Disruption of blood–brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model
Abstract
Inflammatory cytokines and reactive oxygen species are reported to be involved in blood–brain barrier (BBB) disruption. Because there is evidence that ethanol (EtOH) induces release of free radicals, cytokines and inflammatory mediators we examined BBB integrity and matrix metalloproteinase (MMP) activity in postmortem human alcoholic brain and investigated the role of TLR4 signaling in BBB permeability in TLR4-knockout mice under a binge-like EtOH drinking protocol.
Immunohistochemical studies showed reduced immunoreactivity of the basal lamina protein, collagen-IV and of the tight junction protein, claudin-5 in dorsolateral prefrontal cortex of alcoholics. There was also increased MMP-9 activity and expression of phosphorylated ERK1/2 and p-38. Greater number of CD45+ IR cells were observed associated with an enhanced neuroinflammatory response reflected by increased GFAP and Iba-1 immunostaining. To further explore effects of high EtOH consumption on BBB integrity we studied TLR4-knockout mice exposed to the drinking in the dark paradigm. Repetitive EtOH exposure in wild-type mice decreased hippocampal expression of laminin and collagen-IV and increased IgG immunoreactivity, indicating IgG extravasation. Western blot analysis also revealed increased MyD88 and p-ERK1/2 levels. None of these changes was observed in TLR4-knockout mice. Collectively, these findings indicate that chronic EtOH increases degradation of tight junctions and extracellular matrix in postmortem human brain and induces a neuroinflammatory response associated with activation of ERK1/2 and p-38 and greater MMP-9 activity. The EtOH-induced effects on BBB impairment are not evident in the hippocampus of TLR4-knockout mice, suggesting the involvement of TLR4 signaling in the underlying mechanism leading to BBB disruption in mice.
Introduction
The main function of the blood–brain barrier (BBB) is to maintain homeostasis of the brain and protect the CNS from endogenous or exogenous toxins within the circulation (Abbott et al., 2010). Drugs inducing BBB disruption may increase the concentration of CNS active drugs in the brain and may also favor the infiltration of pathological mediators from periphery making the CNS more vulnerable to damage. The primary anatomical substrate of the BBB is the cerebral microvascular endothelium, which, together with pericytes, astrocytes, neurons and the extracellular matrix constitute a neurovascular unit (Hawkins & Davis, 2005; Persidsky et al., 2006; Abdul-Muneer et al., 2015). The endothelial cells of the BBB are characterized by the presence of cell-to-cell tight junctions (TJ) and lack of fenestrations. Pericytes and endothelial cells are encircled by the basal lamina, a membrane composed of collagen-IV, laminin, fibronectin, heparin sulfate proteoglycans and other extracellular matrix proteins (Farkas & Luiten, 2001).
Abundant evidence shows that BBB structure and permeability are altered by a number of factors including increased levels of inflammatory cytokines (Shafel et al., 2007; Tian & Kyriakides, 2009), their downstream signaling mitogen-activated protein kinases (MAPKs) (Tai et al., 2010; Chu et al., 2014) and free radicals (Gasche et al., 2001; Katsu et al., 2010), all of which are increased by ethanol (EtOH) administration (Montoliu et al., 1994; Agoglia et al., 2015; Pascual et al., 2015).
It is well known that MAPKs regulate MMP-9 expression and activity (Kim & Choi, 2010; Urrutia et al., 2013) in such a way that an increase in MAPK phosphorylation increases MMP activity and facilitates BBB disruption (Ralay Ranaivo et al., 2012; Wang et al., 2014; Cai et al., 2015).
Neuropathological studies on brain tissue from alcohol dependent subjects reported that alcoholic brain shows neuroimmune activation reflected by an increase in the expression of proinflammatory cytokines and chemokines, microglial markers and inflammasome proteins (He & Crews, 2008; Zou & Crews, 2012). In addition, postmortem human alcoholic brains show an increase in the protein expression of TLR2, TLR3 and TLR4 in the orbitofrontal cortex which correlates with lifetime alcohol consumption (Crews et al., 2013). Concomitantly, an increase in the expression and immunoreactivity of high mobility group box 1 (HMGB1) was also observed. Similar results were obtained in the orbitofrontal cortex of mice treated for 10 days with a binge-drinking protocol of EtOH (Crews et al., 2013). The fact that EtOH increased the expression of IL-1β mRNA in brain slice culture and that this effect was blocked by a neutralizing HMGB1 antibody or by knockdown TLR4 suggest that EtOH-released HMGB1 contributes to EtOH induction of IL-1β through an activation of TLR4 (Crews et al., 2013).
To our knowledge, there is no study on the integrity of BBB in postmortem brain of alcoholic subjects. In cultured human brain microvascular endothelial cells (BMVEC) EtOH increases phosphorylation of myosin light chain, occludin and claudin-5 (Haorah et al., 2005). Cytoskeletal alterations and TJ changes result in a reduction of transendothelial electrical resistance, BBB impairment and increased leukocyte migration across the BBB (Haorah et al., 2005). These findings are associated with oxidative stress because of the generation of reactive oxygen species derived from EtOH metabolism (Haorah et al., 2005; Haorah et al., 2007; Shiu et al., 2007). In line with these observations it seems reasonable to propose that chronic EtOH intake produces changes in BBB permeability associated with neuroimmune activation.
