Characterization of white matter integrity deficits in cocaine-dependent individuals with substance-induced psychosis compared with non-psychotic cocaine users
Abstract
With sufficient drug exposure, some individuals develop transient psychotic symptoms referred to as ‘substance-induced psychosis’ (SIP), which closely resemble the symptoms observed in schizophrenia spectrum disorders. The comparability in psychotic presentation between SIP and the schizophrenias suggests that similar underlying neural deficits may contribute to the emergence of psychosis across these disorders. Only a small number of studies have investigated structural alterations in SIP, and all have been limited to volumetric imaging methods, with none controlling for the effects of chronic drug exposure. To investigate white matter abnormalities associated with SIP, diffusion tensor imaging was employed in a group of individuals with cocaine-associated psychosis (CAP; n = 24) and a cocaine-dependent non-psychotic (CDN) group (n = 43). Tract-based spatial statistics was used to investigate group differences in white matter diffusion parameters. The CAP group showed significantly lower fractional anisotropy values than the CDN group (p < 0.05) in voxels within white matter tracts of fronto-temporal, fronto-thalamic and interhemispheric pathways. The greatest differences in white matter integrity were present in the corpus callosum, corona radiata, bilateral superior longitudinal fasciculi and bilateral inferior longitudinal fasciculi. Additionally, the CAP group had voxels of significantly higher radial diffusivity in a subset of the previously mentioned pathways. These results are the first description of white matter integrity abnormalities in a SIP sample and indicate that differences in these pathways may be a shared factor in the expression of different forms of psychosis.
Introduction
Psychostimulant drugs produce a range of acute psychological effects in humans, with lower doses generating feelings of increased energy, mood and confidence, while higher doses can result in additional dysphoric effects. With sufficient drug exposure, some individuals develop symptoms that result in a syndrome referred to as ‘substance-induced psychosis’ (SIP), which symptomatically resembles schizophrenia spectrum disorders (Medhus et al. 2013). These SIP episodes are characterized by both positive (hallucinations, delusions, disorganized thinking) and negative (flattened affect, emotional withdrawal, lack of spontaneity) symptoms. Cognitive deficits in SIP similar to those noted in schizophrenia have also been reported (Jacobs et al. 2008). Unlike idiopathic psychoses, SIP is defined as a transient psychotic episode enduring up to 1 month after the drug is metabolized and cleared from the body (Association, AP 2000; Orikabe et al. 2011). SIP is typically associated with exposure to high doses of psychostimulants over an extended duration, with an average latency of 3–5 years between first drug exposure and the initial psychotic episode (Ujike & Sato 2004).
While heavy psychostimulant use may predispose individuals to SIP, many psychostimulant users will never experience a SIP episode. Approximately 25–45 percent of methamphetamine-dependent individuals, most of whom consumed large quantities of the drug, developed psychosis at some point (McKetin et al. 2006)—implying that only a minority of users are vulnerable. The biological basis that confers a predisposition to SIP remains largely unknown. While genetic studies have provided preliminary evidence that particular variants in genes commonly associated with schizophrenia may be involved (Grant et al. 2012), it is also likely that differences in brain structure or function may contribute to individual susceptibility to SIP. This has been addressed empirically in only a small number of neuroimaging studies and only for the psychostimulant drug methamphetamine. Frontal and temporal volume loss (Aoki et al. 2013) and smaller hippocampal and amygdala volumes (Orikabe et al. 2011) were observed in methamphetamine users with SIP compared with healthy controls. However, the effects of methamphetamine on the brain are an ongoing area of study [reviewed in (Thompson et al. 2004; Panenka et al. 2013)]. Thus, without inclusion of drug-using non-psychotic subjects, it is difficult to determine which structural changes are unique to psychosis and which reflect the toxic effects of chronic exposure to the psychostimulant.
