et al. 1999; Miller et al. 2001). Declines or losses of apex predators from habitats can have significant flow-on effects in ecosystems including increased prey populations and a reduction of species diversity through competitive exclusion of subordinate species (Miller et al. 2001). Within arid Australia, varanids (monitor lizards; Reptilia: Varanidae: Varanus) often fill high-order predatory roles, occurring at relatively high species richness (Pianka 1994; Read & Scoleri 2015). Varanids have highly generalist diets, preying on a variety of invertebrate and vertebrate items (Losos & Greene 1988; Cross et al.2020b). Consumption of prey from multiple trophic levels may facilitate the support of increased biodiversity through increased stability of food webs (Gross et al.2009; Bird et al.2013). Varanids occupy a wide range of habitats, including aquatic, terrestrial and arboreal niches. This diversification has led to the largest range of sizes within a single genus of any vertebrate taxon (~20 cm; V. sparnus, to ~3 m; V. komodoensis; Pianka et al. 2004; Doughty et al. 2014). Australian varanids encompass almost the entirety of this size breadth, with the largest species, V. giganteus, growing to around 2.5 m long (Pianka et al. 2004). Due to their diverse range of body sizes and, therefore, home ranges (King et al. 1989), varanids present an ideal group to monitor habitat change and restoration over a range of spatial scales.
Here, we analyse habitat use and movement by varanids within reference (unmined) and early-stage restoration vegetation at a mine site in the Mid West region of Western Australia, approximately 415 km northeast of Perth. We aim to assess (i) whether reference and restoration sites differ in total or type of habitat usage (total of all recorded tracks, diggings and burrows); (ii) whether restoration vegetation supports burrowing and foraging behaviour (diggings), or whether usage is restricted to transitory movement through these areas, and (iii) whether reference and restoration vegetation present different thermal environments.
Methods
Study site and species
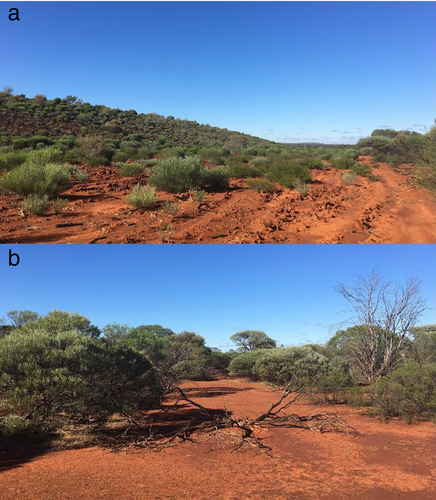
Study sites were located in the semi-arid shrubland communities within the tenement of a magnetite extraction operation in the Mid West region of Western Australia (29°11′31″S, 116°45′36″E). Five sympatric Varanus species co-exist within the study region; the stripe-tailed goanna (V. caudolineatus, arboreal, total length [TL] 0.32 m), black-headed monitor (V. tristis, primarily arboreal, TL 0.76 m), Gould’s goanna (V. gouldii, primarily terrestrial, TL 1.2 m), yellow-spotted monitor (V. panoptes, terrestrial, TL 1.4 m) and the perentie (V. giganteus, terrestrial, TL 2.5 m; Wilson & Swan 2003; Pianka et al. 2004). Territoriality has not been documented among studies tracking the movement and activity of varanids (e.g. Green & King 1978; Auffenberg 1981; Stanner & Mendelssohn 1986; Case & Schwaner 1993), and home ranges of Varanus species often overlap considerably (King & Green 1993). The broad niche overlap of varanids in the Mid West region of Australia suggests little interspecific exclusion (Cross et al. 2020b). The study area comprised two sites previously directly impacted by mining activities, located eight and 12 km from current active mining operations. Both sites were characterised by a restored waste rock dump (an area of ~800 × 500 m with sandy/rocky-loam soils) and were adjacent to reference bushland (unmined, largely flat landscape with sandy loam soils). Restoration of the waste rock areas in each site commenced in 2014 with completion in 2017. The dominant vegetation types within the study area are Acacia shrubland and open Eucalyptus woodland, with sandy rocky-loam soils (Bamford 2006). Restoration sites comprise a similar species composition to the reference habitat; however, the vegetation is at earlier successional stages than the reference community (Fig. 1a,b).