The aim of the present study was to evaluate the integrity of the BBB in the dorsolateral prefrontal cortex (PFC) from postmortem human controls and alcoholics. In order to elucidate the involvement of TLR4 signaling, the structure and permeability of the BBB will also be studied in the brain of TLR4 knockout (TLR4-KO) and wild-type mice following a binge-like EtOH drinking protocol.
Materials and Methods
Animals, drug administration and experimental design
Adult male TLR4−/− mice (n = 11, TLR4-KO; C57BL/6 background kindly provided by Dr. S Akira, Osaka University, Suita, Japan) and C57BL/6 background mice (n = 12; WT; Harlan Iberica) weighing 20–30 g were used. Mice were maintained in conditions of constant temperature (21 ± 2°C) and a 12 h reverse lighting cycle (lights on: 21:00 h). Initially, animals were housed in groups of 4–6 with ad libitum access to food and water.
The paradigm ‘Drinking in the dark’ (DID) was used as a model of binge drinking (Rhodes et al., 2005). Following 10 days of group housing, mice were individually housed and habituated for 7 days to drinking water ad libitum from a 25-ml serological pipette fitted with a drinking spout. For 4 consecutive days, starting 3 h after lights-off, water was replaced by EtOH 20% (v/v), for 2 h during the first 3 days and for 4 h on the 4th day. Following three EtOH-free days, the pattern of EtOH exposure was repeated a further 3 times (a total of 4 cycles of DID), and mice were killed 24 h after the last EtOH exposure. Control mice were exposed to water at all times. This protocol of EtOH exposure led to plasma levels of EtOH of 135.2 ± 16 mg/ml immediately after the 4 h of EtOH exposure.
All experimental procedures were approved by the Animal Welfare Committee of the Universidad Complutense de Madrid (following European Union Directive 2010/63/EU).
Postmortem human samples
Human postmortem samples of dorsolateral PFC were obtained frozen and a subset of these (five control and four alcoholic subjects) as formalin-fixed paraffin embedded blocks from the New South Wales Tissue Resource Centre at the University of Sydney. Subject information was collected through personal interviews, next-of-kin interviews and medical records and is presented in Table 1. Alcoholic patients with a history of chronic (34 ± 3 years) alcohol dependence uncomplicated by liver cirrhosis and/or nutritional deficiencies were included. There was no differences in age (control = 57.1 ± 2.3, alcoholic = 51.6 ± 2.8, p = 0.15), postmortem interval (control = 31.5 ± 3.4 h, alcoholic = 42.8 ± 6.6 h, p = 0.10), brain pH (control = 6.7 ± 0.05, alcoholic = 6.6 ± 0.08, p = 0.46) and RIN (control = 7.1 ± 0.12, alcoholic = 7.1 ± 0.14, p = 0.79).
| Group | Sex | Age | PMI (h) | Brain pH | RIN | Cause of death | Began drinking | Drinking years |
|---|---|---|---|---|---|---|---|---|
| C | Male | 50 | 30 | 6.37 | 8 | Cardiac | — | — |
| C | Male | 55 | 12 | 6.39 | 6.7 | Cardiac | — | — |
| C | Male | 66 | 63 | 6.91 | 7.3 | Cardiac | — | — |
| C | Male | 50 | 40 | 6.87 | 6.8 | Cardiac | — | — |
| C | Male | 69 | 52 | 6.95 | 6.95 | Cardiac | — | — |
| C | Male | 67 | 25 | 6.7 | 7.3 | Cardiac | — | — |
| C | Male | 48 | 17 | 6.62 | 7.4 | Cardiac | — | — |
| C | Male | 59 | 28 | 6.77 | 7.5 | Cardiac | — | — |
| C | Male | 64 | 41 | 6.73 | 7.2 | Cardiac | — | — |
| C | Male | 47 | 27 | 6.66 | 6.5 | Cardiac | — | — |
| C | Male | 61 | 22 | 6.41 | 6.4 | Cardiac | — | — |
| C | Male | 40 | 27 | 6.79 | 6.7 |
Cardiac/ Respiratory |
— | — |
| C | Male | 61 | 30 | 6.69 | 7.7 | Cardiac | — | — |
| C | Male | 66 | 32 | 6.66 | 7.1 | Cardiac | — | — |
| C | Male | 53 | 26 | 6.36 | 7.2 | Cardiac | — | — |
| A | Male | 41 | 54 | 6.4 | 7.3 | Neurological | 25 | 16 |
| A | Male | 45 | 18.5 | 6.57 | 6.7 | Respiratory | 14 | 31 |
| A | Male | 65 | 72 | 6.88 | 7.2 | Stroke | 25 | 40 |
| A | Male | 61 | 59 | 6.57 | 6.9 | Cardiac | 16 | 45 |
| A | Male | 49 | 44 | 6.41 | 7.7 | Cardiac | 16 | 33 |
| A | Male | 49 | 16 | 6.19 | 6.8 | Cardiac | 14 | 35 |
| A | Male | 44 | 59 | 6.87 | 7.5 | Cardiac | 18 | 26 |
| A | Male | 60 | 28 | 6.48 | 7.1 | Infection | 17 | 43 |
| A | Male | 50 | 34.5 | 6.93 | 6.4 |
Respiratory/ toxicity |
16 | 34 |
- C: normal control; A: alcoholic; PMI: postmortem interval; RIN: RNA integrity number.