In idiopathic psychosis such as schizophrenia, symptoms are posited to result from aberrant anatomic connectivity, either globally or via specific white matter tracts (Konrad & Winterer 2008; Melicher et al. 2015). White matter microstructural organization can be quantified using diffusion tensor imaging (DTI) that measures the directionality of water diffusion within tissue to infer structural characteristics and integrity in white matter pathways (Ellison-Wright & Bullmore 2009). In white matter, water diffuses more easily along—rather than across—the fiber tract axis, resulting in anisotropic diffusion. The most common index for quantifying anisotropy is with fractional anisotropy (FA). Other indices of diffusion include axial diffusion (AD; diffusion along the fiber tract axis) and radial diffusion (RD; diffusion perpendicular to the fiber tract axis). Reductions in FA have been interpreted as a disruption in the organization of tracts/white matter integrity (Mädler et al. 2008). DTI has been used extensively to characterize white matter alterations in schizophrenia, with alterations observed predominantly in interhemispheric, fronto-temporal and fronto-thalamic tracts (Kanaan et al. 2005; Ellison-Wright & Bullmore 2009).
The goal of the current study was therefore to investigate white matter integrity in psychostimulant users with SIP. Subjects were recruited as part of the Hotel Study (Vila-Rodriguez et al. 2013), in which subjects underwent detailed psychiatric and neurocognitive assessment, as well as a MRI brain scan, at entry into the study. For the current investigation, we compared a group of cocaine-dependent non-psychotic subjects with a group with cocaine-associated psychosis—to control for general effects of cocaine on the brain. We used a whole brain tract-based spatial statistics (TBSS) approach (Smith et al. 2006) to compare changes in white matter diffusion properties between groups. We hypothesized that patients with cocaine-associated psychosis (CAP) would show reduced white matter integrity compared with cocaine-dependent non-psychotic (CDN) subjects in fronto-temporal and interhemispheric pathways.
Materials and Methods
Participants
Participants were recruited as part of a larger study of 370 subjects living in single room occupancy hotels with a history of mental illness and/or substance abuse in the Downtown Eastside of Vancouver, BC (Vila-Rodriguez et al. 2013). For the current investigation, exclusionary criteria were history of Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnosis of schizophrenia, schizoaffective disorder, psychosis not otherwise specified or bipolar disorder. Further exclusionary criteria included past moderate or severe traumatic brain injury (loss of consciousness >30 minutes or confusion >24 hours after injury), stroke, significant MRI artifacts (motion, major distortion) and other gross morphometric brain abnormalities (i.e. encephalomalacia). All participants met DSM-IV criteria for cocaine dependence, verified by a positive urine toxicology [mean (SD) of 8.4 (6.8) days from imaging acquisition]. When a urine drug screen was unavailable, recent cocaine use was verified according to self report via the timeline followback method (TLFB; n = 4) (Sobell et al. 1986). Subjects were divided into two groups: (1) 24 CAP subjects and (2) 47 CDN subjects. The maximum inclusion age was set at 58 to ensure equal age distribution across groups, resulting in the exclusion of four CDN subjects for a final CDN sample size of 43. In accordance to Tri-Council policy, the study was approved by the University of British Columbia Clinical Research Ethics Board. All participants provided written informed consent.
Demographics
Data including age, gender and education were collected. The Mini-International Neuropsychiatric Interview was administered, and it was supplemented by a clinical interview and mental status examination carried out by a psychiatrist. Diagnoses of psychiatric disorders and substance dependency were made according to the DSM-IV (Association, AP 2000) by an experienced psychiatrist (WGH, OL or FV-R) through consensus evaluation with the Best Estimate Clinical Evaluation and Diagnosis (Endicott 1988). The TLFB (Sobell et al. 1986) was employed by a trained research assistant to quantify recent drug use. Years of regular psychostimulant use and age of first use were provided by self-report. Psychosis severity at time of MRI scan was assessed with the Positive and Negative Symptom Scale [PANSS (Kay, Fiszbein, & Opler 1987)].
Cognition
Neuropsychological tests were administered by trained researchers approximate to the time of imaging acquisition. Premorbid IQ was estimated using the Wechsler Test of Adult Reading (Wechsler 2001). Verbal learning was measured with the immediate recall score of the Hopkins Verbal Learning Test-Revised (Brandt & Benedict 2001). The Cambridge Neuropsychological Test Automated Battery (Fray, Robbins, & Sahakian 1996) was used to index sustained attention with the Rapid Visual Information Processing subtest and mental flexibility with the Intra-dimensional Extra-dimensional subtest. Cognitive inhibition was measured using the Color-Word Trial from the Stroop Color-Word Test. Affective decision-making was assessed using the total net score from the Iowa Gambling Task (Bechara et al. 1994)
MRI acquisition
All participants were scanned on a 3T MRI Scanner (Philips Achieva) at the University of British Columbia MRI Research Centre using an eight-channel SENSE head coil between 2008 and 2014. Each subject was scanned twice within the session using the same protocols (total scan time = 7:32 minutes). Standard orthogonal localizers were performed. Diffusion imaging was performed with the following parameters: 32 diffusion gradient directions were used with b = 700 s/mm2, TE = 60 ms, TR = 6451 ms, flip angle = 90°, FOV = 224 × 224 mm, acquisition matrix = 100 × 100, reconstruction matrix = 112 × 112, voxel dimensions = 2.0 × 2.0 mm, 70 contiguous slices, thickness = 2.2 mm, gap = 0, SENSE = 2.1.