Survey design
Favoured activity temperatures for many Varanus species average around 35°C (King & Green 1999), and as such, we surveyed sites consecutively between September and October 2018, where daily maximum temperatures average between 36.2 and 41.2°C (Bureau of Meteorology, http://www.bom.gov.au/climate/data/). Sites were surveyed for a total of 16 days each, with reference and restoration areas within each site surveyed concurrently. The footprint of restoration activities within each site was ~800 × 500 m, and we surveyed each site using marked transects spaced at 100 m intervals. Each transect ran the width of the restoration area (500 m) and extended the equivalent distance into the reference vegetation (1 km in total). We walked two groups of five transects such that each site contained 10 transects. Each transect group was walked on alternating days, with transects in the second group also spaced 100 m apart but shifted 50 m further along the restoration footprint from transects in the first group. To maximise the ground covered, we surveyed a width of 25 m either side of each transect (10 transects of 50 m width, 1 km length).
Thermal environment
To determine whether the thermal environment differed between reference and restoration sites, we set 10 EasyLog USB temperature loggers (Lascar Electronics Ltd., UK) in each site. We aimed to assess the general differences in ambient temperatures between sites, and as such loggers were placed randomly along transects, such that reference and restoration areas each contained five temperature loggers (five transects each with two loggers, one in each of the reference and restoration sites). Loggers were suspended ~30 cm above the ground within open ended PVC tubes attached to wooden stakes to capture ambient temperature in reference and restoration sites. Loggers were programmed to record temperature at 15-min intervals for the duration of the study period (16 days per site), such that the number of recordings per site totalled 15 360 readings (7680 temperature readings in each of the reference and restoration areas at each site).
Mapping habitat use
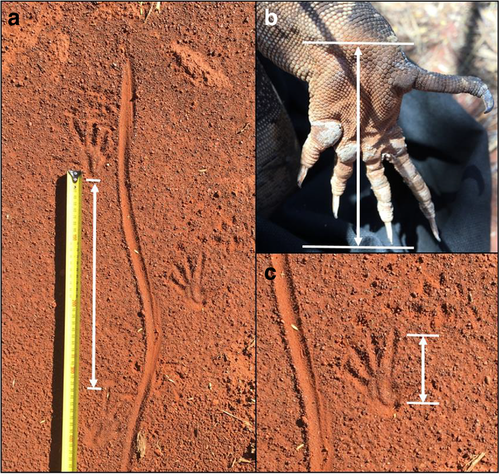
To determine how varanids move through and use reference and restoration vegetation, we GPS mapped all signs of varanid habitat use (tracks, diggings and burrows) along transects. Varanids create visible and distinctive tracks (Blamires 2000), and in the absence of similar-sized burrowing animals in the study region (Bamford 2006), burrows and diggings were easily identifiable. We marked burrows and diggings as fresh activity if there were signs of recently disturbed soil or visible tracks around each use, and tracks were recorded as fresh if footprints were clearly visible. To gauge the size of varanids utilising reference or restoration areas, we recorded the height and width of burrows, and measurements of tracks, including total track length, tail width (TW; width at the thickest section of the tail mark), stride length (SL; distance between the base of the pad of the forelimb and tip of the middle claw of the hindlimb; Fig. 2a) and foot length (FL; base of the pad to the tip of the longest claw; Fig. 2b, c). Where possible, we measured the FL of the hindlimb and SL of five imprints along each track to obtain an average measurement for analyses. We controlled for independence in samples by including tracks only if they were separated by at least 100 m from any other track, or if they were recorded on different dates and therefore verified as fresh usage. While assessments of habitat use by animals using tracks are infrequent, some studies of wide-ranging fauna have suggested 100 m as a suitable distance for independence of samples to reduce the risk of spatial autocorrelation (e.g. Bowman & Robitaille 1997; Proulx & O’Doherty 2006; Proulx et al. 2006).
Statistical analyses
Thermal environment
To determine whether there were any differences in the thermal environment between reference and restoration vegetation, we used paired t-tests with temperature as the dependent variable and vegetation type (reference or restoration) as the independent variable. As varanids are active diurnally (King & Green, 1993), we assessed whether reference and restoration sites presented thermally heterogeneous environments by calculating the coefficient of variation for average day time temperatures between 07.00 and 18.00. As varanid activity is typically highest at temperatures around 35°C, to determine the frequency at which recorded temperatures exceeded optimal activity temperature in reference and restoration vegetation, we calculated the total and average number of days, and minutes each day, where temperatures (recorded by dataloggers within each site) exceeded 35°C.
