Endocannabinoid contributions to alcohol habits and motivation: Relevance to treatment
Abstract
Individuals with alcohol use disorder exhibit compulsive habitual behaviors that are thought to be, in part, a consequence of chronic and persistent use of alcohol. The endocannabinoid system plays a critical role in habit learning and in ethanol self-administration, but the role of this neuromodulatory system in the expression of habitual alcohol seeking is unknown. Here, we investigated the role of the endocannabinoid system in established alcohol habits using contingency degradation in male C57BL/6 mice. We found that administration of the novel diacyl glycerol lipase inhibitor DO34, which decreases the biosynthesis of the endocannabinoid 2-arachidonoyl glycerol (2-AG), reduced habitual responding for ethanol and ethanol approach behaviors. Moreover, administration of the endocannabinoid transport inhibitor AM404 or the cannabinoid receptor type 1 antagonist AM251 produced similar reductions in habitual responding for ethanol and ethanol approach behaviors. Notably, AM404 was also able to reduce ethanol seeking and consumption in mice that were insensitive to lithium chloride-induced devaluation of ethanol. Conversely, administration of JZL184, a monoacyl glycerol lipase inhibitor that increases levels of 2-AG, increased motivation to respond for ethanol on a progressive ratio schedule of reinforcement. These results demonstrate an important role for endocannabinoid signaling in the motivation to seek ethanol, in ethanol-motivated habits, and suggest that pharmacological manipulations of endocannabinoid signaling could be effective therapeutics for treating alcohol use disorder.
1 INTRODUCTION
Alcohol use disorder (AUD) is characterized by the inability to restrict alcohol drinking despite the persistent desire, yet unsuccessful efforts, to cut down or control alcohol use. Habits are defined as actions that happen automatically in response to antecedent environmental stimuli and are insensitive to changes in the desirability of the outcome. Difficulties in abstaining from alcohol—despite persistent desire to do so—can be thought of as habitual because drinking behavior is insensitive to changes in the desirability of alcohol for individuals with AUD. Accordingly, a transition to habitual responding for alcohol is hypothesized to contribute to the compulsive patterns of alcohol-seeking and consumption that are characteristic of AUD.1-3 Moreover, people with AUD show an overreliance on habitual responding in laboratory tasks,4-6 and this strong habitual behavioral control in general is hypothesized to extend to alcohol-motivated behavior. If strong habitual control over alcohol consumption is a core pathological component of AUD, then pharmacological compounds that reduce the expression of well-established alcohol (i.e., ethanol) habits in mice may have translational relevance to mitigate compulsive alcohol seeking in AUD.
Habitual behavior depends on the functioning of the dorsolateral striatum and connected cortico-basal ganglia networks,7, 8 which are highly conserved across species.9, 10 Robust expression of presynaptic cannabinoid receptor type 1 (CB1) in the dorsolateral striatum contributes to forms of short-term and long-term plasticity.11, 12 It is hypothesized that these forms of plasticity are important for habit formation.13 Indeed, transgenic animals with CB1 receptors knocked out globally or within specific nodes of the dorsal striatal network have impaired habit formation.14, 15 Chronic exposure to ethanol alters plasticity in the dorsolateral striatal network, strengthening its control over behavior,16-18 which may promote habitual control over alcohol seeking. Therefore, the CB1 receptor dysregulation that has been observed in alcohol-dependent individuals19, 20 may be a key mechanism by which habitual alcohol use persists in AUD.
Support for this hypothesis comes from evidence demonstrating that pharmacological manipulations of CB1 receptors bidirectionally alter ethanol self-administration: CB1 agonists increase, whereas CB1 receptor antagonists decrease, self-administration of ethanol, and motivation for ethanol.21 The two most studied endocannabinoid ligands are anandamide and 2-arachidonoyl glycerol (2-AG).22 There is evidence indicating that 2-AG is released following administration of ethanol, and that the magnitude of 2-AG release is higher for self-administered than experimenter-administered ethanol.23, 24 Moreover, the magnitude of 2-AG release is correlated with the amount of ethanol consumed in self-administration.24 This suggests that 2-AG release may, in part, encode the motivation to consume ethanol. Although it is currently unknown which endocannabinoid ligand contributes to the necessity of CB1 receptors to habit formation, it is likely that heightened 2-AG signaling at the CB1 receptor contributes to the plasticity underlying the formation of habitual response strategies for ethanol.
