Bees of the Victorian Alps: Network structure and interactions of introduced species
Abstract
Bees are considered the most important plant pollinators in many ecosystems, yet little is known about pollination of native plants by bees in many Australian ecosystems including the alpine region. Here we consider bee pollination in this region by constructing a bee visitation network and investigating the degree of specialism and network ‘nestedness’, which are related to the robustness of the network to perturbations. Bees and flowers were collected and observed from 10 sites across the Bogong High Plains/Mt Hotham region in Victoria. Low nestedness and a low degree of specialism were detected, consistent with patterns in other alpine regions. Twenty-one native and one non-indigenous bee species were observed visiting 46 of the 67 flower species recorded. The introduced Apis mellifera had a large floral overlap with native bees, which may reduce fecundity of native bees through competition. The introduced plant, Hypochaeris radicata (Asteraceae), had the largest and most sustained coverage of any flower and had the most visitations and bee species of any flower. The network developed in this study is a first step in understanding pollination patterns in the alpine/subalpine region and serves as a baseline for future comparisons.
Introduction
Bees are considered to be an indicator species group for ecosystem health. While their necessity to human survival is often overstated (Garibaldi et al. 2013; Rader et al. 2016), they are an excellent species group for studying changes in pollination processes within an ecosystem due to their dependence on flowers for nutrition (Vanbergen et al. 2017), whereas other pollinators, such as flies or wasps, exploit other resources (Burkle et al. 2013).
The interrelationship of bees with flowers results in a structural organization that forms a network, which can then be quantified for its properties, importance and strength (Bascompte et al. 2003; Nielsen & Bascompte 2007; Popic et al. 2013). One component of networks involves specialization, characterized by the number of links a species encounters in a network (Blüthgen et al. 2006; Almeida-Neto et al. 2008; Dormann 2011). Abundant generalists can cover a wide range of floral resources with high connectivity and are therefore important for network stability (Vanbergen et al. 2017). Specialist pollinators are rare (Bascompte et al. 2003; Nielsen & Bascompte 2007; Popic et al. 2013), and considered less redundant than generalists given that their loss can potentially have a greater effect on the plant community (Dormann 2011), however, generalists have a greater effect on the networks structure (Vanbergen et al. 2017) particularly when generalists represent newly introduced, non-indigenous species.
Another related commonly-used network metric, ‘nestedness’, is the degree of asymmetry of the network, and indicates its stability against perturbations; that is, the ability to function when individual species become extinct (Bascompte et al. 2003; Almeida-Neto et al. 2007; Nielsen & Bascompte 2007; Popic et al. 2013). Nestedness reflects a non-random structure (Bascompte et al. 2003) that measures niche width and niche interactions (Guimaraes & Guimaraes 2006; Dormann et al. 2009; Dormann 2011). The presence of nestedness within interactive networks is hypothesized to arise due to uneven distribution of interacting species (Nielsen & Bascompte 2007; Burkle et al. 2013). Highly nested networks occur where specialized species interact with generalized ones (Bascompte et al. 2003; Nielsen & Bascompte 2007; Almeida-Neto et al. 2008). Although debated (Strona & Fattorini 2014), a high degree of nestedness is thought to indicate that a species is less likely to be vulnerable even when other species are eliminated from a network (Bascompte et al. 2003; Almeida-Neto et al. 2007; Nielsen & Bascompte 2007; Vanbergen et al. 2017).
Non-indigenous species in an environment are considered one of the biggest threats to global biodiversity (Goulson 2003; Valdovinos et al. 2009). Together with climate change and habitat deterioration, invasions by non-indigenous species constitute the three main anthropogenic threats to ecosystem processes, including pollination networks (Memmott & Waser 2002; Memmott et al. 2007; Valdovinos et al. 2009). Non-indigenous species can impact directly by predating on native species, or indirectly by causing behavioural shifts, niche displacement and competitive exclusion of natives (Mooney & Cleland 2001; Goulson 2003). The removal of a pollinator species can change foraging specialization in unpredictable ways, such as through reduced floral fidelity and plant reproductive function (Brosi & Briggs 2013). These impacts can cause trophic cascades through an ecosystem that lowers resilience, including through altered nestedness (Folke et al. 2004). However, once established non-indigenous species can become an integral part of the network (Memmott & Waser 2002) and important for species persistence (Valdovinos et al. 2009).
