Effects of repeated social defeat on adolescent mice on cocaine-induced CPP and self-administration in adulthood: integrity of the blood–brain barrier
Abstract
Social stress in adulthood enhances cocaine self-administration, an effect that has been related with an increase in extracellular signal-regulated kinase and p38α mitogen-activated protein kinase phosphorylation. A detrimental effect of cocaine on blood–brain barrier (BBB) integrity has also been reported. This study evaluates the effects of repeated social defeat (RSD) during adolescence on the reinforcing and motivational effects of cocaine in adult mice and the changes induced by RSD on BBB permeability. Cocaine self-administration, conditioned place preference and quantitative analysis of claudin-5, laminin, collagen-IV and IgG immunoreactivity took place 3 weeks after RSD. Mice socially defeated during adolescence developed conditioned place preference and exhibited reinstated preference with a non-effective dose of cocaine (1 mg/kg). RSD mice needed significantly more sessions than control animals for the preference induced by 25 mg/kg of cocaine to be extinguished. However, acquisition of cocaine self-administration (0.5 mg/kg per injection) was delayed in the RSD group. Mice exposed to RSD displayed significant changes in BBB structure in adulthood, with a marked reduction in expression of the tight junction protein claudin-5 and an increase in basal laminin degradation (reflected by a decrease in laminin and collagen-IV expression) in the nucleus accumbens and hippocampus. The detrimental effect induced by cocaine (25 mg/kg) on collagen-IV expression in the hippocampus was more pronounced in RSD mice. In summary, our findings suggest that stress and cocaine can increase the long-term vulnerability of the brain to subsequent environmental insults as a consequence of a sustained disruption of the BBB.
Introduction
Social environment constitutes a key factor of survival and maintenance of health in most animal species (Dunbar, 2010; Weidt et al., 2012). In humans, experience of social stress, especially during childhood or adolescence, increases the risk of suffering mental disorders (Kessler et al., 2010). Appropriate animal models are of great use when exploring the mechanisms by which social stress affects health. The social defeat paradigm has been successfully employed in laboratory rodents to throw light on the neurobiological, physiological and behavioral changes caused by acute or chronic social defeat experience (Tornatzky & Miczek, 1993; Buwalda et al., 1999). The resident/intruder paradigm, in which the intruder is repeatedly exposed to attacks and threats from a dominant rodent, is considered the best model of bullying in laboratory rodents (Vidal et al., 2007; Watt et al., 2009). Stressful events disrupt the extensive re-organization of limbic monoamine systems such as the mesocorticoaccumbal dopamine (DA) system. DA activity in the accumbens and other subcortical DA terminal regions seems to be less pronounced in adolescents than in adults (Andersen & Gazzara, 1993). Basal levels of synaptic DA are lower during this phase of development, although drug-induced DA release is greater and increases faster in adolescents (Badanich et al., 2006; Laviola et al., 2001). Stressful experiences in early life have a great impact on the subcortical (Andersen, 2003) and mesolimbic DA systems, altering their development (Andersen and Teicher, 2009). Specifically, exposure to stress increases DA content and decreases serotonin turnover in the nucleus accumbens (NAc) and produces neural adaptations in the ventral tegmental area (Andersen et al., 1999; Hall et al., 1998; Andersen & Teicher, 2009).
Adult rats defeated during adolescence show lower baseline DA content and attenuation of amphetamine-induced DA increases in the medial prefrontal cortex (Watt et al., 2009), as well as reduced neurogenesis in the dentate gyrus (Kovalenko et al., 2014), while amphetamine-induced DA release has been shown to be enhanced in their NAc core (Burke et al., 2010).
Few reports have addressed the issue of how adolescent exposure to social defeat can increase the probability of compulsive drug taking later in life, as has previously been shown to occur in animals (Ding et al., 2005; Howes et al., 2000). To date, no studies have been performed to evaluate cocaine self-administration or cocaine-induced conditioned place preference (CPP) in animals socially defeated during adolescence. It has, however, been shown that rats deprived of social interaction during adolescence self-administer more cocaine at low unit doses than non-isolated subjects (Ding et al., 2005), but less at high unit doses (Howes et al., 2000).