Image processing
Diffusion-weighted data were corrected for eddy current and head motion using affine registration of all gradient volumes with the first b = 0 volume (FMRIB Linear Image Registration Tool; FMRIB Software Library, Oxford, UK). Slices with extreme intensities were re-estimated from the average of adjacent slices. The two DTI scans were then averaged. A brain mask was created by running Brain Extraction Tool with a fractional intensity threshold of 0.3 (Smith 2002). DTI fitting was performed with Slicer 3 using non-linear least-square fitting with shifted negative eigenvalues.
Tract-based spatial statistics
TBSS (Smith et al. 2006) and randomise (Nichols & Holmes 2002) of the FMRIB Software Library (FSL, Analysis Group, FMRIB, Oxford, UK) were used for comparisons of FA, MD, AD and RD. Individual FA maps were then non-linearly registered via FSL-FNIRT to the John Hopkins University International Consortium Brain Mapping (JHU-ICBM) FA template provided by FSL, followed by creation of a study-specific mean FA skeleton. Individual FA values were then projected onto this mean skeleton. MD, AD and RD values were projected onto the skeleton using the previously created FA transformation. FA threshold was set to 0.20, and voxel-based comparisons of FA, MD, AD and RD were performed on the mean FA skeleton using randomise (Nichols & Holmes 2002) from FSL Version 4.1.9, including age and methamphetamine dependence as covariates, as these variables affect FA (Salo et al. 2009; Bendlin et al. 2010). The analysis performs 5000 random permutations, using threshold-free cluster enhancement (Smith et al. 2006) to threshold the statistical data and family-wise error (FWE) to correct for multiple comparisons before creating a p < 0.05 T-contrast map. The JHU-ICBM-DTI-81 white matter label atlas was used to identify specific anatomical areas implicated by TBSS (Hua et al. 2008).
The JHU-ICBM-DTI-81 white matter label atlas was transformed back onto individual DTI space by reversing the previous non-linear registration for each subject to calculate white matter tract volumes.
Statistical analysis
Demographic and clinical variables were analyzed with chi-squared tests or independent Student's t-tests as appropriate.
To further investigate intergroup FA and RD differences, mean FA or RD was extracted from the clusters where the highest statistical group difference was identified using a p value cutoff threshold of p < 0.05. The anatomical regions to which these clusters belonged were determined using the JHU-ICBM-DTI-81 white matter label atlas. A partial correlation was performed to assess relationships between the mean clusters of FA/RD and PANSS positive subscale, controlling for age and methamphetamine dependence.
White matter tract volume was compared by partial correlation controlling for age, methamphetamine dependence and total brain volume (Jancke et al. 1997).
Statistical analysis was performed with SPSS Vs22.
Results
Demographics
The mean age of the CAP group was 40.4 (±8.2) years and 43.3 (±7.3) years for the CDN group. The CAP group included 15 males and 9 females, while the CDN group included 30 males and 13 females. Age, gender distribution and mean years of completed education were similar across groups (p > 0.05; Table 1). Additionally, there was no difference in HIV status between groups (20.8 percent positive in CAP and 18.6 percent in CDN). Scores on all PANSS subscales were higher in the CAP than CDN group, including positive [CAP: 15.3 (±4.1), CDN: 11.9 (±3.1)], negative [CAP: 18.3 (±5.4), CDN: 15.2 (±4.9)] and general psychopathology subscales [CAP: 37.1 (±7.5), CDN: 33.7 (±6.4)] (Table 2).