Here, we investigated the role for the endocannabinoid system in the expression of habitual responding for ethanol in mice. Using an extended ethanol self-administration habit model, we tested the novel compound, DO34, that degrades the primary enzyme that synthesizes 2-AG.25, 26 We observed a reduction in habitual responding for ethanol and ethanol approach behavior using a contingency degradation paradigm. Furthermore, we observed similar reductions in habitual responding for ethanol following administration of a CB1 receptor antagonist, AM251, and following administration of the putative endocannabinoid transport inhibitor AM404.27-29 We observed no effect of pharmacologically increasing anandamide or 2-AG on habitual responding for ethanol. Consequently, we hypothesized that reduced habitual responding for ethanol was likely due to a reduction in CB1 receptor-mediated signaling via a reduction in 2-AG release. Because these drug manipulations reduced both habitual ethanol seeking responses and approach behaviors for ethanol, the release of 2-AG may encode the motivation to seek and consume ethanol. Indeed, we report that administration of JZL184, an inhibitor of the metabolic enzyme for 2-AG, dose-dependently increased motivation for ethanol using a progressive ratio schedule of reinforcement. Together, our data provide new evidence that 2-AG release may contribute to the motivation for ethanol, and that pharmacological manipulations to reduce 2-AG release or moderate activation of the CB1 receptor can decrease habitual ethanol seeking and approach behavior.
2 MATERIALS AND METHODS
2.1 Animals
Adult male C57BL/6N mice (Charles River Laboratories; Wilmington, MA) were used for all experiments. Males were selected because they are known to form ethanol habits at a faster rate than female mice.30 Mice were delivered at 7 to 8 weeks of age, group-housed in standard cages on ventilation racks (Tecniplast; West Chester, PA) within a climate-controlled vivarium, maintained on a 12-hour light/dark cycle (lights on at 0700 hours). Following 7 days of acclimation to the vivarium, access to food was restricted, and mice were maintained at 85% to 90% of free-feeding body weight for the duration of the experiment. Mice were fed 2.0 to 3.0 g of standard rodent chow (2918 Teklad diet, Envigo, Huntingdon, United Kingdom) per mouse per day. Water was available ad libitum in the home cage but was removed prior to behavioral testing. Mice were fed for 15 minutes without water available before behavioral sessions to induce thirst. Before beginning instrumental training, mice were exposed to ad libitum 10% ethanol, 0.1% saccharin solution in their home cage for 2 days in 1-hour sessions. Intake was measured to ensure that all animals consumed ethanol and to reduce neophobia to the ethanol solution. All behavioral procedures were approved by the Yale University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources.
2.1.1 Drugs
The diacyl glycerol lipase inhibitor DO34 and the comparison compound DO53 were synthesized as previously described.25, 26 Lithium chloride (Sigma-Aldrich [St. Louis, MO]) was dissolved at 0.15 M in saline and injected at 40 mL/kg.30
All endocannabinoid drugs were dissolved in 5% DMSO, 15% Tween 80 in sterile physiological saline, except DO34 and DO53, which were dissolved in 5% DMSO 15% kolliphor in saline. All endocannabinoid drugs were administered via intraperitoneal injection at 10 mL/kg. DO34 and DO53 were administered at 50 mg/kg because they have previously been shown to decrease food intake at these doses.25 AM404 (R&D Systems [Minneapolis, MN] and Fisher Scientific [Waltham, MA]) was administered at 10 mg/kg, a dose reported to decrease presumably goal-directed ethanol self-administration.31 AM251 (Fisher Scientific) was administered at 1 mg/kg based on our previous work demonstrating reduced habitual food seeking at this dose.32 JZL184 (Sigma-Aldrich and Cayman Chemical [Ann Arbor, MI]) was administered at 2, 8, and 18 mg/kg based on previously reported effects on habitual food seeking (2 and 18 mg/kg)32 and anxiety-like behavior (8 mg/kg).33 URB597 (Sigma-Aldrich) was administered at 0.5 mg/kg.34
2.1.2 Behavioral training
Behavioral apparatus and training
Behavior was assessed in standard operant conditioning chambers located within sound-attenuated boxes (Med Associates; St. Albans, VT), which were identical to those described previously.30 Each chamber was equipped with three nosepoke apertures located on the back wall, with a magazine positioned in the center of the front wall. Nosepoke apertures and magazine were each equipped with a light and with a photobeam sensor to record entries. A fan provided ventilation and background noise throughout the behavioral sessions. Liquid reinforcers (10% ethanol v/v, 0.1% saccharin) were presented using a dipper arm that delivered a 10-μL cup of the reinforcer into the magazine. When the dipper arm was raised, magazine entries were recorded as a proxy for ethanol consumption—these entries are referred to as “incentivized entries.” Total magazine entries are reported to measure ethanol approach.