Habitat usage
We used log-linear models to analyse differences in the total and type of habitat use between reference and restoration vegetation, with vegetation type (reference or restoration), and site (8 or 12 km) as the independent variables, and the total number of each habitat usage (numbers of tracks, diggings or burrows) as the dependent variable. We repeated analyses with only fresh habitat usage included, and then with only old usage, to assess whether each vegetation type was more likely to contain fresh or old habitat usage. We used separate two-way generalised linear models with a Gaussian distribution to assess whether track measurements (total length, TW, FL or SL), or the size of burrows differed between reference and restoration vegetation, with vegetation type and site as the independent variables, and track length, TW, FL, SL or burrow size as the dependent variables. As tracks were marked as ‘old usage’ if there were no visible foot imprints, we included only fresh tracks in measurement analyses.
Usage measurements

Results
Temperature
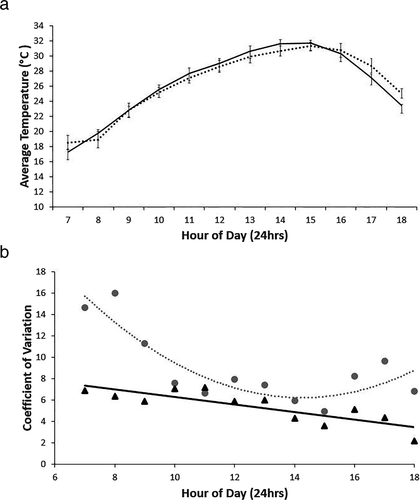
Recorded temperatures ranged between 5.5 and 42.8°C in reference vegetation, and 5.75 and 46.8°C in restoration sites, but reference and restoration sites did not differ significantly in either the average number of days (t = −5.80, df = 1, P = 0.109), or average number of daily minutes (t = −10.98, df = 1, P = 0.058) exceeding 35°C. Although average temperatures did not differ statistically (Fig. 3a), reference vegetation had a higher level of variability in recorded temperatures than restoration vegetation, where the thermal environment was relatively homogenous (Fig. 3b).
Usage of reference and restoration sites
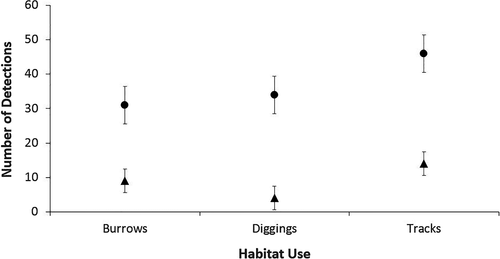
We recorded a total of 138 signs of habitat usage across all sites, with 80% (n = 110) of all usage recorded within the reference habitat (Fig. 4). Tracks were the most frequently recorded evidence of varanid presence within habitats (n = 60, 44%), with diggings and burrows recorded at equal frequencies (n = 39, 28%). Total habitat usage (i.e. the combined total of all recorded burrows, diggings and tracks within each vegetation type) was significantly higher in the reference vegetation, both including (χ2 = 48.72, d.f. = 2, P < 0.001) and excluding points of old habitat usage (χ2 = 17.09, d.f. = 2, P < 0.001). Reference vegetation contained a high proportion of old habitat usage (n = 46/110, 42% of all recorded usage); however, we rarely recorded old habitat usage within restoration vegetation (n = 3/28, 10.7% of all recorded usage). Restoration vegetation rarely contained signs of burrowing or diggings, with tracks recorded significantly more frequently than both diggings and burrows in these areas (χ2 = 6.50, d.f. = 2, P = 0.038). Reference vegetation supported movement, diggings and burrowing at similar frequencies, with no significant differences in the type of habitat usage recorded in reference areas (χ2 = 2.96, d.f. = 2, P = 0.227). We did not note any significant interaction between site (8 or 12 km from active mining) and total habitat usage in reference and restoration vegetation (χ2 = 0.73, d.f. = 1, P = 0.392). There was no significant interaction between type of habitat use (burrows, diggings or tracks) and vegetation type (χ2 = 2.97, d.f. = 2, P = 0.784), or site (χ2 = 3.46, d.f. = 2, P = 0.061).