There are two groups of non-indigenous species in the Australian Alps, the honeybee Apis mellifera L. 1758 (Apidae) and several non-indigenous plant species (Mcdougall et al. 2005) that could influence alpine networks. Apis mellifera is a prolific, polylectic forager that influences networks (Santos et al. 2012; Giannini et al. 2015), often being considered either detrimental or potentially beneficial to native plants (Paini & Roberts 2005). Negative impacts include displacement of native pollinators through resource competition, inefficient pollination of native flowers, and an increase in non-indigenous plant populations due to preferential visitation (Goulson 2003; Hanley & Goulson 2003; Kearns et al. 1998; Paini 2004; Paini & Roberts 2005; Paton 1993). Each of these effects has the potential to lower network nestedness (Strona & Fattorini 2014). Apis mellifera is considered competitive because of its large size, aggressive behaviour and social structure, which provides an advantage over solitary species (Paton 1993; Manning 1997). There are no studies on the impact of honeybees on network structures, in Australia, although they can indirectly alter the structure of a network by changing the connectivity and strength of interactions (Giannini et al. 2015; Vanbergen et al. 2017).
Introduced plants continue to increase in abundance in the Australian Alps, with 175 species now recorded above 1500 m (Mcdougall et al. 2005). Invasive angiosperms often bloom for long periods, attracting pollinators (Memmott & Waser 2002). With strong presence within a pollination web, such species can potentially increase pollinator populations by increasing resources, thereby competitively reducing native flower fecundity and eroding asymmetric structure of networks to cause instability (Memmott et al. 2004; Aizen et al. 2008; Muñoz & Cavieres 2008).
Developing and quantifying a visitation network on the relationship between bees and flowers is a first step in determining pollinator/plant community structure. When species' interconnections have been established, they provide a baseline to assess how any new invasive species might interact within the network, helping to assess their role as a threatening process in alpine ecosystems. Therefore, a visitation network of Victorian alpine/subalpine bee species and their angiosperm hosts across open heathland/grasslands (Mcdougall & Walsh 2007) is developed. We consider the potential impact of Apis mellifera, which has recently been detected (Nash 2013), on the structure of the network. The likely impact of the dominant non-indigenous plant species on bees and the network is discussed.
Methods
Study sites
Ten sites were selected to have similar vegetation and flowering species from across the Bogong High Plains and Mt Hotham/Dinner Plain region, within the Victorian Alps. Sites were from 700 m to 27.9 km apart (Table 1) across alpine (3) and subalpine (7) ecosystems from 1400–1870 m a.s.l (Fig. 1). Varying levels of dissimilarity between site flowering vegetation; excluding wind-pollinated species; were observed (Table 1). The three alpine sites were less dissimilar compared with the subalpine sites. All sites shared at least nine flowering species.
| Site | Buckety | ITEX | Lang | Mt McKay | Cult | Mt Nelse | WH | JB | Mt Hotham | Baldy |
|---|---|---|---|---|---|---|---|---|---|---|
| Buckety | 6.9 km | 8.7 km | 11.3 km | 9.5 km | 10.7 km | 10.0 km | 14.1 km | 19.1 km | 20.0 km | |
| ITEX | 62% | 5.3 km | 4.4 km | 9.1 km | 9.4 km | 14.0 km | 13.8 km | 15.2 km | 15.8 km | |
| Lang | 66% | 49% | 7.5 km | 3.8 km | 4.3 km | 6.8 km | 18.1 km | 20.7 km | 21.4 km | |
| Mt McKay | 63% | 59% | 44% | 10.3 km | 9.3 km | 14.0 km | 16.5 km | 15.3 km | 15.9 km | |
| Cult | 45% | 57% | 55% | 71% | 1.9 km | 4.1 km | 22.0 km | 24.5 km | 25.1 km | |
| Mt Nelse | 62% | 55% | 36% | 50% | 58% | 5.6 km | 22.3 km | 23.8 km | 24.7 km | |
| WH | 60% | 66% | 67% | 76% | 58% | 75% | 23.0 km | 27.0 km | 27.9 km | |
| JB | 69% | 74% | 65% | 59% | 76% | 70% | 67% | 9.5 km | 10.0 km | |
| Mt Hotham | 61% | 60% | 48% | 44% | 56% | 38% | 81% | 76% | 0.7 km | |
| Baldy | 68% | 80% | 73% | 62% | 74% | 67% | 76% | 74% | 69% |
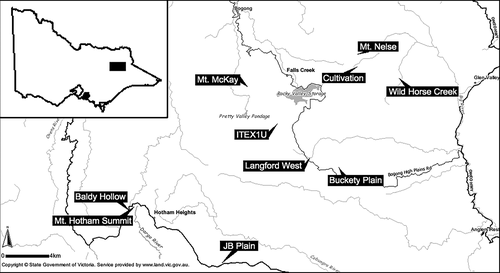
Field methods