Mice underwent 2 days of magazine training to habituate to the operant chambers and to learn the association between ethanol delivery and the magazine. Sixty seconds after the start of the behavioral session, the dipper of ethanol reinforcer became available. The dipper remained available until the mouse entered the magazine and retracted after 10 seconds. Following a 60-second intertrial interval, the dipper became available for 10 seconds after a magazine entry, and this was repeated throughout the 40-minute session.
Following magazine training, mice underwent operant training. One nosepoke aperture, either the left or the right, was assigned to deliver reward (referred to as “active”), and the other two apertures had no programmed consequence (referred to as “inactive”). Assignment of active aperture was counterbalanced across mice and maintained throughout the duration of the experiment. Training sessions began with the illumination of the active nosepoke and ended with the light extinguishing.
Active aperture entries were initially reinforced using a fixed ratio 1 (FR1) schedule, where each entry resulted in delivery of a single reinforcer. These FR1 sessions began with one free dipper that remained available until the mouse entered the magazine and retracted 10 seconds after. Subsequent reinforcers were earned with active responses. Dippers earned during FR1 training sessions remained available until the mouse made an entry into the magazine and then retracted 10 seconds later. FR1 sessions terminated when mice earned 60 dippers, or 45 min elapsed, whichever occurred first. Once individual mice earned 13 dippers of ethanol solution in a single FR1 session (5-13 days), they were then trained on a variable interval (VI) schedule of reinforcement. Mice that required 6 or more days to achieve the performance criterion underwent remedial FR1 sessions that were extended to a duration of 2 hours. Mice that took >13 days to reach FR1 criteria were excluded (n = 19 excluded out of n = 111 for all experiments).
Once mice achieved the performance criterion on the FR1 schedule, VI training began. VI schedules were determined by randomly selecting the duration of the interval from an exponential array32 that had an average of 30 seconds for the VI30 schedule and an average of 60 seconds for the VI60 schedule. The first active nosepoke response made after the interval elapsed resulted in delivery of the dipper full of ethanol solution. During VI sessions, dippers were available for 10 seconds following the active response, regardless of whether the mouse entered the magazine. The duration of the next interval was then randomly selected from the exponential array. This continued for the duration of the 45-minute session. Mice received 3 days of VI30 training and were subsequently trained on a VI60 schedule for at least 21 days.
Contingency degradation test
Contingency degradation tests were conducted as previously described.32, 35 Briefly, these sessions appeared similar to the operant training session, but dippers of ethanol were noncontingently delivered. Active aperture responses had no programmed consequence. Dippers were delivered at equal intervals, matching the total number of ethanol reinforcers mice earned the day prior. Sessions terminated when 45 minutes had elapsed.
For most experiments, the first contingency degradation test was administered following 21 days of VI60 training to confirm that responding was habitual (see statistical analysis below) prior to any pharmacological intervention. Following each test, mice received additional VI60 sessions to stabilize response rates before additional testing. Cohorts that were not habitual on their first contingency degradation test received additional VI60 training (3-10 days), until habitual responding was confirmed.
Individual logistic regression analysis
We applied a novel statistical technique for classifying individual animals' pattern of responding as goal-directed or habitual.32 Response rate for each mouse during the contingency degradation session was compared with that mouse's response rate for all previous VI60 sessions using a generalized linear model (GLM) with a Poisson distribution, which is the most appropriate distribution for count data. A session-type regressor (ie, VI60 session coded as 0, test coded as 1) and a linear covariate regressor were included. Mice with significant negative regression coefficients for the session-type regressor were classified as goal-directed, because a significant negative regression coefficient indicates that the decrease in responding during the test session was beyond the normal range of variability in response rates on VI60 sessions for that animal. In contrast, mice with a nonsignificant or positive coefficient for the session-type regressor were classified as habitual, because a nonsignificant or positive coefficient indicates that responding had not substantially decreased during the test session. Only mice confirmed to be habitual were included in the pharmacological studies.
Pharmacological studies
Following confirmation of habitual responding and re-establishment of stable responding on the VI60 schedule (2-8 days), the pharmacological studies began. Vehicle was administered to all mice prior to the VI60 session on the day immediately before the contingency degradation tests with pharmacological manipulations. This served as the nondegraded operant session (referred to as “Baseline”). If vehicle administration drastically reduced the number of rewards earned compared with previous VI60 sessions, mice underwent additional training on the VI60 schedule (2-14 days) until rewards earned in the Baseline were approximately equivalent to previous sessions. The day after completing the Baseline session, drug was administered and responding was assessed in a contingency degradation test. AM404, AM251, and the vehicle conditions for those experiments were administered 30 minutes prior to the session as reported previously.31 JZL184, URB597, DO34, DO53, and their respective vehicle conditions were administered 2 hours prior to the behavioral session as reported previously.25, 33 Drug order was counterbalanced with a Latin square for all experiments, except for the DO34 experiment, where vehicle or DO34 were given in a counterbalanced order, with the control compound DO53 administered as the final condition for all animals. This design was used to avoid any potential carryover effects from previous drug administration that might impact responding following administration of DO34.