Although there are no data available on the effect of social stress on blood–brain barrier (BBB) permeability, there is substantial evidence to show that repeated social defeat (RSD) modifies the expression of antioxidant enzymes and levels of oxidative stress markers (Patki et al., 2014) and induces neuroinflammation (Wohleb et al., 2011, 2013; Hanke et al., 2012). In several models, a rise in proinflammatory cytokine levels (Shaftel et al., 2007) or free radical formation (Gasche et al., 2001) has been shown to disrupt the integrity of the BBB through an increased phosphorylation of the downstream signaling pathways mitogen-activated protein kinases (MAPKs) and matrix metalloproteinase (MMP) activation (Tian & Kyriakides, 2009; Katsu et al., 2010). The primary anatomical substrate of the BBB is the cerebral microvascular endothelium, which, together with pericytes, astrocytes, neurons and the basal lamina, constitutes a neurovascular unit (Hawkins & Davis, 2005). The endothelial cells of the BBB are characterized by the presence of cell-to-cell tight junctions formed by transmembrane molecules such as claudins (claudin-5 appears to be the most abundant), occludins and junction adhesion molecules. Endothelial cells and pericytes are surrounded by a basement membrane made up of extracellular matrix molecules, including collagens, laminins and heparan sulfate proteoglycans (Persidsky et al., 2006). Laminin and collagen-IV have been identified as substrates for several MMPs (Lee et al., 2009).
Cocaine induces expression of the adhesion molecules ICAM-1 and VCAM-1 (Gan et al., 1999) and mediates transcriptional and translational induction of ALCAM in microvascular endothelial cell cultures of the human brain (Yao et al., 2011). In addition, cocaine enhances leucocyte adhesion to endothelial cells and subsequently increases leucocyte transmigration across the cerebral vessel wall, in particular under inflammatory conditions (Gan et al., 1999; Yao et al., 2011). Together, these findings constitute evidence that cocaine impairs BBB integrity.
The present study was aimed to determine the effect of RSD on (1) acquisition and reinstatement of cocaine-induced CPP; (2) acquisition and motivation for operant self-administration of cocaine; and (3) BBB structure and permeability in the NAc and hippocampus and their regulation by the subsequent administration of cocaine.
Material and Methods
Animals
Male OF1 (n = 124) or CD1 (n = 60) (Charles River, Barcelona, Spain) mice arrived at our laboratory at 21 days of age. CD1 mice were used for self-administration studies owing to their greater sensitivity to this technique. All animals (except those used as aggressive opponents) were housed in groups of four in plastic cages (25 × 25 × 14.5 cm) for 8 days before the experiments began. Aggressive opponents were housed individually in plastic cages (23 × 13.5 × 13 cm) for a month prior to experiments in order to heighten aggression (Rodríguez-Arias et al., 1998) (30 OF1 and 10 CD1 adult mice). After the RSD procedure, CD1 mice were moved to the CEEA-PRBB (ethical committee for animal experimentation of the Center of Biomedicine Research of Barcelona) for the self-administration procedure. Mice were housed individually in controlled laboratory conditions at a constant temperature of 21 ± 1°C and 55 ± 10 percent humidity. All experiments were conducted under a reversed cycle (lights off at 08:00 hours and on at 20:00 hours), and mice were tested during the first hours of the dark phase. Food and water were available ad libitum to mice in the cocaine experiment. All procedures were conducted in compliance with the guidelines of the European Council Directive 2010/63/UE regulating animal research and were approved by the local ethics committees (CEEA-PRBB and University of Valencia).
Drugs
For the CPP study, animals were injected intraperitoneally with 1 or 25 mg/kg of cocaine hydrochloride (Laboratorios Alcaliber, Madrid, Spain) in a volume of 10 ml/kg of weight. Physiological saline (NaCl 0.9 percent) was used to dissolve the drug. The doses of cocaine were selected on the basis of previous studies (Rodríguez-Arias et al., 2009; Vidal-Infer et al., 2012; Arenas et al., 2014; Montagud-Romero et al., 2014).
For the self-administration study, cocaine hydrochloride was dissolved in sterile 0.9 percent physiological saline. Ketamine hydrochloride (100 mg/kg) (Imalgene 1000; Rhone Merieux, Lyon, France) and xylazine hydrochloride (20 mg/kg) (Sigma, Madrid, Spain) were mixed and dissolved in ethanol (5 percent) and distilled water 95 percent. This anesthetic mixture was administered intraperitoneally in an injection volume of 20 ml/kg of body weight. Thiopental sodium (5 mg/ml) (Braun Medical S.A., Barcelona, Spain) was dissolved in distilled water and delivered by infusion of 0.1 ml through the intravenous catheter.