Gel zymography
Frozen samples were homogenized and lysed in NP-40 lysis buffer (150 mM NaCl, 50 mM Tris–HCl, 1% NP-40, pH 8.0) supplemented with 5% protease inhibitor cocktail (Sigma-Aldrich, Spain). Activities of MMP-9 and MMP-2 were assessed by gelatin zymography. Similar protein quantities were mixed with non-reducing Laemmli buffer for 15 min at 37°C and subjected to SDS-PAGE using 9% acrylamide gel containing 0.1% gelatin (Sigma-Aldrich). Gels were washed and incubated for 1 h at room temperature in modified enzymatic activation buffer (50 mM Tris–HCl, 6 mM CaCl2, 1.5-μM ZnCl2, pH 7.4) containing 2.5% Triton X-100. Gels were then incubated at 37°C for 24 h in buffer without Triton X-100. Gels were stained in Coomasie Brilliant Blue R-250 (Bio-Rad) and destained in a solution containing 40% methanol and 10% acetic acid and then placed in 10% acetic acid. Gels were dried and digitized for densitometric analysis.
Western blot
Frozen samples were homogenized and lysed as detailed above. The samples were boiled in Laemmli buffer, and proteins were resolved by 10% SDS-PAGE and transferred to PVDF membranes. Non-specific binding was blocked by incubation in TBS-buffer containing 0.1% Tween 20 and 5% skimmed milk. Membranes were incubated overnight at 4°C with the following primary antibodies: anti-MMP9 (Millipore, Spain, 1:1000), anti-tERK1/2 (Cell Signaling, USA, 1:1000), anti-pERK1/2 (Cell signaling, USA, 1:500 for human and 1:1000 for mouse), anti-tp38 (Cell signaling, USA, 1:1000), anti-p-p38 (Santa Cruz, USA, 1:500), anti-MyD88 (Abcam, UK, 1:800) followed by incubation for 2 h with the appropriate HRP-conjugated secondary antibodies: goat anti-rabbit IgG (Santa Cruz, USA; 1:2000), rabbit anti-goat IgG (Sigma-Aldrich, Spain; 1:5000) and goat anti-mouse IgG (Amersham GE, Spain; 1:5000). Band densities were normalized by referring it to their β-actin (Sigma-Aldrich, 1:1000) signal.
IgG extravasation
IgG leakage from serum into the brain was assessed in the CA1 subfield of the hippocampus as a marker of vasculature damage. Mice were anesthetized with sodium pentobarbital and perfused transcardially with 0.1 M PBS (pH 7.4) followed by 4% paraformaldehyde-PBS. Brains were removed, post-fixed in the same solution and cryoprotected by immersion in 30% sucrose-PBS at 4°C. The brains were sliced at 20 µm in the coronal plane through the hippocampus. Following washing with 0.1 M phosphate-buffered saline, sections were blocked by incubation with 0.5% BSA, 10% horse serum and 0.1% Triton X-100, incubated at 4°C overnight with the antibody Alexa Fluor® 594 donkey anti-mouse IgG (1:1000) and covered with ProLong®Gold (Life Technologies). Images were acquired with a Zeiss Axio Imager A1 microscope taking eight fields of 40× magnifications per animal and condition. Images were converted to gray scale, and blood vessels were outlined to provide an integrated gray scale value for image analysis with ImageJ Software (version 1.43; NIH, Maryland, USA). Animals from each of the treatments were processed together; investigator was blind to the experimental groups.
Immunohistochemistry
For human immunohistochemistry, formalin-fixed paraffin embedded samples of the PFC region were cut in 8-µm-thick sections. Sections were deparaffinized by incubating at 56°C overnight, then rehydrated in EtOH. To avoid lipofuscin granule autofluoresence and improve the specific signal-to noise ratio, sections were stained with 1% Sudan Black diluted in EtOH 70% for 5 min and then washed in 70% EtOH. Sections treated in this way but not incubated with primary or secondary antibodies were examined to confirm that no autofluorescence remained. Antigen unmasking was performed by incubating in citrate sodium solution (Vector Labs, USA) in a pressure cooker for 2 min. Non-specific binding was blocked by incubation in TBS-buffer containing 1% Triton and 10% BSA. Sections were then incubated at 4°C with primary antibodies: anti-Iba1 (Wako, Germany, 1:1000), anti-GFAP (BD Pharmingen, Spain, 1:500), anti-laminin (Sigma-Aldrich, 1:1000) anti-collagen-IV (Abcam, 1:250), anti-claudin-5 (Life Technologies, 1:500), anti-CD45 (BD Pharmingen, 1:500) and anti-CD31 (Santa Cruz, 1:500), followed by Alexa Fluor® 488 donkey anti-rabbit IgG (1:1000) or Alexa Fluor® 594 donkey anti-mouse IgG (1:1000).
For mice histological studies, free-floating sections containing the hippocampal CA1 subfield were blocked with 0.5% BSA, 10% normal horse serum and 0.1% Triton X-100 and incubated at 4°C with appropriate primary antibodies (anti-laminin, Sigma-Aldrich, 1:1000; anti-collagen-IV, Abcam, 1:250) followed by an Alexa Fluor® 488 donkey anti-rabbit IgG (1:1000).