Lithium chloride devaluation experiment
Habitual behavior is defined by a loss of representation of the action-outcome relationship, which can be tested for by contingency degradation, and by a loss of sensitivity to the value of the outcome, which can be tested for by reinforcer devaluation. Following assessment of effects of AM404 on habitual ethanol seeking during contingency degradation, habitual responding was further assessed using lithium chloride (LiCl) devaluation as an additional test for habitual behavior. Following completion of counterbalanced contingency degradation tests with vehicle and AM404, mice were assigned to groups (devalued or valued) balanced according to prior response rates during the contingency degradation test following administration of AM404. The lithium chloride devaluation procedures have been described elsewhere.30 Mice were fed at least 3.5 hours prior to being placed in a novel cage for a 30-minute exposure to ad libitum 10% ethanol, 0.1% saccharin solution. Immediately following removal from the cage, devalued group mice were injected with LiCl, and the nondevalued group with saline. Three hours after the ethanol exposure, mice received a crossover injection in order to counterbalance the experience of malaise. These devaluation procedures were repeated for 3 days. Devaluation was assessed the following day with a habitual responding test and a conditioned taste aversion test. First, in the habitual responding test, mice were returned to the operant chamber for a 5-minute session in extinction, where the active nosepoke was illuminated, but had no programmed response. Conditioned taste aversion was confirmed directly after the habitual responding test, in a novel cage with access to ad libitum 10% ethanol, 0.1% saccharin solution for 30 minutes.
Progressive ratio experiment
We hypothesize that 2-AG release contributes to the motivation for ethanol, so to directly test this hypothesis we examined whether increasing 2-AG levels with JZL184 administration prior to a progressive ratio paradigm would increase motivation. A separate cohort of mice was trained on the progressive ratio schedule of reinforcement after achieving the FR1 performance criterion described above. This ratio schedule followed the formula: response ratio = (5*e0.2*dipper)-5, rounded up to the nearest integer as described previously (e.g., 36). This task was modified for ethanol reinforcers by changing the session timeout to 504 seconds from the last active response, which was three times the average inter-reinforcer interval during FR1 self-administration.37 The last ratio that the mouse completed is referred to as the “breakpoint” and is thought to be an index of the motivation for the reinforcer. Because breakpoint is not a continuous measure, we report rewards earned, which is proportional to the log-transformed breakpoint.37 Mice underwent several days on the progressive ratio schedule until they achieved the same breakpoint on two sequential days. Animals that took >10 days to achieve a stable breakpoint were excluded from the experiment (n = 5). The remaining animals (n = 12) took 5 to 10 days to stabilize for the first time on the progressive ratio schedule of reinforcement, and there were 2 to 9 days between drug conditions. Data from each progressive ratio session following drug administration were compared with the drug-free progressive ratio session from the previous day, referred to as “Baseline,” and to data from the vehicle treatment session.
2.2 Statistical analysis
Data were analyzed using Prism 7 (Graphpad, San Diego, CA), SPSS 21 (IBM, Armonk, NY), and MATLAB 2016b (MathWorks, Natick, MA). Data are presented as the mean ± standard error of the mean (SEM), unless otherwise specified. Our threshold for significance was set at P < .05. Response rates, total magazine entries, and incentivized entries were analyzed using repeated measures GLM with a Poisson distribution. Regression coefficients were tested with Wald χ2 to determine if they were significantly different from zero. Significant interactions were followed up using post hoc, pairwise comparisons with Sidak adjustment for multiple comparisons.