For intravenous catheter surgery, the mice were anesthetized with a ketamine/xylazine mixture (20 ml/kg of body weight) and implanted with indwelling intravenous silastic catheters, as previously described (Soria et al, Neuropsychopharmacology, 29: 1122-1133, 2005). In brief, a 6-cm length of silastic tubing (0.3 mm inner diameter, 0.6 mm outer diameter) (Silastic®, Dow Corning, Houdeng-Goegnies, Belgium) was fitted to a 22-gauge steel cannula (Semat, Herts, England) that was bent at a right angle and then embedded in a cement disk (Dentalon Plus, Heraeus Kulzer, Germany) with an underlying nylon mesh. The catheter tubing was inserted 1.3 cm into the right jugular vein and anchored with suture. The remaining tubing ran subcutaneously to the cannula, which emerged from the midscapular region. All incisions were sutured and coated with antibiotic ointment (Bactroban, GlaxoSmithKline, Madrid, Spain).
Procedure and apparatus
Repeated social defeat encounters
Animals in the corresponding group were exposed to four episodes of social defeat lasting 25 minutes each on postnatal days 27, 30, 33 and 36. Each episode consisted of three phases and began by placing the experimental animal or intruder in the home cage of the aggressive opponent or resident for 10 minutes. During this initial phase, the intruder was protected from attack by a wire mesh wall that permitted social interaction and species-typical threats from the male aggressive resident (Covington & Miczek, 2001). In the second phase, the wire mesh was removed, and a 5-minute period of confrontation began. In the third phase, the wire mesh was replaced for a further 10 minutes to allow social threats from the resident. The exploration group underwent the same protocol, but without the presence of a ‘resident’ mouse in the cage. Following this last phase, animals were kept in the vivarium for 3 weeks, after which the behavioral tests began. The second phase of each social defeat protocol was video recorded and ethologically analyzed. Threat and attack behaviors were scored in resident mice, and avoidance/flee and defensive/submissive behaviors were evaluated in intruder mice.
Three different sets of mice were employed in this study. A detailed description of the experimental procedure is provided in Table 1.
| Groups | n | Social defeat | 3 weeks | Experimental procedure | Reinstatement | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | ||||||
| PND | 27 | 30 | 33 | 36 | 58–64 | 66 | |||
| 17 | CPP: 1 mg/kg cocaine | Post-C test | |||||||
| 15 | CPP: 25 mg/kg Cocaine | 12.5 and 6.25 mg/kg | |||||||
| Control | 5 | Exploration without cospecific | 1 mg/kg cocaine | ||||||
| 5 | 25 mg/kg cocaine | Brain samples | |||||||
| 25 | Cocaine self-administration | ||||||||
| 18 | CPP: 1 mg/kg cocaine | Post-C test | 0.5 mg/kg | ||||||
| 14 | CPP: 25 mg/kg cocaine | 12.5 and 6.25 mg/kg | |||||||
| RSD | 5 | Social defeat | 1 mg/kg cocaine | Brain samples | |||||
| 5 | 25 mg/kg cocaine | ||||||||
| 25 | Cocaine self-administration | ||||||||
- CPP = conditioned place preference; PND = postnatal day; RSD = repeated social defeat.
Conditioned place preference
Details of the apparatus and the procedure of cocaine CPP are described in the Supporting Information and follow the protocol described previously (Rodríguez-Arias et al., 2009; Vidal-Infer et al., 2012).
Intravenous cocaine self-administration
Cocaine self-administration sessions were performed in accordance with the previously described protocols (Soria et al., 2005). Details of the apparatus, surgery and the cocaine self-administration procedure are included in the Supporting Information.
Corticosterone measurements (enzyme-linked immunosorbent assay)
Corticosterone determination was performed as previously described (García-Pardo et al., 2014), and a detailed description is included in the Supporting Information.
Immunohistochemistry
Mice were anesthetized with sodium pentobarbital and perfused transcardially through the left ventricle with 100 ml of phosphate-buffered saline (0.1 M PBS, pH = 7.4) followed by 100 ml of 4 percent paraformaldehyde–PBS. Brains were removed, postfixed in the same solution for 4 hour at room temperature and cryoprotected by immersion in 30 percent sucrose–PBS at 4°C. The brains were sliced at 30 µm in the coronal plane and stored in cryoprotectant solution. They were then frozen and stored at −20°C. Immunohistochemical studies of the NAc and hippocampus were performed, and the sections were localized using a mouse brain stereotaxic atlas (Franklin & Paxinos, 1997).