Images were acquired sequentially on a Leica TCS-SP2AOBS confocal microscope (Leica Microsystems, Germany) taking 10 fields of 40× magnifications per subject and condition by an investigator blind to the experimental groups.
Statistical analysis
Data are presented as mean ± S.E.M. Data from human samples were analyzed using Student's t-test, data from mice samples were analyzed using two-way ANOVA followed by Bonferroni test and correlations were analyzed using the Pearson product–moment correlation coefficient (GraphPad Prism 5.0, GraphPad Software Inc., USA).
Results
Reduced claudin-5 and collagen-IV expression and increased CD45 positive cells in postmortem human alcoholic brain
By means of immunohistochemical studies we examined the effect of alcohol consumption on the immunoreactivity of the TJ protein claudin-5, the basal lamina proteins laminin and collagen-IV, an endothelial cell marker CD31 and the leukocyte marker CD45 (Fig. 1a–j). Figure 1f shows fluorescence images of representative claudin-5 immunostained sections of PFC. Quantitative image analysis by Student's t-test revealed a reduction in the immunoreactivity of claudin-5 in alcoholics compared with controls (Fig. 1a; F1,7 = 5.130, p = 0.0014). Figure 1g shows fluorescence images of representative laminin-immunostained sections of PFC. Quantitative images analysis by Student's t-test revealed no changes in the immunoreactivity of laminin in alcoholics compared with controls (Fig. 1b; F1,7 = 0.4929, p = 0.6372). Figure 1h shows fluorescence images of representative collagen-IV immunostained sections of PFC. Quantitative image analysis by Student's t-test revealed a reduction in the immunoreactivity of collagen-IV in alcoholics compared with controls (Fig. 1c; F1,7 = 3.259, p = 0.0139).
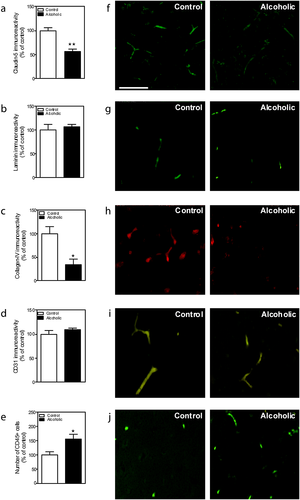
To ensure that the changes observed in claudin-5 immunoreactivity were not because of changes in the number of blood vessels, the effect of alcohol consumption on endothelial cells was determined by examining CD31 immunoreactivity. Figure 1i shows fluorescence images of representative CD31-immunostained sections of PFC. Quantitative image analysis by Student's t-test revealed no effect of alcohol on the immunoreactivity of CD31 (Fig. 1d; F1,6 = 1.070, p = 0.356).
Leukocyte infiltration was visualized and quantified by means of CD45 positive cells. Figure 1j shows fluorescence images of representative CD45 positive cells in immunostained sections of PFC. Quantitative image analysis by Student's t-test revealed an increase in the immunoreactivity of CD45 positive cells in alcoholics compared with controls (Fig. 1e; F1,7 = 3.186, p = 0.0154).
Increased expression and activity of MMP-9 in postmortem human alcoholic brain
Figure 2a,c illustrates a representative zymogram of the active forms of MMP-9 and MMP-2 and Western blot of the pro-active and active forms of MMP-9 in the PFC of control and alcoholic subjects. Gelatin zymography reveals a band at approximately 82 kDa corresponding to the active form of MMP-9 (Fig. 2a). Quantitative image analysis by Student's t-test revealed a significant effect of alcohol consumption on MMP-9 activity (Fig. 2b; F1, 19 = 2.535, p = 0.0202).
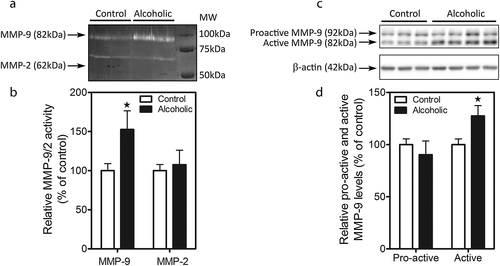
To study the possibility that augmented MMP-9 activity was because of increased enzyme expression, Western blot analysis was performed. Pro-active and active MMP-9 bands were detected at 92 and 82 kDa, respectively (Fig. 2c). Quantitative image analysis of each of the two bands by Student's t-test revealed a significant effect of alcohol consumption on the expression of active MMP-9 (Fig. 2d; F1,22 = 2.66, p = 0.0142).
Increased ERK1/2 and p38 phosphorylation in postmortem human alcoholic brain
We analyzed the activation of ERK1/2 and the stress kinase p38 which have been shown to play a role in alterations of the BBB. No differences in the expression of the total MAPKs were found between control and alcoholic subjects (Fig. 3a,b,e,f; for tERK1/2: F1,22 = 1.68, p = 0.1065 and for t-p38: F1,22 = 0.88, p = 0.3928). Interestingly, robust increases in phosphorylation of ERK1/2 (Fig. 3c,e) and p38 (Fig. 3d,f,) were detected in PFC lysates. Student's t-test indicated that there was a significant effect of alcohol consumption on p-ERK1/2 (F1,22 = 2.12, p = 0.0459) and p-p38 (F1,22 = 2.54, p = 0.0194) expression.