| CDN (n = 43) | CAP (n = 24) | Statistic | P value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Age | 43.3 | 7.3 | 40.4 | 8.2 | 1.606 | 0.110 |
| Gender (F) | 10.4 | 2.5 | 10.1 | 1.3 | 0.601 | 0.550 |
| Years regular meth use | 4.1 | 10 | 2.6 | 3.6 | 0.647 | 0.520 |
| Age first meth use | 23.5 | 9.8 | 24.4 | 13.1 | −0.275 | 0.784 |
| Days cocaine use in past month | 17.1 | 10.0 | 19.3 | 10.9 | −0.714 | 0.480 |
| Years regular cocaine use | 12.4 | 9.0 | 11.8 | 8.4 | 0.472 | 0.640 |
| Age cocaine first use | 17.7 | 18.8 | 19.2 | 5.8 | 0.752 | 0.460 |
| Cigarette pack years | 13.2 | 14.800 | 12.600 | 11.800 | 0.122 | 0.900 |
| N | % | N | % | X2 | p | |
| Gender (F) | 13 | 30.2 | 9 | 37.5 | 0.449 | 0.510 |
| Meth dependency | 6 | 14.0 | 6 | 24.0 | 1.380 | 0.250 |
| Alcohol dependency | 8 | 18.6 | 5 | 20.8 | 0.071 | 0.790 |
| Heroin dependency | 21 | 48.8 | 12 | 50.0 | 0.000 | 1.000 |
| Methadone dependency | 24 | 55.8 | 16 | 66.7 | 0.629 | 0.440 |
| Marijuana dependency | 11 | 25.5 | 10 | 41.7 | 2.021 | 0.160 |
| CDN | CAP | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | p value | Mean T-score | |
| WTAR (premorbid IQ) | 43 | 97.4 | 9.0 | 24 | 94.3 | 9.6 | 0.19 | 50.0 |
| Stroop (inhibition) | 42 | 37.5 | 9.9 | 21 | 34.5 | 7.7 | 0.22 | 49.9 |
| HVLT (verbal learning) | 42 | 20.4 | 5.5 | 22 | 18.5 | 5.4 | 0.21 | 31.7 |
| RVP A′ (attention) | 37 | 0.869 | 0.052 | 20 | 0.86 | 0.058 | 0.55 | 39 |
| IED (mental flexibility) | 36 | 128.4 | 40.9 | 20 | 142.5 | 51.5 | 0.27 | 38.5 |
| IGT net score (decision-making) | 38 | 1.6 | 30.4 | 22 | −13.0 | 43.6 | 0.17 | 44.8 |
| Positive PANSS | 43 | 11.9 | 3.1 | 24 | 15.3 | 4.1 | 0.000 | N/A |
| Negative PANSS | 43 | 15.2 | 4.9 | 24 | 18.3 | 5.4 | 0.025 | N/A |
| General PANSS | 43 | 33.7 | 6.4 | 24 | 37.1 | 7.5 | 0.057 | N/A |
| Total PANSS | 43 | 60.8 | 12.0 | 24 | 70.8 | 14.4 | 0.004 | N/A |
- Because of invalid tests, sample sizes of cognitive tests were diminished in a case-by-case basis.
The atypical antipsychotic quetiapine was prescribed for 20 percent of the CAP and 9 percent of the CDN group, mostly for hypnotic effects.
Drug use
DSM-IV TR substance dependence was diagnosed by a qualified psychiatrist. Aside from cocaine use, tobacco dependence was highly prevalent at 93 percent of the pooled sample. Methamphetamine (CAP = 24 percent, CDN = 14 percent) and marijuana (CAP = 42 percent, CDN = 26 percent) use were non-significantly greater in the CAP group. Heroin, methadone and alcohol dependence were approximately even between groups (Table 1).
Cognition
For cognitive analyses, sample sizes vary because of invalid performances by some participants on select tests (Table 2). No significant group differences or trends were observed for any of the cognitive measures (all p > 0.10), including estimated premorbid IQ, verbal learning, inhibition, sustained attention, mental flexibility and decision-making. For descriptive purposes, demographically corrected (age and/or education) T-scores were calculated for the group-pooled neurocognitive measures using available normative data (mean of 50, SD of 10; Table 2). Briefly, the sample exhibited greatest impairments in verbal learning (>1.5 SDs below the mean), with milder impairment for sustained attention and mental flexibility (>1 SD below the mean). In contrast, estimated premorbid IQ, inhibitory control and decision-making ability fell within normal limits.