For labeling studies, cerebral free-floating sections were blocked by incubation with 0.5 percent BSA, 10 percent normal goat serum and 0.1 percent Triton X-100 for 1 hour and incubated at 4°C with the appropriate primary antibodies (Claudin-5, Life Technologies (Carlsbad, CA, Estados Unidos), 1:500; Laminin, Sigma, 1:1000; Collagen IV, Abcam, 1:500 (Cambridge, Reino Unido)) followed by the secondary antibody Alexa Fluor™ 488 donkey antimouse IgG (1:1000) and Alexa Fluor™ 594 donkey antirabbit IgG (1:1000). They were then mounted in ProLong®Gold with DAPI (Life Technologies).
IgG leakage from serum into the brain was assessed as a marker of vasculature damage. After three washes with 0.1 M PBS, sections were blocked by incubation with 0.5 percent BSA, 10 percent goat serum and 0.1 percent Triton X-100 for 60 minutes and then incubated at 4°C overnight with the antibody Alexa Fluor™ 594 donkey antimouse IgG (1:1000) and covered with ProLong®Gold (Life Technologies). Images were acquired with a Zeiss Axio Imager A1 (Jena, Alemania) microscope with eight fields of 40× magnification per animal and condition. All images were converted to gray scale, and blood vessels were outlined to provide an integrated gray scale value for image analysis with imagej software (version 1.43; NIH, New York, NY, USA).
Figure representative images were acquired sequentially using a Leica TCS-SP2AOBS confocal microscope (Leica Microsystems, Heidelberg, Germany) for each fluorophore in order to avoid any cross-signal between them. Control experiments were performed in which sections were stained with each of the secondary antibodies or with a combination of them to rule out the possibility of reaction between them, and images were taken using the same settings for each antibody staining.
Statistical analyses
For the CPP data corresponding to each cocaine dose, the time spent in the drug-paired compartment during pre-C and post-C tests was analyzed with a mixed two-way ANOVA, with one between-subjects variable [Stress, with two levels (RSD and Control)] and a within-subjects variable [Days, with two levels (pre-C and post-C)]. In all cases, post hoc comparisons were performed with Bonferroni tests. In the groups showing CPP, extinction and reinstatement values were analyzed with Student's t tests. The time required for the preference to be extinguished in each animal was analyzed by means of the Kaplan–Meier test, with Breslow (generalized Wilcoxon) comparisons when appropriate (Daza-Losada et al., 2009). Although the mean of the group as a whole determined the day on which extinction was considered to have been achieved, preference was confirmed to have been extinguished when a mouse spent 380 seconds or less in the drug-paired compartment on two consecutive days. We chose this time based on the values of all the pre-C tests performed in the study (mean = 370 seconds).
Corticosterone levels at minute 0 (immediately after) and 30 of the first and fourth social defeat and 3 weeks after the last social defeat encounter were analyzed with a mixed ANOVA with one between-subjects variable [Stress, with two levels (RSD and Control)] and a within-subjects variable (Time, with five levels). Post hoc comparisons were performed with Bonferroni tests.
An ANOVA with one within-subjects variable [Days, with two levels (first and fourth encounter)] was employed to evaluate each of the behaviors during the social encounter.
Data obtained within the different experimental groups of cocaine self-administration were compared using a three-way ANOVA with a between variable [Stress, with two levels (RSD and Control)] and repeated measures (day and hole as within group factors) followed by subsequent one-way ANOVA (hole as within group factor) when a main effect was revealed. Statistical significance criterion was P < 0.05. Data are expressed as number of reinforcers (mean + SEM) during each daily self-administration session for FR1 and FR3, and value of the breaking point (mean + SEM) obtained in the PR schedule.
Data of IgG extravasation and claudin-5, laminin and collagen-IV expression were analyzed using two-way ANOVA followed by a Bonferroni multiple comparison test (graphpad prism 5.0, GraphPad Software Inc., San Diego, CA, USA) with two between-subjects variables [Stress, with two levels (RSD and Control), and Treatment, with three levels (saline, 1 and 25 mg/kg)]. Differences were considered significant when P < 0.05. graphpad prism 5.0 and spss (Armonk, NY, Estados Unidos) version 19 software were used.
Results
Behavioral characterization of social defeat in adolescent mice
The times opponent mice spent engaged in aggressive behaviors are shown in Supporting Information Table 2. In aggressive opponent mice, ANOVA revealed a significant effect of the variable Days for the time spent in attack [F(1, 7) = 9.497; P < 0.01], and the latency of threat [F(1, 7) = 7.450; P < 0.05] and attack [F(1, 7) = 15.511; P = 0.01]. During the fourth social defeat encounter, aggressive opponent mice threatened and attacked earlier and spent more time in attack behavior than during the first social defeat.