Burrow and track measurements
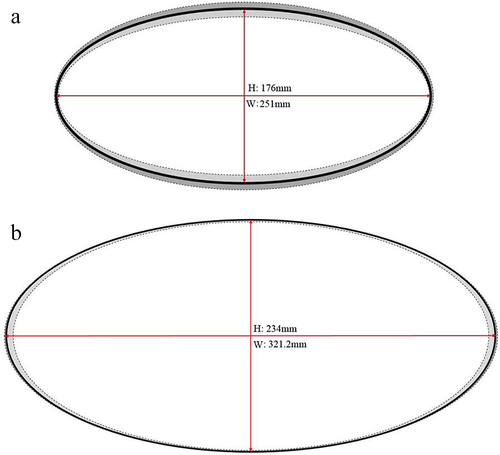
Burrows within the restoration vegetation were significantly larger (234.0 ± SE 22.4 mm height, 321.20 ± 24.70 mm width) than those within the reference vegetation (176.0 mm ± SE 62.10 height, 251.0 ± 10.0 mm; F1, 35 = 6.06, P = 0.019; Fig. 5). However, we did not detect any significant difference between each area for total track length (F1, 46 = 0.12, p = 0.733), TW (F1, 46 = 0.34, P = 0.546), FL (F1, 46 = 0.79, P = 0.376) or SL of tracks (F1, 46 = 0.29, P = 0.595). Fifty-five per cent of all tracks crossed through any given area without deviation, and we did not detect any significant difference between reference and restoration vegetation in the proportion of travel (F1, 56 = 2.41, P = 0.126). While there were no significant differences in proportion of travel of varanids between reference and restoration sites, tracks in the reference habitat displayed the largest range of travel proportion, ranging between 1 (straight line, no deviation) and 4.27 (proportion travelled ~ 4 times greater than the straight-line distance between the track start and end), in comparison with restoration areas, which only ranged between 1 and 1.26.
Discussion
Ambient temperature ranges did not differ significantly between reference and restoration sites; however, early-stage restoration lacked the thermal variability present within reference vegetation. Restoration vegetation in the study area is being used by varanids; however, usage appears to be infrequent and largely opportunistic. The metabolic costs of thermoregulating within spatially homogenous landscapes can be significantly higher than those within heterogeneous landscapes, even in cases where recorded temperatures do not differ between landscapes (Sears et al. 2011; Tuff et al. 2016). We recorded tracks significantly more frequently than both burrows and diggings within restoration vegetation, in comparison with the reference vegetation where tracks, diggings and burrows were recorded in similar proportions. The lack of diggings and burrowing activity in restoration vegetation may indicate that these areas lack key resources, such as food, thermal refuges and a diversity of microclimates, and may have increased metabolic costs associated with their use. While burrows were infrequently recorded in restoration vegetation, their use appeared to be restricted to larger-bodied varanids. Early-stage habitat restoration may be more restrictive to smaller-bodied varanids, which have lower thermal tolerances, lower thermal inertia, and increased reliance on an availability of thermal refuges and a diversity of microhabitats (Huey & Bennett 1990). However, it is also possible that larger-bodied varanids colonise restoration first and restrict use by smaller individuals through competitive exclusion, although evidence in the literature to support this is sparse.
Habitat usage
We recorded signs of varanid activity significantly less frequently within restoration habitat than within the reference habitat, with restoration areas containing just 20% of all recorded habitat usage. Sites undergoing restoration following discontinuation of mining activities can require long periods of time for vegetation to become established, or for vegetation to approach a state comparable to pre-disturbance structure and floristics (Grant & Loneragan 1999; Tuff et al. 2016). Furthermore, restoration, particularly early-stage restoration, is typically more homogeneous than reference habitats, as the reinstatement of vegetation structure can be a slow process (Pywell et al. 2002; Baer et al. 2004).Survival of reptile populations is often dependent on spatially heterogeneous habitats with an abundance of microclimates to support foraging and thermoregulatory behaviours (Hertz et al. 1993; Basson et al. 2017). Reptile populations are negatively impacted by a loss of native vegetation and decreasing habitat structure and complexity (Smith et al. 1996; Cunningham et al. 2007; Brown et al. 2008). Therefore, spatially and structurally homogenous landscapes often present increased metabolic costs through a lack of suitable thermal refuges and diverse microhabitats (Attum & Eason 2006).