A stratified sample design was used. At all sites, surveys were conducted three times each month from November 2013 to March 2014 (N = 15), within the same pre-defined 100 × 100 m areas. Each survey consisted of eight randomly placed 5 × 2 m transects within this area. Percentage abundance of each flowering species was estimated in each transect, and each transect patrolled for 15 min to record visitation; that is, a total of 2 h was spent on observations per site. Air temperature was recorded at the start of each sampling at each site. Only bees were recorded, although it is acknowledged that flies, moths and other insects can also be important pollinators. Individual bees observed on a flower were caught with a butterfly net. For each capture, the bee and flower species were recorded. If the individual was not identifiable, a voucher was retained for subsequent identification. As permit restrictions did not allow for destructive sampling, the collection of pollen from flowers and bees did not yield results adequate for properly quantifying floral resources or a pollination network, hence the focus of this study was on visitation networks despite their limitations (Popic et al. 2013).
Flowers
Flowers in plots were measured in three ways: (i) total coverage as estimated by the number of floral units/m2; (ii) floral unit that bees were known to visit; and (iii) floral unit on which bees were caught. Floral units were defined as non-connected florets, with flowering occurring when the flower is open and more than 50% of the anthers are showing, or more than 50% of the ray florets have opened.
Network analysis
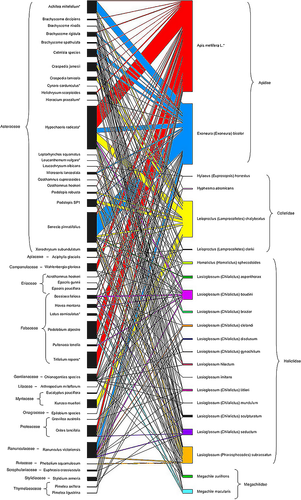
Due to the low number of visitations per individual survey at each site and non-significance of networks (Appendix S1), a network was created by pooling all observed visitation data to create a matrix, showing the number of times each bee species (A) visited each plant species (P) for Victorian alpine/subalpine open heathland/grasslands of the Bogong High Plains and Mt Hotham region. The network was constructed with the Bipartite package (Dormann et al. 2009) in R (R Core Team 2014), with nestedness and associated significance analysed in the ANINHADO program. Only bipartite connections between the two groups were considered, as unconnected pollinator species can skew results towards specialism due to a lack of information (Dormann 2011). To visualize networks, rectangles were generated in two columns that represent species, with their width proportional to how many interactions they have with the opposing group. Lines linking the two groups show the number of interactions. GIMP (GNU Image Manipulation Program 2001–2013) was used to colour the network, to make it easier to interpret and to arrange family phylogenies (Fig. 2).
The network analysis involves two levels of indices. The first-order indices look at the number of plant and bee species, the average number of links per species, and how many visits and number of species were observed. The second-order indices are calculated from qualitative data and affected by network size (Dormann et al. 2009; Popic et al. 2013), and represent the degree of the networks’ nestedness and connectance. Connectance is the proportion of all possible links within the network (Dormann et al. 2009). Nestedness is a measure of the temperature (T) of a matrix. If a matrix is cold (0) then it has high nestedness, but if it is hot (100) then it is random. T was converted into the nestedness index, N, where N = (100 − T)/100. In this index 0 is random and 1 is perfectly nested (Bascompte et al. 2003). In a perfectly nested network the most generalist bee visits all flowers and the most generalist flower is visited by every bee (Almeida-Neto et al. 2008). When these distributions of occurrence are arranged into a matrix, it is considered ‘perfectly nested’ if most of the presences are in the top left corner of the matrix, forming a triangle (Ulrich et al. 2009). Any metric that quantifies the arrangement of a network aims to determine how much it deviates from the perfectly nested arrangement (Almeida-Neto et al. 2008). Nestedness is relatively insensitive to sampling effort (n) and is more affected by the number of species and links in the network (Nielsen & Bascompte 2007).