In defeated mice, the ANOVA revealed a significant effect of the variable Days on defense/submission [F(1, 7) = 24.828; P = 0.01] and latency of defense/submission [F(1, 7) = 66.741; P = 0.001] and avoidance [F(1, 7) = 6.028; P = 0.05]. Defeated mice spent more time engaged in submissive behavior and exhibited defensive/submissive and avoidance behaviors sooner during the fourth encounter than in the first encounter.
Effect of repeated social defeat on corticosterone levels
Blood concentrations of corticosterone are shown in the Supporting Information (Table S1).
Effects of repeated social defeat on the acquisition and reinstatement of cocaine-induced CPP
Conditioned place preference induced by 1 mg/kg of cocaine
The ANOVA revealed a significant effect of the Interaction Days × Stress [F(1, 30) = 6.458; P < 0.01] (Fig. 1a). The RSD-C1 group spent more time in the drug-paired compartment in the post-C test than in the pre-C test (P < 0.01). Extinction of preference was achieved after five extinction sessions and administration of a priming dose of 0.5 mg/kg of cocaine-induced reinstatement of the preference (P < 0.05). This preference was extinguished after four more sessions, and a further priming dose of 0.25 mg/kg of cocaine did not reinstate the preference.
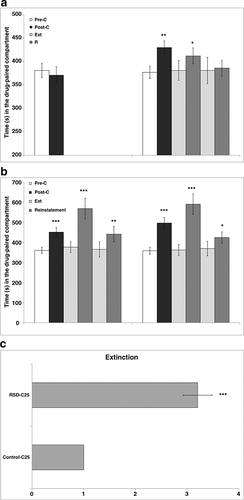
Conditioned place preference induced by 25 mg/kg of cocaine
The ANOVA revealed a significant effect of the variable Days [F(1, 27) = 62.215; P < 0.001]. Both groups developed CPP, as they spent more time in the drug-paired compartment in the post-C test than in the pre-C test (P < 0.001) (Fig. 1b).
Kaplan–Meier analysis of the data recorded during the extinction test revealed that more time was required to achieve extinction in the RSD-C25 group (four sessions) than in the Control-C25 group (one session) (χ2 = 27.000; P < 0.001) (Fig. 1c). Once the preference had been extinguished, it was reinstated in all the adolescent groups with a priming dose of 12.5 mg/kg of cocaine (P < 0.001 in both cases). This new preference was extinguished after one session in both groups. A further priming dose of 6.25 mg/kg of cocaine reinstated once again the preference in both groups (P < 0.01 for Control-C25 and P < 0.05 for RSD-C25).
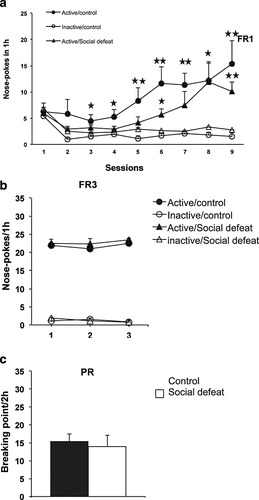
Effects of repeated social defeat on cocaine self-administration
The reinforcing properties of cocaine were also evaluated in mice that underwent RSD using an operant self-administration paradigm. Similar percentages of the mice exposed to social defeat (27 percent in 8.40 ± 0.51 days) and control animals (35 percent in 8.00 ± 0.37 days) reached the acquisition criteria of the operant responding during FR1 training. A significant discrimination between the active and inactive holes was revealed in social defeat (days 6, 8 and 9) and control (from day 3 to 9) mice during FR1 (Fig. 2a) and FR3 (days 1, 2 and 3 in both groups) training periods. A progressive increase in the number of active nose-poking responses was observed in both groups during FR1 training. No significant differences in the number of active nose pokes were detected between social defeat and control groups during FR1 and FR3. In the PR schedule, the breaking points achieved by both groups were also similar (Fig. 2b and c). More information is presented in Tables S3 to S5.