Reptiles experience trade-offs between time spent foraging and time spent engaged in thermoregulatory behaviour (Tuff et al. 2016). This trade-off can be particularly high in hot, open landscapes that impose increased metabolic costs and predation risk (Tuff et al. 2016), and we rarely recorded burrows or diggings in restored vegetation. The lower variability in daily temperatures in restored areas indicates that these sites present increased homogeneity in the thermal landscape and may present increased thermal costs (Sears & Angilletta 2015; Cross et al.2020c). Foraging densities can be indicative of both prey abundance and habitat quality, with habitat patches of higher quality and an increased abundance of resources tending to support increased foraging activity (Wellenreuther & Connell 2002; Kilgo 2005; Lindell 2008). Mapping diggings may not fully encapsulate foraging activity by varanids in reference and restored habitats as larger-bodied varanids can hunt and capture vertebrate prey items without digging. However, many varanid species are primarily insectivorous and often dig for food resources (Losos & Greene 1988). Varanids of a range of body sizes in the Mid West region of Western Australia primarily prey upon invertebrates and small reptilian species (Cross et al. 2020b), suggesting diggings accurately represented foraging activity. Invertebrate richness has previously been reported to be positively correlated with increasing vegetation structure and diversity (Muren et al. 2003; Robinson et al. 2018). Increased metabolic costs and a reduction in vegetation structure in restoration areas may present less favourable habitat for both predator and prey species, limiting foraging efficiency by varanids in these areas. High metabolic costs associated with homogenous landscapes can be particularly restrictive for smaller reptile species, which rapidly reach a temperature equilibrium with the surrounding environment (Huey & Bennett 1990). As with signs of foraging, we rarely recorded burrows in restoration vegetation; however, when present, burrows in these areas appeared to be restricted to larger-bodied varanids. Larger-bodied reptiles have greater thermal inertia, requiring longer time periods to reach maximum thermal levels (Cowles & Bogert 1944), and are typically able to withstand greater temperature fluctuations than smaller individuals (Spotila et al. 1973; Stevenson 1985; Huey & Bennett 1990).
Movement ecology
Tracks were the most frequently recorded sign of varanid presence within restoration vegetation; however, we recorded tracks in these areas significantly less frequently than within the reference vegetation. The structural complexity of landscapes, and the perceived costs associated with their use, has substantial impacts on the movement ecology of animals (Morales & Ellner 2002; Jeanson et al. 2003; Fahrig 2007). Boundaries of habitat patches can impose hard constraints to movement and dispersal, with animals unlikely to leave habitat patches if the surrounding habitat is of lower quality (Fahrig 2007). The decreased availability of refuges and established vegetation cover within restoration areas may account for the reduction in movement of varanids within these areas. Animals minimise time spent in high-risk environments, tending to cross these areas rapidly and infrequently, in contrast with higher quality habitats that facilitate slower and non-uniform movement and can be crossed with less selectivity (Fahrig 2007). While we did not detect any significant differences in the proportion of travel between reference and restoration vegetation, tracks within restoration areas rarely deviated from straight-line movement, whereas tracks in the reference bushland had greater variability in the proportion of travel. While use of longer-term signs of habitat usage (e.g. burrows) may be restricted to larger-bodied varanids, we did not find any significant difference in the size of varanids traversing through restoration and reference areas. Early-stage restoration appears to support infrequent, opportunistic use by individuals of a range of body sizes, with use by smaller-bodied varanids largely confined to simply traversing through the restoration habitat. This disparity in use may result in restored landscapes serving as an ecological trap, with smaller-bodied varanids capable of moving through these landscapes but not persisting within restored areas. Ecological traps may affect the long-term viability and persistence of populations within habitats (Battin 2004). However, our study represents a snapshot of usage in early-stage habitat restoration and it is likely that habitat usage by both predator and prey species will increase as vegetation structure becomes established and heterogeneity increases, creating an availability of suitable microhabitats.