A null network model was used to compare the structure of networks with varying size (Popic et al. 2013); this accounts for sensitivity to the number of species in the higher and lower trophic levels, the asymmetry of network dimensions and the number of interactions when determining statistical significance of the degree of nestedness of the matrix (Dormann et al. 2009). Matrix T was recalculated using a Monte Carlo randomization analysis (1000 randomizations) to create null networks, and compared with actual networks to determine how often the network could be produced by chance (Dormann 2011). A nestedness metric based on Overlap and Decreasing Fill (NODF) (Guimaraes & Guimaraes 2006) and CE null model (Strona & Fattorini 2014) were also used to test whether observing an interaction between bees and flowers being specialized was greater than expected by chance (Alarcón 2010).
The second-order indices included a measure of the degree of specialization of species in a network (H2′) and specialization at the species level (d′). Both indices are robust to variation in sampling effort and variation in the matrix (Blüthgen et al. 2006). H2′ is based on how much each species deviates from its actual number of interactions to an expected number, given the total interactions within the web. If there is no specialization, H2′ will be close to 0, but if there is high specialization, H2′ is closer to 1. The d′ measure accounts for the importance of the availability of each niche proportionally (Dormann 2011). If a pollinator uses all niches in the same proportion available in the environment, it is a more generalist species, being more opportunistic. If a pollinator uses few niches in contrast to their availability in the environment, it is a specialist. For the most generalized bee species d′ = 0, and for the most specialist bee species d′ = 1 (Blüthgen et al. 2006; Dormann 2011).
Two factors were thought to influence bee activity directly, hence likely to influence network measures. Temperature has a direct influence on bee activity, consequently, the association between temperature recorded when bees were sampled was tested directly using a Pearson correlation. Regression analysis did not indicate elevation being a significant factor for either the indices of nestedness (F1,9 = 0.77; P = 0.406) or connectiveness (F1,9 = 0.02; P = 0.889). ANOVA was used to determine the effect of flower coverage, as quantified by flora units/m2 on visitation by a bee. The coverage of native and introduced flowers were kept as separate independent variables, and the percentage of native and introduced flowers on which bees were caught, relative to their coverage, was used to test if non-indigenous bees favour non-indigenous flowers.
Results
Structure of the visitation network
A total 2262 captures for 22 bee species (Table 2) was recorded on 15 flower families (Table 3 and Fig. 2). Four bee families were represented (relative abundance): Apidae (63.09%), Colletidae (15.69%), Halictidae (18.66%) and Megachilidae (2.56%) (Fig. 2). Two species of Apidae visited the most flower species: A. mellifera (37.98% of all bees) and Exoneura bicolor (25.11% of all bees). Four species of Colletidae were recorded (Fig. 2), with Leioproctus chalybeatus (Colletidae, Erichson 1842) a relatively common species representing 95% of Colletidae caught (14.99% of all bees). Halictidae had the most species (Fig. 2), with Lasioglossum subrussatum (Cockerell 1922) the most common (6.81% of all bees), followed by L. baudini (3.76% of all bees). Five Lasioglossum spp. were not observed on invasive plant species, however, three of these species were least abundant: L. gynochilum (Michener 1965) (0.09% of all bees), L. imitans (Cockerell 1914) (0.09% of all bees) and L. mundulum (Cockerell 1916) (0.13% of all bees). Two species of Megachilidae, Megachile aurifrons (Smith 1853) and M. macularis (Torre 1896), were recorded preferring Fabaceae flowers (1.72% coverage).