3 RESULTS
3.1 Measuring the impact of 2-AG release on habitual responding
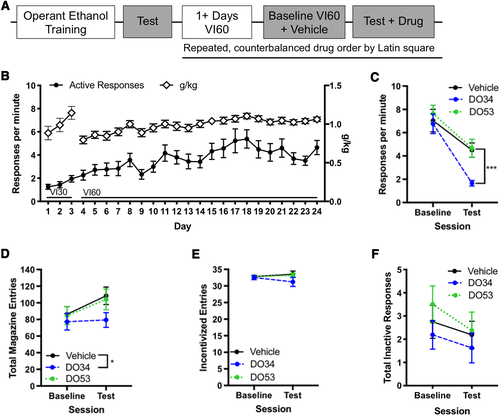
2-AG levels have been reported to increase after ethanol administration,23, 24 so we sought to determine if inhibiting 2-AG release reduced habitual responding for ethanol. We examined the effect of the novel compound DO34, which is an inhibitor of diacyl glycerol lipases (DAGLα and DAGLβ), the enzymes that synthesize 2-AG, on habitual responding for ethanol. Because DO34 is not completely selective for DAGLs, but also inhibits ABHD6, we compared the effect of DO34 to a control compound, DO53, which shares the same off-target activity as DO34 but does not inhibit DAGLs.25, 26 The experimental timeline for measuring the impact of DO34 on habitual responding for ethanol is presented in Figure 1A. Active response rates increased across VI30 and VI60 sessions (Figure 1B). We then conducted a contingency degradation test and classified mice (n = 16) as goal-directed or habitual based on our individual regression analyses, ensuring all mice were habitual prior to pharmacological testing. DO34 significantly reduced response rates in the contingency degradation test compared with vehicle-administration (Figure 1C; P < .001), whereas the control compound DO53 was not different from vehicle administration (P = .6). During the DO34 condition, mice made significantly fewer magazine entries (Figure 1D; main effect of DO34: χ2 = 10.9, P = .001), but magazine entries following administration of the control compound DO53 were not significantly different from vehicle (main effect of DO53: χ2 = 0.3, P = .6). No effects of drug were observed for incentivized entries (Figure 1E) or inactive responses (Figure 1F).
3.2 Impact of endocannabinoid drugs on habitual behavior
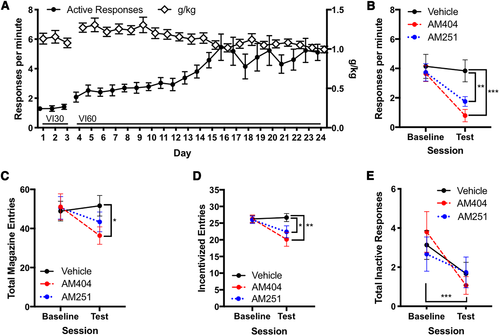
Next, we tested the hypothesis that increasing endocannabinoid concentrations and subsequent CB1 receptor-mediated signaling induces ethanol self-administration by testing the endocannabinoid transport inhibitor AM404 and the CB1 receptor antagonist AM251. A naïve cohort of mice (n = 15) underwent habit training (Figure 2A) and was confirmed to be habitual. Then, mice underwent contingency degradation testing following administration of AM251, AM404, or vehicle. AM404 administration reduced responding in the test compared with vehicle (Figure 2B; P < .001), as did AM251 (P = .006). Responding following AM404 and AM251 administration were not significantly different from each other (P = .09). AM404 reduced total magazine entries compared with vehicle (Figure 2C; P = .03), whereas AM251 administration did not differ from vehicle (P = .31) or AM404 (P = .6). Both AM404 (Figure 2D; P = .008) and AM251 (P = .04) reduced incentivized entries compared with vehicle treatment but were not significantly different from each other (P = .6). Inactive responses decreased from an average of 3.2 ± 0.5 SEM per session during the baseline to an average of 1.5 ± 0.4 SEM per session during the contingency degradation test (Figure 2E; main effect of session: χ2 = 17.98, P < .001), but no effects of drugs or interactions were observed for inactive responses.
3.3 AM404 reduces habitual responding that is insensitive to devaluation
As AM404 robustly reduced habitual ethanol seeking and consumption in our contingency degradation paradigm, we next sought to confirm these findings using a second type of test for habit: reinforcer devaluation via LiCl pairing. A naïve cohort of mice (n = 23) first underwent habit training (Figure 3A) and were confirmed to be habitual by contingency degradation and then underwent contingency degradation testing in the presence of AM404 or vehicle. Consistent with our previous experiment, AM404-administration significantly reduced habitual responding in the contingency degradation test compared with the vehicle condition (Figure 3B; χ2 = 103.6, P < .001). Administration of AM404 significantly reduced total magazine entries during contingency degradation compared with that following vehicle (Figure 3C; χ2 = 19.8, P < .001). Mice made fewer incentivized entries following AM404-treatment compared with vehicle treatment (Figure 3D; χ2 = 32.9, P < .001). There was a significant reduction in inactive responses following administration of AM404 (Figure 3E; P < .001), from an average of 1.17 ± 0.3 SEM inactive responses per session in baseline, to an average of 0.39 ± 0.2 SEM inactive responses per session during test with AM404. In summary, AM404 reduced habitual responding for ethanol, with a corresponding decrease in consumption of ethanol and ethanol-approach behavior, thereby replicating the previous behavioral results with AM404.