Changes induced by social defeat in the expression of claudin-5 and basal laminin proteins and in IgG extravasation in the NAc and effect of cocaine
Quantitative analysis of claudin-5, laminin, collagen-IV and IgG immunoreactivity by two-way ANOVA (Fig. 3a–d) revealed a significant effect of Stress and Treatment, but not Interaction, indicating that stress had a similar effect in saline-treated and cocaine-treated mice (Table S6). Mice exposed to social defeat showed a decrease in claudin-5, laminin and collagen-IV expression and an increase in IgG immunoreactivity with respect to the control group, effects that were not modified by cocaine administration. Cocaine at the dose of 25 mg/kg significantly reduced claudin-5, laminin and collagen-IV and increased IgG immunostaining compared with the saline group. At the dose of 1 mg/kg, cocaine induced a reduction only of laminin and an increase of IgG immunostaining with respect to the saline group. Mice exposed to social defeat and receiving cocaine 1 mg/kg exhibited a decrease in claudin-5 and collagen-IV immunoreactivity compared with the corresponding cocaine control group.
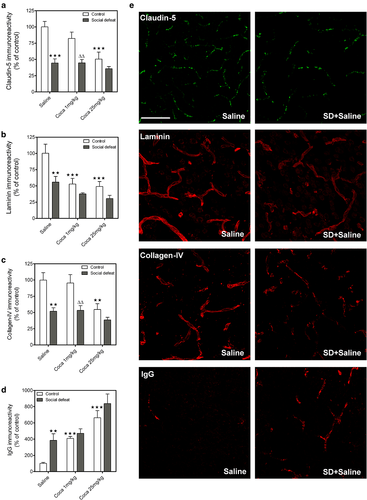
Figure 3e shows fluorescence images (40×) of representative claudin-5, laminin and collagen-IV immunostained sections of NAc.
Changes induced by social defeat on the expression of claudin-5 and basal laminin proteins in the hippocampus and effect of cocaine
Quantitative analysis of claudin-5, laminin and collagen-IV in the dentate gyrus, CA1 and CA3 by two-way ANOVA (Fig. 4a–d) revealed a significant effect of Stress and Treatment, but not Interaction, indicating that stress exerted a similar effect in saline-treated and cocaine-treated mice (for more details, see Supporting Information, Table S7). Mice exposed to social defeat showed a decrease in claudin-5, laminin and collagen-IV expression in the three hippocampal areas studied compared with the control group. Cocaine at the dose of 25 mg/kg significantly reduced laminin in the dentate gyrus and CA3 with respect to saline treatment. Mice exposed to social defeat and given cocaine 1 mg/kg showed a decrease in claudin-5 and collagen-IV immunoreactivity in the three areas studied (except for collagen-IV in the dentate gyrus) compared with their cocaine-treated counterparts. Social defeat plus 25 mg/kg of cocaine reduced claudin-5 in the CA3, laminin in the CA1, and collagen-IV in the dentate gyrus and CA1 with respect to the corresponding cocaine control group.
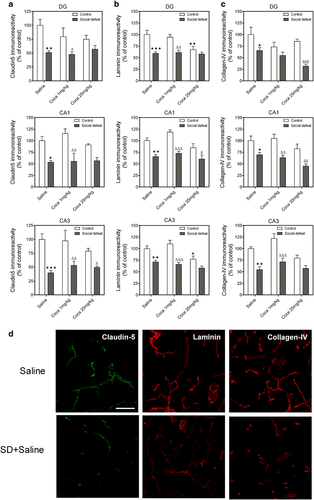
Figure 4d shows fluorescence images (40×) of representative claudin-5, laminin and collagen-IV immunostained sections of CA1.
Discussion
This study provides the first evidence that RSD during adolescence impairs the structure and permeability of the BBB and modifies the rewarding effects of cocaine in adulthood. The novelty of our results lies in the evaluation of the long-lasting effects of RSD during adolescence on (1) the conditioned reinforcing effects of cocaine during adulthood and (2) BBB integrity by means of the quantification of claudin-5 (a tight junction protein), laminin and collagen-IV (main proteins forming basal lamina) immunoreactivity and IgG extravasation in the NAc and hippocampus.
Conditioned place preference and self-administration
Mice that experienced social defeat during adolescence developed CPP with a dose of cocaine (1 mg/kg) that was not effective in control animals. In addition, a priming dose of 0.5 mg/kg of cocaine reinstated the extinguished preference in RSD mice. An effective dose of cocaine (25 mg/kg) induced CPP in all groups, but RSD mice needed significantly more sessions than control animals for the preference to be distinguished. Only one study has previously evaluated the effect of chronic social defeat stress during adolescence on the CPP induced by amphetamine. In accordance with our results, Burke et al. (2011) observed that RSD during adolescence increased preference for amphetamine-paired cues in adulthood.