| Apidae: Apis mellifera L. (1758) | 0.470 |
| Apidae: Exoneura (Exoneura) bicolor (Smith, F. 1854) | 0.449 |
| Colletidae: Hylaeus (Euprosopsis) honestus (Smith, 1879) | 0.458 |
| Colletidae: Hyphesma atromicans (Cockerell, 1913) | 0.359 |
| Colletidae: Leioproctus (Lamprocolletes) chalybeatus (Erichson, 1842) | 0.452 |
| Colletidae: Leioproctus (Lamprocolletes) clarki (Cockerell, 1929) | 0.370 |
| Halictidae: Homalictus (Homalictus) sphecodoides (Smith, 1853) | 0.391 |
| Halictidae: Lasioglossum (Chilalictus) disclusum (Cockerell, 1914) | 0.337 |
| Halictidae: Lasioglossum (Chilalictus) asperithorax (Cockerell, 1910) | 0.365 |
| Halictidae: Lasioglossum (Chilalictus) baudini (Cockerell, 1915) | 0.284 |
| Halictidae: Lasioglossum (Chilalictus) brazier (Cockerell, 1916) | 0.254 |
| Halictidae: Lasioglossum (Chilalictus) clelandi (Cockerell, 1910) | 0.396 |
| Halictidae: Lasioglossum (Chilalictus) gynochilium (Michener, 1965) | 0.266 |
| Halictidae: Lasioglossum (Chilalictus) littleri (Cockerell, 1914) | 0.389 |
| Halictidae: Lasioglossum (Chilalictus) mundulum (Cockerell, 1916) | 0.247 |
| Halictidae: Lasioglossum (Chilalictus) sculpturatum (Cockerell, 1930) | 0.161 |
| Halictidae: Lasioglossum (Chilalictus) seductum (Cockerell, 1914) | 0.304 |
| Halictidae: Lasioglossum (Pharasphecodes) subrassatum (Cockerell, 1922) | 0.310 |
| Halictidae: Lasioglossum hilactum (Smith, 1853) | 0.428 |
| Halictidae: Lasioglossum imitans (Cockerell, 1914) | 0.524 |
| Halictidae: Lasioglossum culpturatum (Cockerell, 1930) | Not Calculated |
| Megachilidae: Megachile aurifrons (Smith, 1853) | 0.271 |
| Megachilidae: Megachile macularis (Torre, 1896) | 0.579 |
| Network (all months) | |
|---|---|
| Number of bee species | 22 |
| Number of plant species | 46 |
| Links per species | 2.956 |
| Visits | 2262 |
| C (Connectance) | 0.199 |
| Nestedness Index (N) | 0.528 |
| P(Ce) | <0.01 |
| H2′ | 0.406 |
| Mean bee specialization index d′ | 0.367 |
- C (Connectance): the proportion of all possible links within the network. N (Nestedness): an index reflecting the temperature of a matrix. Under high nestedness (N = 1), generalist bees visit all flowers and generalist flowers are visited by all bees. Under low nestedness (N = 0) the association is random. P(Ce): represents probability of an interaction between bees and flowers being specialized was greater than expected by chance. H2′: degree of specialization of species in a network. If there is no specialization the index approaches 0. d′: degree of specialization at the species level taking into account available niches. If species have a high level of specialization, d′ approaches 1.
Bees were captured on 46 of the 67 flower species recorded. Visited flowers had greater coverage within sites than non-visited flowers (92.06% of total coverage including non-visited flowers). The plant family Asteraceae had the most species and the most bee visits (55.53%; Fig. 2). The second largest family, Fabaceae, had fewer than half the bee visits of the Asteracea (18.7%). The other families of flowers had very low coverage and bee visits, except for Ranunculaceae; 10.71% coverage, 6.59% total bee visitation.
The network had low connectance, mid-range nestedness, with H2′ being of mid-range specialization and the network being more generalist (Table 3). The monthly nestedness data (N = 1) contradicted overall nestedness (N = 0.528), which is most likely due to the low number of species and links in the network (Nielsen & Bascompte 2007), hence the pooling of data. The d′ index for bees and plants was low (Table 3), suggesting alpine species maximize niche utilization. Overall, bees were found to be opportunists, but for individual species the d′ index had a large range (Table 2). The most generalist bee species was L. sculpturatum (Halictidae, Cockerell 1930) and the least opportunistic was M. macularis.
The non-indigenous honeybee, A. mellifera, was caught on more flower species than any other bee (30); three of the species visited were not visited by other bee species (Fig. 2). Three invasive flowering species were visited by A. mellifera (42.5% of its visits), whereas native bees visited six non-indigenous species (24.23% of native bee visits). Apis mellifera was the only visitor to the non-indigenous Triflolium repens L. (Fabaceae) (6.81% of its visits), but was also caught on the native species Podolobium alpestre (F.Muell.) (Fabaceae) (6.19%), Wahlenbergia gloriosa Lothian (Campanulaceae)(3.05%), Hovea montana (Hook.f.) (Fabaceae) (0.75%), Orites lancifolia F.Muell (Proteaceae) (3.27%) and Stylidium armeria (Labill.) (Stylidiaceae) (1.02%).