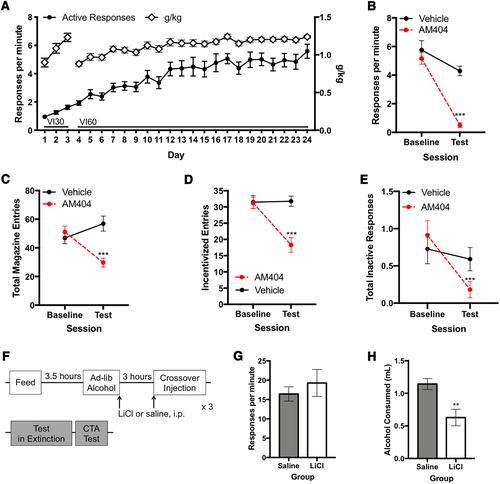
To confirm habitual responding in these mice with a secondary assessment of habit, the mice were then divided into two conditions matched on response rates during contingency degradation following AM404 (valued vs devalued group: χ2 = 0.002, P = .96). Mice underwent 3 days of devaluation (pairing LiCl with exposure to the ethanol solution) or a control procedure (pairing saline with exposure to the ethanol solution, see timeline Figure 3F), followed by an operant habit test and a conditioned taste aversion test. There was no difference in response rates between saline-paired and LiCl-paired animals during the habitual responding test (Figure 3G; main effect of drug: χ2 = 0.6, P = .4). However, LiCl-paired animals developed a conditioned taste aversion for ethanol: LiCl-paired animals drank less ethanol than the saline-paired ones (Figure 3H; t12 = 3.4, P = .006), indicating devaluation procedures were successful. Thus, the responding for ethanol (Figure 3G) was decoupled from the value of the ethanol, confirming that the responses were habitual. These data indicate that responding for ethanol in the contingency degradation was insensitive to reinforcer devaluation yet was reduced following AM404 administration in the contingency degradation test.
3.4 Measuring the impact of increased 2-AG and anandamide on habitual responding
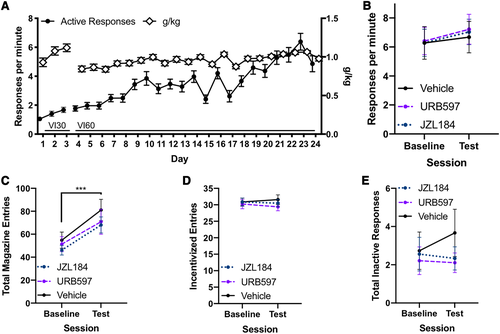
In order to better understand the mechanism by which AM404 produces its effects on responding, we tested whether increasing 2-AG or anandamide levels would produce similar reductions in habitual responding. We tested JZL184, a drug that degrades the metabolic enzyme for 2-AG resulting in increased 2-AG, and URB597, a drug that degrades the metabolic enzyme for anandamide resulting in increased anandamide, on habitual responding. We chose the 2 mg/kg dose of JZL184 because previously we reported that this dose partially blocked the effect of AM404 on habitual food responding in mice.32 A naïve cohort of mice (n = 26) underwent habit training (Figure 4A), were confirmed to be habitual, and then administered JZL184, URB597, and vehicle in a counterbalanced manner. There were no effects of either drug on response rate (Figure 4B; P = .955), magazine entries (Figure 4C; P = .11), incentivized entries (Figure 4D; P = .6), or inactive responses (Figure 4E; P = .3).
3.5 Measuring the impact of 2-AG release on progressive ratio responding
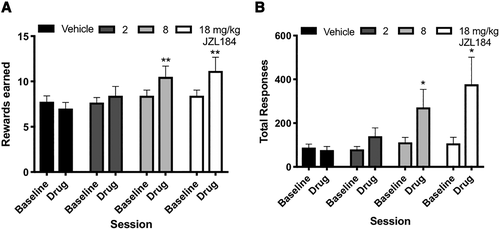
Because AM404, AM251, and DO34 reduced approach behaviors along with ethanol-seeking responses, we hypothesized that 2-AG may mediate motivation to seek ethanol. One behavioral metric for quantifying motivation is measuring responding using a progressive ratio schedule of reinforcement, where the effort required to earn a single reinforcer progressively increases across a session. To determine if increasing 2-AG increased motivation for ethanol, we trained a naïve cohort of mice (n = 12) on a progressive ratio schedule of reinforcement, and administered, in a counterbalanced order, vehicle and three doses of JZL184. The rewards earned following 2 mg/kg JZL184 were not different from vehicle treatment (Figure 5A; χ2 = 3.6, P = .2). The 8 and 18 mg/kg doses of JZL184 increased rewards earned compared with vehicle treatment (8 mg/kg: χ2 = 8.8, P = .009; 18 mg/kg: χ2 = 10.9, P = .003). The 2 mg/kg dose of JZL184 did not significantly alter response rate on progressive ratio compared with vehicle treatment (Figure 5B; χ2 = 3.7, P = .2), but both 8 mg/kg (χ2 = 6.3, P = .04) and 18 mg/kg (χ2 = 6.7, P = .03) doses of JZL184 increased total responses made compared with vehicle treatment.