TBSS—global WM
The CAP group had lower FA than the CDN group (p < 0.05, FWE) in (i) fronto-temporal, (ii) fronto-thalamic and (iii) interhemispheric white matter tracts (voxel-based differences shown in Fig. 1). Specifically, deficits were present in (i) bilateral inferior longitudinal fasciculus, bilateral superior longitudinal fasciculus, (ii) right anterior limb of the internal capsule, right posterior limb of the internal capsule, bilateral posterior corona radiata, left superior corona radiata, left minor forceps and (iii) splenium and body of the corpus callosum. There were no areas of increased FA in the CAP group compared with the CDN group.
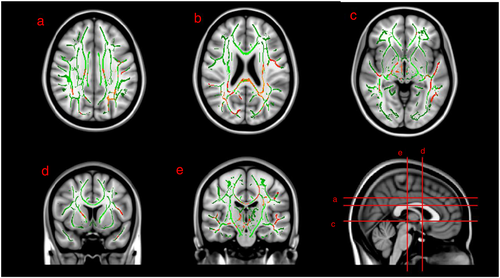
Radial diffusivity (RD) was significantly higher in the CAP group compared with the CDN group in white matter tracts of the (i) bilateral superior longitudinal fasciculus, (ii) left anterior, superior and posterior corona radiata and (iii) splenium and body of corpus callosum (Fig. 2). There were no areas of significantly decreased RD in the CAP group.
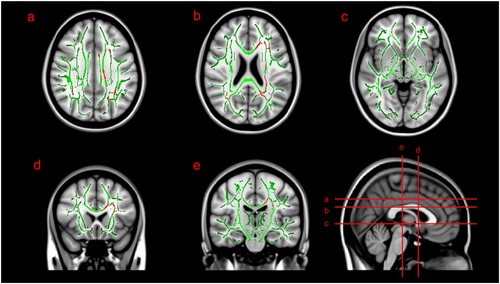
No differences were present between groups comparing either mean diffusivity or axial diffusivity.
Associations between symptom severity and white matter integrity
Mean FA/RD was extracted from the clusters where the highest statistical group difference was identified using a p value cutoff threshold of p < 0.05. PANSS positive subscale was related to (1) splenium (FA: r = −0.251, p = 0.044) and (2) body of the corpus callosum (FA: r = −0.304, p = 0.014; RD: r = 0.333, p = 0.007). FA correlations were negative, with greater symptom severity associated with lower FA values. RD correlations were positive, with higher RD values associated with greater symptom severity. No findings were statistically significant after applying Bonferroni correction for multiple comparisons.
WM tract volume
There were no WM tract volume differences between groups in any of the tracts identified to have FA differences after correcting for multiple comparisons (all p values >0.05).
Discussion
The present study showed decreased FA and increased RD values in cocaine-dependent subjects with psychosis compared with those without psychosis, in the body and splenium of the corpus callosum, bilateral superior longitudinal fasciculi, left posterior corona radiata, left superior corona radiata and right anterior limb of the internal capsule. There were no regions of greater FA or reduced RD in the CAP group compared with the CDN group, nor were there group differences in mean or axial diffusivity. The alterations in radial, but not axial, diffusivity may reflect deficits in white matter microstructural integrity, including damage to myelin or the cellular membrane (Song et al. 2003; Song et al. 2005). These changes were inversely correlated with symptom severity in the splenium and body of the corpus callosum, although this finding did not withstand correction for multiple comparisons. Cognitively, the combined sample exhibited substantial impairment in the domains of verbal learning, mental flexibility and sustained attention compared with normative data, although no significant group differences were observed.