Although numerous studies show that social defeat in adulthood increases cocaine self-administration (e.g. Miczek et al., 2004), to date, no study has evaluated the effects of social defeat when experienced during adolescence. Social stressors experienced in adulthood can significantly shorten the latency to acquire cocaine self-administration and to maintain this behavior at low unit doses (Kabbaj et al., 2001; Yap et al., 2015; Han et al., 2015). Studies in this line have been performed mainly in rats, and cocaine self-administration has been evaluated immediately after the stressful experience or after a maximum of 10 days. Our results show that RSD experienced during adolescence delays the acquisition of cocaine self-administration when an effective dose is administered in adulthood. Control animals identified the inactive hole in the third session, while defeated mice required six sessions to discriminate the active hole. We cannot rule out that this delay may have been due to deficits in learning or memory processes. Although several studies have shown that RSD during adolescence does not affect aversive memory in the passive avoidance task or spatial learning (Buwalda et al., 2005; Rodriguez-Arias et al., 2015), previous findings have shown that social stress in adolescence results in deficits in hippocampal-based spatial memory (McCormick et al., 2012; Sterlemann et al., 2010). In line with this, a recent report by Novick et al. (2013) showed that rats defeated during adolescence displayed long-lasting deficits in spatial working memory performance. In our study, once self-administration was established, adolescent RSD did not seem to affect performance in a progressive schedule of reinforcement, suggesting that it does not contribute to the motivational properties of cocaine. Exposure during adolescence to other types of stress, such as early social isolation, resulted in heightened acquisition of cocaine self-administration at low doses in adulthood, while a recent dose–response analysis has revealed that sensitivity to cocaine reinforcement is not altered (Baarendse et al., 2014). Moreover, a single episode of early maternal deprivation has been shown to impair motivation for cocaine in adolescent mice; deprived mice require more time to achieve acquisition criteria, and the maximal effort required to obtain cocaine infusion is also significantly reduced (Martini & Valverde, 2012).
One possible explanation for the increased preference observed in our CPP study and the slow acquisition of self-administration is that RSD increased the sensitivity of the mice to the conditioned rewarding effects of cocaine. The low dose of cocaine administered in the CPP may have been experienced more intensely by defeated mice, which would have been more sensitive than their non-defeated counterparts. Consequently, these mice would have acquired CPP with a non-effective dose. However, as we employed an effective dose in the self-administration procedure, defeated mice would have experienced a stronger subjective effect, which would have induced them to self-administer this cocaine dose at a lower rate. In support of this hypothesis, previous studies have reported that although amphetamine-induced DA increases in the medial prefrontal cortex are attenuated in adult rats socially defeated in adolescence, the NAc core DA response to amphetamine is more pronounced than in non-defeated controls (Burke et al., 2010). Moreover, it should be taken into consideration that social defeat during adolescence can differ to that observed in adult animals. In addition to the fact that adolescent rodents display lower physiological responses to social stressors (Adriani et al., 1998), the first social encounter was less intense than the fourth, with aggressive opponents exhibiting less aggression. In line with previous reports (García-Pardo et al., 2014), the corticosterone levels of the mice defeated in adolescence did not rise after the first social defeat encounter; in fact, the increase was significant only after the fourth social defeat. Defeated mice also exhibited higher levels of defense and submission in the last encounter. Importantly, each procedure was performed in a different strain of mice, which could be responsible for the different results obtained. Finally, the inconsistencies in the effects of RSD in the CPP and self-administration procedures (increased CPP and slower acquisition of nose-poking response) may also be due to the fact that these paradigms evaluate different aspects of reward. Self-administration models drug-taking behavior and evaluates the primary rewarding properties of drugs, while CPP assesses the incentive value of drug-associated cues for maintaining addictive behavior. We have found that exposure to RSD during adolescence, although not modifying the primary hedonic properties of cocaine in adult mice, increases the sensitivity of these animals to the conditioned rewarding effects of the drug, thus enhancing the ability of drug-related cues to maintain drug-seeking behavior.
Blood–brain barrier integrity
The current study shows for the first time that mice exposed to RSD undergo significant changes in BBB structure. RSD during adolescence induces a marked reduction in expression of the tight junction protein claudin-5 and an increase in basal laminin degradation (reflected by a decrease in laminin and collagen-IV expression) in the NAc in adulthood. Concomitantly, there is an increase in IgG extravasation, indicating that social defeat increases BBB permeability, probably through alterations in structural proteins. It is worth noting that the effect of stress on the disruption of BBB in our experimental animals was not brain region specific, as similar results were obtained when the subfields of the hippocampus, dentate gyrus, CA1 and CA3 were analyzed. In addition, social defeat induced the same alterations independent of previous cocaine exposure. Although there is a lack of studies on the effect of RSD on BBB integrity, there is abundant information regarding the effect of cocaine on BBB permeability. Our results confirm the detrimental effect of cocaine on the integrity of the BBB. Four administrations of the higher dose of cocaine on the post-C test day also affected BBB integrity in the NAc and increased the effect of social defeat on the hippocampus.