4 DISCUSSION
These data provide novel evidence for the role for 2-AG in regulating the expression of habitual ethanol seeking, as well as in the motivation to obtain ethanol. We combined the innovative application of statistical methods with advanced behavioral analyses and pharmacology to provide empirical support that endocannabinoid-mediated CB1 receptor signaling is necessary for the expression of habitual ethanol seeking. This is the first study, to our knowledge, to examine effects of DO34, a novel DAGLs inhibitor, on ethanol-motivated behavior. These data provide insights into the physiology of habitual ethanol seeking in mice and may provide a novel therapeutic mechanism for research aimed at the development of treatments for AUD.
Here, we show that the endocannabinoid transport inhibitor AM404 results in a robust and replicable reduction in habitual ethanol seeking and ethanol approach behavior. This finding extends previous results demonstrating that AM404 decreases ethanol self-administration on an FR schedule of reinforcement that was presumably goal directed because this schedule of reinforcement is unlikely to produce habitual responding.31 Previous work from our lab has shown that this dose of AM404 does not affect locomotor activity.32 Critically, we found that AM404 was able to reduce habitual ethanol seeking and incentivized entries for ethanol. Our data showing that AM404 could attenuate ethanol habits also were replicated in a second cohort of mice that were shown to be insensitive to LiCl devaluation of ethanol, which confirms that the ethanol-seeking behavior was no longer tied to the value of the ethanol. These results may suggest a high translational value for the effect of AM404 because the compulsive, habitual alcohol seeking that is observed in AUD is hypothesized to result from drinking that is no longer tied to the value of the alcohol.
The mechanism of action of AM404 in alcohol self-administration models is currently unknown. Previously, it has been shown that the mechanism of AM404-induced reductions in ethanol self-administration was not due to indirect activation of the cannabinoid CB1, CB2, nor the transient receptor potential vanilloid-1 (TRPV1) receptors.31 This suggests that AM404 does not increase endocannabinoid levels in the synapse within the context of ethanol self-administration. This possibility aligns with our observed lack of effect of administration of URB597 or low-dose JZL184 on ethanol habit—if AM404 was acting by increasing these endocannabinoids, we would have observed a similar reduction in habitual responding. Previously, it has been found that AM404 can prevent the release of endocannabinoids under certain conditions via inhibition of the putative endocannabinoid transporter.38-40 Given that ethanol self-administration does increase 2-AG release,24 we tested the hypothesis that a reduction in synaptic release of 2-AG and subsequent reduced CB1 receptor-mediated signaling may reduce ethanol self-administration. Consistent with this hypothesis, we report that inhibition of 2-AG biosynthesis with DO34 and antagonism of the CB1 receptor with AM251 potently reduced habitual ethanol seeking. Thus, in light of these findings, we suggest that the ability of AM404 to reduce habitual ethanol-seeking behavior may be due to inhibition of 2-AG release from the postsynaptic site, thereby mimicking the effect of DO34 and AM251. However, we cannot rule out the possibility that AM404 could be signaling at other targets such as the TRPV1 receptor.41 Intriguingly, our previous work has demonstrated that AM404 reduced habitual food seeking, yet this effect was abrogated with pretreatment of with a high dose of JZL184,32 suggesting bidirectional modulation of habitual responding by endocannabinoids. More extensive characterization of potential dose-dependent effects of anandamide and 2-AG signaling are warranted.