To date, neuroanatomical characterization of SIP has been limited to a small number of structural MRI studies that have investigated gray matter abnormalities, specifically in methamphetamine-associated psychosis [MAP (Sekine et al. 2002; Orikabe et al. 2011; Aoki et al. 2013)], with a paucity of data on CAP. One study observed reduced amygdala and hippocampal volumes in MAP subjects compared with healthy controls (Orikabe et al. 2011), while another reported gray matter volume reductions within frontal and temporal regions (Aoki et al. 2013)—akin to prior reports of idiopathic psychosis (Shenton et al. 2001). Our observation of decreased white matter FA values in chronic cocaine users with SIP compared with cocaine users without psychosis adds a novel and important dimension to the SIP literature. Of considerable interest, these white matter alterations closely resemble those observed in schizophrenia. Numerous studies using DTI have provided evidence of lower FA values in schizophrenia: meta-analysis has reported the most consistent deficits in left frontal and left temporal deep white matter regions—specifically within the genu/splenium of the corpus callosum, cingulum bundle, left inferior fronto-occipital fasciculus, left anterior thalamic radiation and left inferior longitudinal fasciculus (Ellison-Wright & Bullmore 2009). In the current study, we identified FA reductions in a subset of these tracts, including the splenium of the corpus callosum, inferior longitudinal fasciculus and anterior limb of the internal capsule (Ellison-Wright & Bullmore 2009). Interestingly, although FA reductions were seen bilaterally, RD increases were notably more prevalent on the left than the right side—with group differences present in left but not right splenium, genu, cingulum, anterior corona radiata, superior corona radiata and posterior corona radiata. This left-sided lateralization of abnormalities has also been observed in studies of schizophrenia (Shenton et al. 2001; Ellison-Wright & Bullmore 2009). However, a recent DTI study of white matter pathways in a relatively large sample of first episode psychosis patients (Melicher et al. 2015) observed that changes in schizophrenia were diffuse rather than regionally specific in nature. Thus, caution should be reserved about concluding the necessity for localized white matter FA changes in the expression of psychosis, especially when group sizes are modest.
The different indices of diffusion provide an expanded insight into the observed white matter differences. Increased radial diffusivity is thought to reflect deficits in myelin integrity (Song et al. 2003), whereas decreased axial diffusivity is thought to reflect axonal damage (Song et al. 2005). Our finding of reduced FA concomitant with increased radial diffusivity, and no differences in axial diffusivity or white matter volume, may implicate aberrant myelination without axonal damage.
This pattern of diffusion changes parallels those observed in schizophrenia (Seal et al. 2008; Levitt et al. 2012) and is consistent with findings suggestive of myelin pathology in postmortem tissue (Flynn et al. 2003). Thus, the overlap between the present findings in SIP and those of schizophrenia suggest that reduced structural integrity of white matter tracts within fronto-temporal, fronto-thalamic and interhemispheric pathways may be a common neuroanatomical substrate for the development and expression of psychosis. However, whether the white matter deficits reported presently are of a similar magnitude to those seen in schizophrenia remains unknown.
Symptoms and cognitive profiles have been compared between SIP and schizophrenia (Jacobs et al. 2008; Medhus et al. 2013). It is generally agreed that psychostimulant-induced positive symptoms are almost indistinguishable from the positive symptoms of schizophrenia (Medhus et al. 2013; Panenka et al. 2013). Similarly, both MAP and paranoid schizophrenia subjects show comparable neurocognitive deficits across similar cognitive domains (Jacobs et al. 2008). Given that cocaine abuse is associated with cognitive impairment, it may be expected that comorbid cocaine use and psychosis would have additive negative effects on cognition (Potvin et al. 2008). However, we did not observe differences between groups, although both groups showed impairments compared with population norms. Studies of schizophrenia and comorbid substance abuse have generated contrary findings; many do not demonstrate additive cognitive deficits in schizohprenia patients with comorbid drug use, while others actually report the counterintuitive finding that those with comorbid substance use have better cognition (Potvin et al. 2008). Our present findings suggest that comorbidity is not associated with additive cognitive deficits.