Study limitations
There are some limitations to assessments of behaviour and movement ecology of animals through indirect measures, such as monitoring habitat usage. Although providing an effective method of assessing habitat usage by populations, determining usage by individuals can be challenging, and habitat usage is likely to vary among individuals of different ages and sexes (Garshelis 2000). While some studies suggest 100 m as an appropriate distance between tracks for sample independence (e.g. Bowman & Robitaille 1997; Proulx & O’Doherty 2006; Proulx et al. 2006), varanids can move over large distances (e.g. Green et al.1986; Cross et al.2020c) and difficulties in identifying between usage of individuals risks introducing spatial autocorrelation. Furthermore, given the varying effects of environmental factors, such as fluctuating temperatures, on small and large-bodied reptiles (Spotila et al. 1973; Stevenson 1985; Huey & Bennett 1990), it is likely that Varanus species, or juveniles and adults of the same species, are impacted by habitat degradation and restoration to varying extents. Conclusions drawn at only a population level may therefore not fully represent the ecological impacts of habitat degradation and subsequent restoration on their behaviour and movement ecology. This method may also present a bias towards animals occupying terrestrial niches, with habitat usage by primarily arboreal species less likely to be recorded on the ground. Furthermore, substrate and vegetation density can impact the detectability of habitat usage (Garshelis 2000). Soil compaction following the use of heavy machinery is common in areas undergoing restoration (Bradshaw 1997) and may have reduced the probability of detecting use by varanids in these areas. Lastly, our results may have been affected by the differing proximity of our sites to the active mine pit. However, as we did not detect any interaction effects between distance of sites from active mining (8 or 12 km) and habitat usage of reference and restoration vegetation, we concluded that proximity to the active mine pit was unlikely to have influenced our results.
Conclusions
Restoration of discontinued mine sites within the study area appeared to be supporting a level of usage by varanids. However, usage appeared to be largely movement through restored areas, or where burrows were present, usage was restricted primarily to larger-bodied individuals. Our data suggest that restoration areas may contain a paucity of some fundamental resources, such as food resources, thermal refuges and a diversity of microclimates. Decreased spatial heterogeneity in restoration likely presents unfavourable thermal conditions, reducing the abundance of both varanids, and the prey they are reliant on. Returning fauna refuges, for example hollow logs and debris piles, may aid in facilitating colonisation and long-term use of restoration sites by varanids, and increase their resilience to habitat disturbance, particularly during the initial stages of vegetation establishment (Koch 2007; Robinson et al. 2013; Christie et al. 2013; O'Connell & Keppel 2016). Most studies of wildlife responses to mine site restoration only consider presence or abundance of animals (Cross et al.2019, 2020a). Had this study considered only the presence of varanids as being indicative of habitat use, we might have concluded that restoration and reference sites were utilised similarly. However, by assessing the features associated with movement ecology and burrow use, we show that restoration sites may lack some key resources required to sustain reptile populations, particularly small-bodied varanids. Further research to identify the key resources promoting and aiding the return of fauna groups from a variety of taxa and trophic levels over multiple temporal scales is key to returning functional and diverse fauna populations to habitats undergoing restoration. Understanding how animals respond to habitat change and restoration is critical to their conservation in the face of ever-increasing rates of habitat degradation and loss.
Acknowledgements
This research is a product of the Australian Research Council Industrial Transformation Training Centre for Mine Site Restoration (ICI150100041) and was further supported by the Ecological Society of Australia & Holsworth Wildlife Research Endowment. Site access from Karara Mining Ltd. is gratefully acknowledged, and the authors thank J. Baker, J. Sansom, A. Freeman and D. Juniper for their support throughout field seasons. This work was approved by the Curtin University Animal Ethics Committee (approval no. ARE2016-2) and the Department of Parks and Wildlife (licence no. 01-000141-2). The views expressed herein are those of the authors’ and are not necessarily those of the Australian Government or Australian Research Council.
Author Contribution
Sophie Cross: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Funding acquisition (supporting); Investigation (lead); Methodology (lead); Project administration (lead); Resources (equal); Validation (equal); Visualization (lead); Writing-original draft (lead); Writing-review & editing (lead). Michael D Craig: Conceptualization (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (equal); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing-review & editing (supporting). Sean Tomlinson: Conceptualization (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing-review & editing (supporting). Kingsley Dixon: Funding acquisition (lead); Project administration (supporting); Resources (supporting); Supervision (supporting); Validation (supporting); Visualization (supporting); Writing-review & editing (supporting). Bill Bateman: Conceptualization (supporting); Formal analysis (supporting); Funding acquisition (supporting); Investigation (supporting); Methodology (supporting); Project administration (supporting); Resources (equal); Supervision (lead); Validation (supporting); Visualization (supporting); Writing-review & editing (supporting).
Conflicts of Interest
The authors declare no conflicts of interest.