Temperature was associated with the number of bees observed visiting a flower, with native bees having a slightly stronger correlation (0.339, R2 = 0.115, N = 108, P < 0.01) than A. mellifera (0.23, R2 = 0.53, N = 108, P = 0.016).
Non-indigenous flower visitations
Though non-indigenous flowering plant species (7) were present across all 10 sites in varying amounts, all except Hypochaeris radicata L. (Asteraceae) were in low abundance. Bees visited five non-indigenous species (Fig. 2). Coverage of native and non-indigenous species influenced bee choice (ANOVA F2,133 = 74.83, P < 0.01); that is, the greater flower coverage, the more bees visited that species. Hypochaeris radicata had the most coverage (9.82%) and was the only species flowering every month of this study, hence it had the greatest number of pollinator species caught on it (14) and highest percentage of visitations (17.95%; Fig. 2). Other introduced flowers had relatively low coverage and few visits. The non-indigenous Viola bicolor Pursh (Violaceae) and Verbascum thapsus L. (Scrophulariaceae) were not visited within the study plots. Apis mellifera and native bees visited H. radicata to a similar extent (A. mellifera 51.23%, natives 48.77%). Exoneura bicolor was caught on more flowers than any other native bee and favoured Achillea millefolium L. (Asteraceae) (4.2%); it was the only bee caught on Hieracium praealtum L. (Asteraceae) (0.13%), which had low coverage (0.06%). Leioproctus chalybeatus visited 19 flowers (41.3%), favouring H. radicata (3.54%), and was the only visitor to Leucanthemum vulgare Lam. (Asteraceae) (0.04%). Megachilidae (2 spp.) were the only group observed on Lotus corniculatus L. (Fabaceae) (0.93% coverage).
Discussion
Nature of the visitation network
Bees found in the Victorian Alps have a strong, positive visitation rate associated with the amount of floral coverage, have low connectivity, low specialism and mid-range nestedness. Comparison with other alpine networks is difficult due to the most efficient pollinators in alpine ecosystems, Bombus spp. (Bergman et al. 1996; Bingham 1998), not being present on the Australian mainland as confirmed in this study. This is compounded by limited information from the southern hemisphere (e.g. Primack 1978, 1983), with only one study from the Australian mountains (Inouye & Pyke 1988). That study recorded 13 bee species from the Snowy Mountains, NSW, on 43 flowering species. Of the bee species recorded, only two were considered polylectic, with the other 11 species monolectic (Inouye & Pyke 1988). However, the data available in that study were not used in a network analysis, precluding a direct comparison.
Our results (Table 3) can be loosely contrasted with a visitation network from the Simpson Desert, Australia (Popic et al. 2013). That visitation network had nearly twice the bee species (50) detected from a similar number of plant species (52), but half the number of sites (5); a similar mid-range degree of specialization was found in this study (H2′ 0.493), with a slightly higher degree of bee (d′ 0.46–0.56) and plant (d′ 0.33–0.47) specialization and a lower realized proportion of links (connectance 0.065); however, nestedness in that network was very high (0.948) (Popic et al. 2013).
Although not as low as a desert network, the low connectivity observed here is consistent with observations from other alpine regions. In a comparison of networks from altitudinal gradient studies (Olesen & Jordano 2002), connectivity was found to decrease with an increase in altitude. Aizen et al. (2008) found a negative correlation between connectance and altitude. Species richness can influence indices from a network limiting comparisons between networks (Blüthgen et al. 2006), in that smaller networks have fewer asymmetric interactions potentially influencing this pattern (Fang & Huang 2012).
Low nestedness is thought to be largely due to plant species being more generalized at higher altitudes (Olesen & Jordano 2002), due to the limited period in which flowering (and thus pollination) can occur. This contrasts with the mid-range nestedness value (0.528) for our Victorian Alps visitation network (Table 3), and other alpine pollination networks such as Fang and Huang (2012) who found nestedness of approximately 0.96 at 3500 m. This demonstrates that the literature does not unequivocally support low nestedness in alpine areas. It does, however, support low connectance (Aizen et al. 2008).