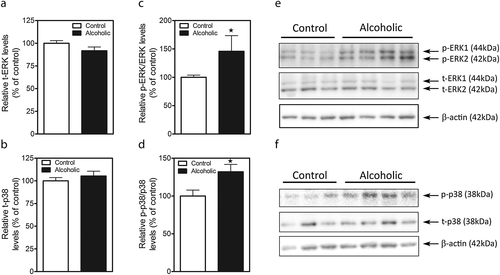
In addition, correlation analysis reveals a negative correlation between the age when subjects began drinking and p-ERK1/2 expression (Pearson r = −0.68, p < 0.05).
Microglia and astroglia activation in postmortem human alcoholic brain
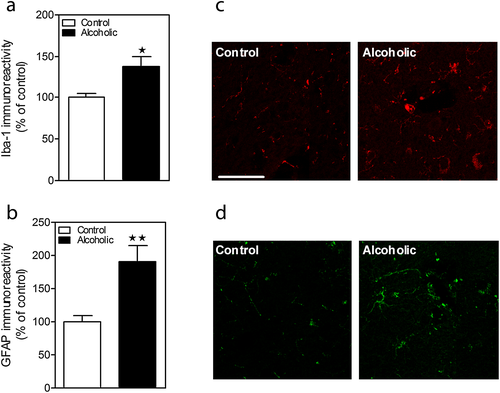
To examine the effect of alcohol consumption on glial activation, we analyzed microglial (Iba-1) and astrocytic (GFAP) markers. Figure 4a–d shows Iba-1 and GFAP immunostaining in PFC. Quantitative image analysis by Student's t-test revealed an increase in both Iba-1 (Fig. 4a–c; F1,7 = 3.397, p = 0.0115) and GFAP (Fig. 4b–d; F1,7 = 3.834, p = 0.0064) immunoreactivity.
Reduced laminin, collagen-IV and IgG immunoreactivity following binge-like EtOH consumption in CA1 of wild-type but not TLR4-KO mice
Figure 5d shows fluorescence images of representative laminin immunostained sections of CA1 24 h after binge EtOH. Quantitative image analysis by two-way ANOVA revealed a significant effect of EtOH (F1,18 = 5.96, p = 0.0252) but not of genetic deletion (F1,18 = 0.988, p = 0.333) nor interaction between the two factors (F1,18 = 1.392, p = 0.254) (Fig. 5a). Post-hoc analysis indicated that EtOH significantly reduced laminin immunostaining in CA1 (29%) in wild-type but not in TLR4-KO mice.
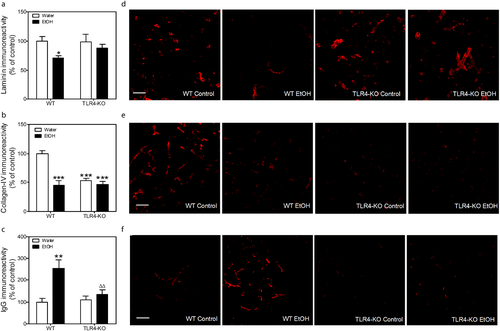
Figure 5e shows fluorescence images of representative collagen-IV immunostained sections of CA1 24 h after binge EtOH. Quantitative image analysis by two-way ANOVA revealed a significant effect of EtOH (F1,18 = 25,21, p < 0.0001), genetic deletion (F1,18 = 14.76, p = 0.0012) and an interaction between the factors (F1,18 = 15.89, p = 0.0009) (Fig. 5b). Post-hoc analysis indicated that EtOH significantly reduced collagen-IV immunostaining in CA1 (54%) in wild-type but not in TLR4-KO mice. In addition, TLR4-KO showed reduced collagen-IV immunoreactivity (47%) compared with wild-type mice.
BBB permeability was visualized and quantified by means of IgG immunostaining. IgG expression in the brain parenchyma is indicative of disruption of the BBB. Figure 5f shows fluorescence images of representative IgG immunostained sections of CA1 24 h after binge EtOH. Quantitative image analysis by two-way ANOVA revealed a significant effect of EtOH (F1,15 = 11.94, p = 0.0035), genetic deletion (F1,15 = 4.826, p = 0.0442) and an interaction between the two factors (F1,15 = 6.342, p = 0.0236) (Fig. 5c). Post-hoc analysis indicated that EtOH significantly increased IgG immunostaining in CA1 (155%) in wild-type but not in TLR4-KO mice.
Increase in MyD88 and p-ERK1/2 expression following binge-like EtOH consumption in wild-type but not in TLR4-KO mice
MyD88 is an adapter protein essential in Toll-like receptor signaling. Figure 6a shows a representative Western blot of MyD88 expression in the hippocampus of wild-type and TLR4-KO mice 24 h after the last exposure to EtOH. Quantitative image analysis by two-way ANOVA revealed a significant effect of EtOH (F1,16 = 0.031, p < 0.05) and genetic deletion (F1,16 = 20.58, p = 0.0003) but no interaction between the two factors (F1,16 = 0.197, p = 0.664) (Fig. 6b). Post-hoc analysis indicated that EtOH increased MyD88 expression (18%) in wild-type but not in TLR4-KO mice. In addition, MyD88 expression in the hippocampus of control TLR4-KO was lower than that observed in control wild-type mice.