Although the mechanisms underlying these effects have not been evaluated in the current study, we believe that the effect of social defeat on BBB could have been produced by an increase in the activation of MMPs, specifically gelatinases such as MMP-9 and MMP-2. An increased gelatinolytic activity may disrupt BBB through proteolytic activity in the tight junctions of endothelial cells and basal lamina. In fact, laminin, collagen-IV and fibronectine are substrates of gelatinases. Numerous stimuli are reported to be involved in increased MMP activity and the basal laminin degradation that occurs as a consequence. Release of proinflammatory cytokines, particularly IL-1β, could be involved in augmented MMP-9 activity (Gottschall & Deb, 1996; Vecil et al., 2000). Previous studies have shown that social defeat influences inflammatory immune processes, including concentrations and mRNA expression of the proinflammatory cytokines IL-6, IL-1β and TNF-α in both plasma and brain (Bartolomucci et al., 2003; Audet et al., 2011; McQuaid et al., 2013), which may induce an increment in MMP expression, as observed in other conditions.
In addition to BBB disruption, mice socially defeated in adolescence take longer to acquire cocaine self-administration and are more sensitive to the rewarding properties of cocaine conditioning in adulthood. Recent studies performed in socially defeated adult animals have demonstrated that this stress enhances DA release from the NAc shell in response to an acute dose of d-amphetamine or cocaine (Han et al., 2015; Shimamoto et al., 2015). This effect may be attributable to a high concentration of d-amphetamine or cocaine in the NAc owing to the increased BBB permeability induced by social defeat. Recently, it has been shown that RSD increases extracellular signal-regulated kinase phosphorylation in the ventral tegmental area and that inhibition of extracellular signal-regulated kinase activation prior to each social defeat attenuates the development of stress-induced sensitization and prevents stress-induced enhancement of cocaine self-administration during a continuous access binge (Yap et al., 2015). Social defeat also induces phosphorylation of p38α MAPK in the dorsal raphe nucleus, and p38α MAPK deletion in serotonergic neurons prevents stress-induced reinstatement of cocaine seeking (Bruchas et al., 2011). It is well known that MAPKs—downstream signaling pathways of proinflammatory cytokines—regulate MMP-9 expression and activity (Kim & Choi, 2010; Urrutia et al., 2013) in such a way that an increase in MAPK phosphorylation increases MMP activity and facilitates BBB disruption. Inhibition of MAPK activity could prevent the effects induced by social defeat on BBB permeability and on the rewarding properties of drugs of abuse.
Together, these findings suggest that stress and cocaine can increase the long-term vulnerability of the brain to subsequent environmental insults as a consequence of sustained disruption of the BBB. This interaction between RSD and cocaine is particularly relevant, as chronic stress is a common contributing factor to the high rate of comorbidity between cocaine abuse and mental disorders.
Acknowledgements
This work was supported by the Spanish Ministerio de Economía y Competitividad ‘Instituto de Salud Carlos III’, RETICS: RD12/0028/0005, RD12/0028/0023, RD12/0028/0002, PSI2011-24762, PSI2014-51847-R, SAF2011-29864 and SAF2013-40592-R.

It is also supported by the Catalan Government (2014SGR1547) and the Valenciano Government (PROMETEOII/2014/063). The research leading to these results has also received funding from the European Community's Seventh Framework Programme (NEUROPAIN, HEALTH-F2-2013).
Disclosure/Conflict of Interest
None.
Authors Contribution
All named authors have made an active contribution to the conception, design, analysis and drafting of the article. MRA, SMR, MAA and JM performed the social defeat protocol and evaluated social, emotional and cognitive behavioral parameters; furthermore, they performed and evaluated the CPP paradigm and the corticosterone measurements. ARA, FP and MIC were responsible for the immunohistochemistry analysis. EMG, RC and RM were responsible for the cocaine self-administration experiments. All authors critically reviewed the content and approved the final version for publication. All authors have contributed equally to the overall coordination of the whole study.