Influence of non-indigenous Hypochaeris radicata
Hypochaeris radicata was a consistent presence across the survey period and was the most visited flower, particularly favoured by the introduced bee A. mellifera. Originally from Morocco, H. radicata (Asteraceae) is considered naturalized across temperate Australia (Ortiz et al. 2008). This dominant species has spread from roadsides to become established across alpine/subalpine grasslands and is now a dominant species across the Australian Alps. Non-indigenous plants in pollinator networks are primarily generalists that seemingly persist by being attractive to pollinators (Memmott & Waser 2002). In pollination networks, these plants may compete with other flowers and/or facilitate pollinator populations, or have no impact (Valdovinos et al. 2009). All these factors have the potential to lower the nestedness strength of a network so other perturbations have a greater negative effect that can lead to species extinction and ultimate collapse of the whole network (Memmott & Waser 2002).
The higher the coverage an introduced flower species has, the greater its attraction to pollinators (Primack 1983; Kearns et al. 1998; Packer et al. 2005; Aizen et al. 2008; Muñoz & Cavieres 2008). This changes visitation rates and connectance within a network (Valdovinos et al. 2009). Aizen and Harder (2008) found that networks with high introduced flower presence exhibited weaker nestedness than networks with fewer introduced flowers. Introduced flowers can change the strength of asymmetrical mutualism within the pollination network, particularly if they have a large coverage, as was the case in this study for H. radicata.
This is an issue for oligolectic bees that not only visit fewer flowers, but also have reduced genetic variation compared with polylactic bees, so are at greater risk in a network (Aarssen 1981; Packer et al. 2005). With higher nestedness, an oligolectic species is less likely to become isolated and its extinction risk decreases. Therefore, the bees in the Victorian Alps network may be at a higher risk of becoming extinct, because of the instability of the network as a whole. However, although nestedness of the network is unusually low, H. radicata may also be having a positive effect on the bees that visit it. Introduced flowers are known to increase populations and redistribute pollinators because they offer large rewards for extended periods of time (Memmott & Waser 2002; Lopezaraiza-Mikel et al. 2007). Sustained availability is attractive to bees because of floral constancy, but also because bees need large amounts of nectar and pollen. Müller et al. (2006) found that for the 41 bee species found in Europe, 85% needed all the pollen from 30 flowers to rear a single larva. One female bee needs 28 475 flower heads over a lifetime. The nutritional content of pollen is also important, and proteins are essential for a bee to reproduce and for its longevity (Weiner et al. 2010). This can be an issue in monoculture crops, where bees are forced to feed on flowers that are low in protein and amino acids (Vanenglesdorp et al. 2007). Hypochaeris radicata has high levels of protein and amino acids (Weiner et al. 2010), and is therefore particularly suitable for bee populations.
Such findings suggest that once introduced species become established in an ecosystem, consideration of their removal should include multiple factors beyond the simplified perception that they have negative effects (Lopezaraiza-Mikel et al. 2007). In fact, the removal of introduced flowers can increase extinction risk for pollinators, particularly if they have generalized effects (Valdovinos et al. 2009). Because H. radicata was the most connected in the network, it forms the core of the visitation network. The removal of a core species increases the extinction risk for bee species (Vanbergen & Initiative 2013). Of course, removal of H. radicata may have a positive effect on native plants for other reasons, but there are also many other non-indigenous species incursions in this region that could benefit from its removal.
With invasive non-indigenous species impacting alpine environments, along with other factors including climate change and associated fire incidence effecting vegetation (Wahren et al. 2013; Williams et al. 2015; Camac et al. 2017), local extinctions of bee species are probably inevitable, but this may be buffered by the presence of additional energy sources provided by naturalized flowers. With warmer conditions habitat resistance lowers, hence increasing the invasion potential of non-indigenous plants (Beaumont et al. 2009). Although such flowers may sustain bee populations, they can change the dynamics of an ecosystem, increasing the risk of a regime shift and extinction cascade (Memmott & Waser 2002; Folke et al. 2004). Ultimately, long-term monitoring will be required to measure these changes and identify potential shifts in the network that threaten particular plant species. Competition experiments would also be useful to assess the impact of Apis mellifera on native populations; both in the presence and absence of non-indigenous flowering species.
Acknowledgements
Thank you to Karen Stott for identifying the many flower species, Nancy Cunningham and Graham Lyons for editing that paper, and the many volunteers that helped collect data. This project was supported by the Long Term Ecological Research Network (LTERN), the Australian Research Council and the Field Naturalists of Victoria. Bees were collected under Department of Environment and Primary Industries permit 10006611.