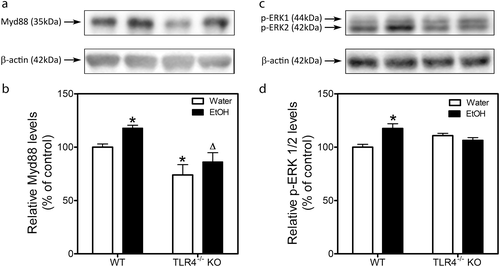
Interestingly, correlation analysis reveals a positive correlation between alcohol intake (g/kg) on the fourth day of the last cycle and MyD88 expression (Pearson r = 0.99, p < 0.01).
Figure 6c shows a representative Western blot of p-ERK1/2 in the hippocampus of wild-type and TLR4-KO mice 24 h after the last exposure to EtOH. Quantitative image analysis by two-way ANOVA revealed a significant effect of EtOH (F1,17 = 4.653, p = 0.0456) and an interaction (F1,17 = 13.21, p = 0.0020) but no effect of genetic deletion (F1,17 = 0.0035, p = 0.9534) (Fig. 6d). Post-hoc analysis indicated that EtOH increased p-ERK1/2 expression (18%) in wild-type mice but not in TLR4-KO mice.
EtOH intake
Ethanol consumption in mice subjected to 1 cycle of DID was constant for the first three days and increased on the 4th day in agreement with the number of hours of exposure to the drug. The pattern repeated itself in the three successive cycles. There were no differences in consumption between wild-type and TLR4-KO mice (Fig. 1S, Supporting Information).
Discussion
The current study shows for the first time that postmortem human alcoholic brain shows changes in the integrity of BBB which are reflected by a marked reduction in the expression of the TJ protein, claudin-5 and the basal lamina protein collagen-IV. Collagen type IV is a component of the extracellular matrix and is one of the proteins most important for the structural integrity of small vessels. Collagen type IV together with laminin form two overlapping polymeric networks (Coelho et al., 2011). The reduced collagen implies basement membrane degradation and has previously been described as indicative of BBB alteration (Rosell et al., 2008). The TJs provide structural integrity and confer low permeability and high electrical resistance to the monolayer (Romero et al., 2003) thus restricting access of substances to brain parenchyma. In fact, claudin-5 is critical for the endothelial–endothelial junction seal (Nitta et al., 2003). Matrix proteins can influence the expression of endothelial TJ proteins (Tilling et al., 1998; Savettieri et al., 2000), indicating that although the TJs constitute the primary impediment to paracellular diffusion, the proteins of the basal lamina are likely involved in their maintenance. The reduction in the expression of claudin-5 and of collagen-IV cannot be attributed to a loss in endothelial cells because there is no change in CD31 expression.
An important limitation to this study is that we do not have direct evidence of permeability in the postmortem human alcoholic brain; however, we did observe increased immunoreactivity of CD45 indicating infiltration of leukocytes which reflects functional changes at BBB (Engelhardt, 2008).
The current study also shows enhanced MMP-9 proteolytic activity in the postmortem human alcoholic brain. Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases specialized in the degradation of components of extracellular matrix (collagens and fibronectin) and basement membrane (laminin and heparan sulfate proteoglycans) (Sluijter et al., 2006) and an enhanced activity of MMPs is associated with a greater degradation of collagen-IV and laminin, disruption of TJ assembly, leukocyte infiltration and activation of resident brain macrophages (microglia) (Rosell et al., 2006; Jin et al., 2010). Increased MMP-9 activity might therefore be contributing to the BBB changes and leukocyte infiltration observed in alcoholics.
Basal MMP-9 protein expression is usually low and tightly controlled under physiological conditions. However, its expression can be upregulated by several factors including proinflammatory cytokines and reactive oxygen species (Vecil et al., 2000; Jian Liu & Rosenberg, 2005; Katsu et al., 2010). This regulation appears to be mediated mainly at the transcriptional level and because the promoter region of MMP-9 contains AP-1 transcription factor sites, mitogen-activated protein kinases (MAPKs) are likely involved (Arai et al., 2003; Wu et al., 2004). Consistent with this, we found an increase in ERK1/2 and p38 phosphorylation in the postmortem brain of alcohol abusers supporting a role for these MAPKs in the increase in MMP-9 activity observed.
The cellular source of MMP-9 is not clear but we observed that postmortem brain of alcohol abusers showed microglial and astrocytic activation. Glial cells and in particular astrocytes and microglia are an important source of MMP-9, and several studies have shown upregulation of MMP-9 in these cells in response to stimuli such as IL-1β and tumor necrosis factor α (Arai et al., 2003; Wu et al., 2004; Ralay Ranaivo et al., 2012). Furthermore, the MAPK pathways have been shown to participate in the increase in MMP-9 induced by proinflammatory cytokines in both these types of cells (Ralay Ranaivo et al., 2011). It is interesting to note that other authors have found increases in proinflammatory cytokines including IL-1β in postmortem human alcoholic brain (He & Crews, 2008; Zou & Crews, 2012) and cerebral cortex of mice exposed to alcohol in drinking water for 5 months (Alfonso-Loeches et al., 2010). Thus, our results are consistent with a role for IL-1β in the induction of MMP-9 mediated by the MAPK signaling pathway in glial cells.