Of note, previous studies using in vivo microdialysis have reported a potentiation of ethanol-induced 2-AG release following AM404 administration.23 There are several differences in experimental design that may explain the disparity between this study and our data. First, ethanol was administered by the experimenter, rather than self-administered as in our studies, and there is evidence that route of ethanol administration has divergent effects on 2-AG.24 Second, 2-AG release was assessed in a new context, whereas our experiments took place in the same context as ethanol self-administration. Alcohol-associated contextual cues are sufficient to produce habitual responding42 and are therefore important to consider. Additionally, Alvarez-Jaimes and colleagues23 used a model of ethanol physical dependence prior to the microdialysis measurements, whereas our operant model may not create such physical dependence. Furthermore, if AM404 potentiated the 2-AG release in our experiment, this result would be in direct conflict with several of our other findings for the following reasons: if higher levels of synaptic 2-AG were responsible for the reductions in habitual ethanol seeking and approach observed with AM404, then we would expect DO34 (which inhibits the enzymes that synthesize 2-AG) to increase habitual responding for ethanol. DO34, however, reduced habitual responding. Moreover, if AM404 increased synaptic levels of 2-AG, we would expect that JZL184 (which inhibits the enzymes that metabolize 2-AG) would decrease motivation for ethanol; however, JZL184 increased motivation for ethanol as assessed by progressive ratio responding. Collectively, our results are more consistent with the hypothesis that AM404 blocked the release of 2-AG, which reduced the motivation to seek and drink ethanol by reducing signaling through the CB1 receptor. Nevertheless, this hypothesis warrants further investigation.
Our results provide support for pharmacological strategies to reduce pathological habitual behaviors in AUD that may benefit from moderating the influence of 2-AG release. Clinical studies assessing treatment efficacy of CB1 receptor antagonists for reducing alcohol consumption or preventing relapse have not reached statistical significance, perhaps due to the incomplete occupancy of the CB1 receptor at the moderate dose administered in the studies.43, 44 Unfortunately, higher doses of the CB1 antagonist could not be tested due to negative on-target psychiatric side effects.43 It is possible that drugs that inhibit the putative endocannabinoid transporter might have a more favorable risk-to-benefit profile than CB1 receptor antagonists. Indeed, AM404 does not change synaptic levels of endocannabinoids on its own45 but instead inhibits the release or clearance of endocannabinoids. AM404 may hold particular promise in treating AUD because it is an active metabolite of acetaminophen/paracetamol46 and, thus, could potentially be used clinically.
The effects observed with JZL184 and DO34 are particularly important for understanding the role for 2-AG in ethanol-motivated behavior. DO34, but not DO53, reduced habitual ethanol seeking and approach behavior, which demonstrates the necessity of 2-AG for the expression of ethanol habits. Previous work demonstrated that both DO34 and DO53 were able to reduce food intake and alter metabolism,25 which suggests that, in contrast to food, the effect of DO34 on ethanol-motivated behavior may be a 2-AG specific effect. Increasing 2-AG levels with JZL184 increased breakpoint for ethanol in our study, and previously similar results were found with progressive ratio for food,47 suggesting that 2-AG may contribute to the motivation to work for caloric reinforcers in general. Furthermore, a recent study demonstrated that stress-induced increases in 2-AG signaling within the prelimbic cortex promoted cocaine seeking behavior,48 suggesting the possibility that 2-AG signaling is involved in hedonic reinforcement aside from caloric reinforcers as well. Our progressive ratio data with JZL184 may complicate MAGL inhibition as an anxiolytic target, because alcohol use disorder or other substance use disorders are often comorbid with anxiety disorders, particularly posttraumatic stress disorder.49, 50 It would be problematic if administration of a MAGL inhibitor alleviated the anxiety disorder, but worsened the AUD. A recent study reported low doses (1 and 3 mg/kg) of JZL184 alleviated anxiety-related behaviors in ethanol-dependent mice.51 Our progressive ratio results may suggest caution for the use of higher doses of JZL184 for treatment of anxiety to prevent increasing motivation for ethanol; however, the low 2 mg/kg dose of JZL184 did not increase motivation for ethanol or alter the expression of ethanol seeking habits. Together, these results indicate a putative critical and selective role for 2-AG in habitual ethanol seeking.
The current study provides evidence for endocannabinoid signaling processes that mediate habitual alcohol seeking and consumption in mice. We propose that 2-AG release contributes to the motivation to seek ethanol and to the expression of ethanol habits, and argue that pharmacological approaches to moderating 2-AG signaling may be fruitful for the treatment of pathological habitual alcohol seeking characteristic of AUD.
ACKNOWLEDGEMENTS
The authors declare no conflict of interest. This study was supported by public health service grant AA012870 from the National Institute on Alcohol Abuse and Alcoholism. Additional support was provided by DA041480, DA043443 from the National Institute on Drug Addiction, a National Alliance for Research on Schizophrenia and Depression award, and the Charles B.G. Murphy Fund. This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
AUTHORS CONTRIBUTION
CAG, SMG, and JRT conceived of the studies. CAG and SLT performed the experiments and analyzed these data. MJ and MvdS synthesized DO34 and DO53. CAG, SMG, SLT, MJ, MvdS and JRT wrote the paper.