Understanding the nature of the relationship between chronic cocaine use, white matter integrity and psychosis remains a complex challenge. As noted previously, heavy and sustained use of psychostimulants may lead to SIP, but only 25–45 percent of methamphetamine-dependent users will later experience drug-induced psychosis (McKetin et al. 2006), indicating that the etiology of SIP is not entirely dependent on substance abuse. The present findings suggest that white matter deficits may play a role in the expression of SIP, although the nature of that association could take several forms. Firstly, the lower white matter integrity in the CAP group may reflect a difference between groups that existed prior to drug exposure. White matter structural deficits in the CAP group may be the result of earlier abnormal neurodevelopment, resulting in a weakened network left vulnerable to environmental stressors, akin to the neurodevelopmental hypothesis of schizophrenia (Weinberger, Berman, & Zec 1986). This hypothesis is consistent with research reporting fronto-temporal dysconnectivity in subjects at ultra-high risk for developing schizophrenia (Karlsgodt et al. 2009). Secondly, lower white matter integrity may be the result of differential sensitivity to the neurotoxic effects of drugs of abuse (Hsieh et al. 2014). Psychostimulant abuse can damage white matter tracts, including the corpus callosum, internal capsule, association fibers and frontal white matter regions (Thompson et al. 2004; Lim et al. 2008), all of which have been implicated in idiopathic psychosis. Thus, the CAP group may reflect a population that is more vulnerable to the neurotoxic effects of cocaine, in which chronic and sustained use of cocaine results in greater damage to white matter tracts that subserve psychosis. After a critical threshold, damage may become sufficient to sustain psychosis in the absence of the drug. The hypothesis that some minimum degree of drug-induced damage must occur before onset of psychosis is supported by studies of MAP that have reported an average latency from first use of methamphetamine to onset of psychosis of 3–5 years (Ujike & Sato 2004). After remission, psychosis relapse occurred promptly after subsequent doses of methamphetamine, with 60 percent of subjects relapsing within 1 week and 80 percent relapsing within 1 month of drug use (Ujike & Sato 2004). Spontaneous MAP relapse has also been reported after significant stressors, such as severe insomnia (Sato, Numachi, & Hamamura 1992; Ujike & Sato 2004), suggesting that following a certain threshold of damage or sensitization of neural substrates, subjects remain prone to future relapse. Finally, white matter deficits may also be a consequence of psychosis or the effects of a third factor responsible for both presentation of psychotic symptoms and white matter alterations. With the current cross-sectional study design, the temporal order of drug use, white matter changes and emergent psychosis cannot be definitively determined, and future longitudinal studies will be required to address this issue.
A potential limitation of this study is the absence of a cocaine-naive control group. Although this would not change the observation that SIP is associated with lower white matter integrity compared with non-psychotic cocaine users, it would be useful in revealing how large these deficits are compared with drug-naive subjects. However, it is highly likely that a cocaine-naive control group would not be matched on the multitude of factors that are inherent to being part of a marginalized population (high infection rates, homelessness, limited formal education, other substances of abuse, etc.), and thus, separation of drug versus other environmental effects on the brain would not be feasible. A second potential limitation is that acute substance use (previous 48 hours) was not controlled for, as the urine drug screen did not occur on the date of the scan for most subjects. However, the authors are not aware of any literature suggesting that white matter FA values are susceptible to acute drug exposure. In this vein, information regarding dosage consumed, route of administration or pattern of ingestion (e.g. ‘bingeing’) was not available, which may play a role in the development of SIP.
Conclusions
The present study is the first to suggest a structural white matter biomarker in SIP, controlling for the effect of substance abuse. We detected reductions in white matter integrity in cocaine-dependent subjects with SIP, manifest as decreases in FA characterized by increases in radial diffusivity in fronto-temporal, fronto-thalamic and interhemispheric white matter pathways, compared with cocaine-dependent subjects without psychosis. These pathways parallel those implicated in schizophrenia, suggesting that damage to these pathways may be a shared factor in the expression of different forms of psychosis. Longitudinal studies will help address whether such white matter abnormalities are pre-existing and reflect a natural diathesis or reflect individual differences in the vulnerability to the neurotoxic effects of psychostimulant drugs.
Acknowledgements
The study was funded by the Canadian Institutes for Health Research (CBG-101827, MOP-137103) and the British Columbia Mental Health and Substance Use Services (an Agency of the Provincial Health Services Authority).
Disclosure
Drs. Lang, Vila-Rodriguez, Thornton, Leonova, Rauscher, MacEwan and Panenka report no competing interests. Mr(s). Willi, Gicas, Su and Giesbrecht report no competing interests.
Dr. Honer has received consulting fees or sat on paid advisory boards for In Silico, Otsuka/Lundbeck, Roche and Eli Lilly; received honoraria from Rush University, University of Ottawa, University of Calgary, University of Hong Kong, British Columbia Health Authorities, the British Association for Psychopharmacology and the Canadian Psychiatric Association; and received grants from the Canadian Institutes of Health Research (CIHR).
Dr. Procyshyn has received consulting fees from Janssen, Lundbeck, Otsuka, Pfizer and Sunovion and is on the speaker's bureau for AstraZeneca, Janssen, Lundbeck, Otsuka and Pfizer; and received grants from the Canadian Institutes of Health Research
Dr. Barr has received grant support from BMS Canada.