The findings in postmortem human alcoholic brain largely agree with those obtained in a number of studies using in vitro models of BBB and primary brain microvascular endothelial cells (BMVEC) exposed to EtOH. EtOH or its metabolite acetaldehyde in nontoxic concentrations decreased BBB tightness via activation of myosin light chain (MLC) kinase, leading to phosphorylation of MLC and TJ proteins (occludin and claudin-5) and monocyte migration across BBB models (Haorah et al., 2005). Antioxidant treatment or suppression of EtOH metabolism in BMVEC prevented these phenomena, suggesting that alcohol-associated BBB impairment is mediated, in part, by oxidative stress (Haorah et al., 2005). Reactive oxygen species have been shown to increase activity and expression of MMPs in several types of mesodermal cells, including hepatic stellate cells (Galli et al., 2005), human monocytes (Lu & Wahl, 2005) and human coronary smooth muscle cells (Valentin et al., 2005).
Treatment of BMVEC with EtOH or acetaldehyde increased MMP-1, -2 and -9 activities and decreased the levels of tissue inhibitors of MMPs (TIMP-1, -2) in a protein tyrosine kinase-dependent manner. Up-regulation of MMP activities and protein contents paralleled a decrease in collagen-IV content and, inhibitors of EtOH metabolism, MMP-2 and -9 or protein tyrosine kinase reversed all these effects (Haorah et al., 2008).
In order to further explore the effects of ethanol exposure on the BBB we carried out studies in mice. The DID model exposes mice to repetitive binge-like consumption of ethanol (Rhodes et al., 2005). It is a simple limited-access procedure which does not involve food or severe water restriction and leads to pharmacologically significant drinking (Rhodes et al., 2005). Mice were repeatedly exposed to the DID model for 4 consecutive weeks. Repeated cycles of DID lead to increases in subsequent voluntary ethanol consumption and preference which may reflect the early stages of ethanol dependence and are thus useful in studying the transition to ethanol dependence (Cox et al., 2013). It is interesting to note that under our experimental conditions, neuroinflammation seems not to impact alcohol consumption, because no differences in alcohol intake were noted between WT and TLR4-KO animals. These results agree with those previously described (Alfonso-Loeches et al., 2010; Pascual et al., 2015). However, other studies indicate that a single injection of LPS produces a long-lasting increase in alcohol consumption in the two-bottle choice test and that deletion of immune signaling components, such as CD14, a key component of TLR4 signaling, decreases alcohol consumption (Blednov et al., 2011) suggesting that activation of immune signaling may have a role in promoting excessive alcohol intake. The reasons for the discrepancy between the latter findings and the lack of changes in voluntary alcohol intake in WT and TLR4-KO mice are presently unknown. In a subsequent study, in the absence of a priming neuroinflammatory stimulus CD14-null mutant mice showed no change in alcohol consumption in the one-bottle DID model (Blednov et al., 2012). Thus, it seems that the role of neuroinflammation in alcohol consumption is complex and may depend, among other factors, on the presence or absence of inflammatory triggers and on the specific drinking test used. Twenty-four hours after EtOH withdrawal mice exhibited alterations in the hippocampal expression of the basement membrane proteins, laminin and collagen IV (also reduced in postmortem human alcoholic brain). These changes were not observed in TLR4-KO mice suggesting that the factors involved in basal lamina alterations mediate their effects through TLR4. In addition to these changes in the basal lamina, wild-type but not TLR4-KO mice exhibited an increase in the extravasation of IgG to the surrounding brain parenchyma indicating EtOH-induced alterations in the permeability of the BBB which are prevented by the absence of TLR4.
Toll-like receptors are a family of pattern recognition receptors responsible for the recognition of diverse pathogen- and damage-associated molecular patterns. Their activation initiates immune responses mediated either by myeloid differentiation factor 88 (MyD88)-dependent or independent pathways. In particular, TLR4 plays a critical role in the neuroinflammation induced by EtOH, and studies have shown that EtOH-induced activation of MAPK and NFκB signaling pathways and production of inflammatory mediators is prevented by treatment with small interfering RNA and is absent in TLR4-KO mice (Alfonso-Loeches et al., 2010). In the current study, binge-like EtOH drinking increased MyD88 and p-ERK1/2 expression in wild-type mice and this was prevented in TLR4-KO mice suggesting that TLR4 plays an important role in the cellular response to binge-like EtOH. The mechanism by which EtOH activates TLR4 receptors is not completely understood but studies have shown that EtOH can promote the translocation and clustering of TLR4 and signaling molecules such as MyD88 and ERK into lipid rafts (Blanco et al., 2008; Fernandez-Lizarbe et al., 2008). Correlation analysis found an association between alcohol intake on the final day of exposure and increases in MyD88 expression in wild-type but not TLR4-KO mice, thus further supporting TLR4 activation in our model. Alternatively, EtOH can also stimulate the release of the alarmin HMGB1 by glial and neuronal cells (Maroso et al., 2010; Maroso et al., 2011), activating TLR4 signaling (Crews et al., 2013) and contributing to the neuroimmune activation and brain damage. Taken together, these findings indicate that EtOH increases TLR4 signaling and that these effects are involved in the disruption of BBB observed in brain mouse after binge drinking.
Interestingly, increased immunoreactivity of TLR2, TLR3 and TLR4 has been found in postmortem human alcoholic brain (Crews et al., 2013). In addition and, in a similar manner to that found in EtOH-treated mouse brain, postmortem alcoholic brain showed an increase in the immunoreactivity and expression of HMGB1 (Crews et al., 2013). Although we cannot demonstrate causality in postmortem brain which is an important limitation to the study, it is tempting to speculate that the impairment in the structural support of the BBB observed in the current study in alcoholic brain might be mediated by an increase in HMGB1/TLR signaling. Leukocyte infiltration might be interpreted as a secondary outcome of the neuroinflammatory response to limit activation of innate immunity. It is important to note that the study in mice was carried out in hippocampus and the post-mortem samples obtained were of dorsolateral PFC. Although both areas are important targets of ethanol-induced damage, further studies are needed to confirm similar mechanisms in both brain areas.
An important observation arising from our study is that similar BBB alterations appear to occur in alcoholic subjects and mice exposed to EtOH. In our DID model the total ethanol exposure for mice was approximately 45 g/kg. This is markedly less exposure than that observed in the alcoholic subjects. Data from studies on human postmortem brain from alcoholics report that lifetime alcohol consumption rates vary between 506 000 and 5811 000 g (Crews et al., 2013) which is equivalent to 7229–83 014 g/kg (taking an average adult weight of 70 kg). Although this observation should be interpreted with caution in particular because we have no information on the lifetime consumption of this particular group of subjects nor on the particular pattern of consumption it might be suggesting that BBB alteration in humans could arise after relatively limited exposure.
It is interesting to note that there is a negative correlation between the age when subjects began drinking and p-ERK1/2 expression indicating that the lower the age of onset of ethanol consumption, the greater the activation of ERK1/2 in adulthood. It has been shown that during withdrawal, following continuous and intermittent ethanol exposure, ERK phosphorylation was increased in several brain areas (Sanna et al., 2002). An intermittent model of exposure resulted in significantly higher levels of ERK activation during withdrawal in the amygdala when compared to animals treated continuously which is consistent with the notion that repeated withdrawal produces a sensitizing effect (Gonzalez et al., 2001; Sanna et al., 2002). Obviously, we cannot speculate on the number of withdrawal episodes suffered by alcoholics but our results suggest that the dysregulation of the ERK pathway could contribute to the molecular adaptations which are thought to be involved in the development of addiction.
In conclusion, results reported in the current study indicate that chronic EtOH consumption increases degradation of TJ and extracellular matrix in postmortem human brain that could be attributed to increased MMP-9 activity. These effects are associated with activation of ERK1/2 and p-38 and a marked neuroinflammatory response that might be promoting leukocyte infiltration. The EtOH-induced impairment of BBB is not evident in TLR4-KO mice exposed to a binge-like EtOH protocol, suggesting the involvement of TLR4 signaling in the underlying mechanism leading to BBB disruption.
Acknowledgments
This work was supported by grants from the Spanish Ministerio de Economia y Competitividad (MINECO) (SAF2010-21529 and SAF2013-40592-R to MIC and SAF 2011-23420 to JM), Ministerio de Sanidad, Servicios Sociales e Igualdad (PNSD 2011/043 to JM, PNSD 2013I077 to EOS and PNSD 2014I015 to MIC), ISCIII RETICS RTA (RD06/0001/0006 and RD12/0028/0002 to MIC and RD06/0001/1004 and RD12/0028/0019 to JM), Universidad Complutense (UCM 910258 to MIC) and Consejeria de Educacion y Empleo, Comunidad de Madrid. (CAM S2010-BMD-2308 to MIC). ARA is the recipient of a RETICS RTA post-doctoral contract; MPH and FP are recipients of pre-doctoral fellowships from the Ministerio de Educacion (Spain) and Regione Autonoma Della Sardegna (Italy), respectively. The authors are entirely responsible for the scientific content of this article.
Tissue samples were received from the New South Wales Tissue Resource Centre at the University of Sydney, supported by the National Health and Medical Research Council of Australia, Schizophrenia Research Institute and the National Institute of Alcohol Abuse and Alcoholism (AA012725).
Conflict of Interest
The authors declare no competing financial interests.
Author Contributions
Ana Rubio-Araiz, Francesca Porcu and Mercedes Pérez-Hernández contributed substantially to conception, design and acquisition of data. Mª Salud García-Gutiérrez and María Auxiliadora Aracil-Fernández contributed to analysis of data. María Dolores Gutierrez-López contributed to interpretation of data. Consuelo Guerri provided TLR4-KO and wild type mice and revised the article critically for important intellectual content. Jorge Manzanares provided human brain samples and revised the article critically for important intellectual content. Esther O'Shea and María Isabel Colado contributed to design, interpretation of data, drafted the article and revised it critically for important intellectual content. All authors critically reviewed content and approved final version for publication.




