A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm
Abstract
Concurrent use of cocaine and heroin is a major public health issue with no effective relapse prevention treatment currently available. To this purpose, a combination of buprenorphine and naltrexone, a mixed very-low efficacy mu-opioid receptor agonist/kappa-opioid receptor antagonist/nociceptin receptor agonist, was investigated. The tail-withdrawal and the conditioned place preference (CPP) assays in adult Sprague Dawley rats were used to show that naltrexone dose-dependently blocked the mu-opioid receptor agonism of buprenorphine. Furthermore, in the CPP assay, a combination of 0.3 mg/kg buprenorphine and 3.0 mg/kg naltrexone was aversive. A combination of 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone was neither rewarding nor aversive, but still possessed mu-opioid receptor antagonist properties. In the CPP extinction and reinstatement method, a combination of 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone completely blocked drug-primed reinstatement in cocaine-conditioned rats (conditioned with 3 mg/kg cocaine, drug prime was 3 mg/kg cocaine) and attenuated drug-primed reinstatement in morphine-conditioned rats (conditioned with 5 mg/kg morphine, drug prime was 1.25 mg/kg morphine). These data add to the growing evidence that a buprenorphine/naltrexone combination may be protective against relapse in a polydrug abuse situation.
Introduction
There is currently no medication licensed in Europe or the United States for treatment of cocaine dependence, and while there are treatments available for opioid dependence, no single treatment is effective for everyone. As many users of crack cocaine are also dependent on heroin, a relapse prevention medication that is effective in the polydrug user population would be a notable step forward.
In this article, the ability of a combination of buprenorphine and naltrexone to inhibit reinstatement of morphine and cocaine conditioned place preference (CPP) was investigated. Buprenorphine is a partial agonist at the mu-opioid receptor, an antagonist at the kappa-opioid receptor and a partial agonist at the nociceptin (NOP) receptor (Huang et al. 2001). Buprenorphine, like methadone, is widely used as a substitution therapy for treatment of opioid addicts (Maremmani & Gerra 2010), but clinical studies have shown that buprenorphine, not methadone, is also effective in reducing cocaine use (Kosten, Kleber & Morgan 1989). Naltrexone is an antagonist at both mu- and kappa-opioid receptors (Giordano, Nock & Cicero 1990) and has been shown to reduce both opioid (Comer et al. 2006) and cocaine use (Schmitz et al. 2001).
Encouraging results have been observed in two clinical trials using a buprenorphine/naltrexone combination therapy (Rothman et al. 2000; Gerra, Fantoma & Zaimovic 2006); significant reduction of both heroin and cocaine use was demonstrated. Currently, buprenorphine is licensed as an opioid substitution therapy, but is in itself rewarding via activation of the mu-opioid receptor (Greenwald et al. 2007). Combination of buprenorphine with sufficient naltrexone can block buprenorphine's mu-opioid receptor agonism (Dum & Herz 1981; McAleer et al. 2003) thus, increasing regulatory acceptability and feasibility of its use in cocaine addicts. Naltrexone is itself licensed as an abstinence-promoter, but treatment success is hindered by low compliance. Naltrexone provides no reinforcement or pleasure, but, as a mu-opioid receptor antagonist, is likely to block rewards caused by release of endogenous opioid peptides (Mucha, Millan & Herz 1985; Kirchmayer et al. 2002). Indeed, in laboratory animals, naltrexone alone has been shown to be aversive at high doses (Parker & Rennie 1992; Suzuki et al. 1992).
Thus, the combination of buprenorphine and naltrexone may be neither rewarding nor aversive, but still an effective anti-addiction therapy. In addition to the effects on mu-opioid receptors, the buprenorphine/naltrexone combination acts as an antagonist at the kappa-opioid receptor. Co-morbid affective disorders can emerge on cessation of use of drugs of abuse (Gerra et al. 2006), and antagonism of the kappa-opioid receptor appears to counter depression in rodents (Mague et al. 2003; Beardsley et al. 2005). Further, the kappa-opioid system and its endogenous agonist dynorphin have been shown to mediate many stress responses; in pre-clinical studies, inhibition or genetic ablation of kappa-opioid receptors has been shown to inhibit stress-induced, but not drug–prime-induced, reinstatement to cocaine-seeking behavior (reviewed by Bruchas, Land & Chavkin 2010).
Another component of the pharmacology of a buprenorphine/naltrexone combination is to act as a partial agonist at the NOP receptor. Selective NOP agonists are neither rewarding nor aversive (Le Pen et al. 2002), and although the mechanism is poorly understood, they have been shown in rodents to oppose the effects of cocaine and morphine (Kotlinska et al. 2002; Sakoori & Murphy 2004), inhibiting drug-primed reinstatement of morphine CPP (Shoblock, Wichmann & Maidment 2005). The effect of NOP agonists on reinstatement of cocaine CPP has not been studied to date.
Overall, a mixed very-low efficacy mu-opioid receptor agonist/kappa-opioid receptor antagonist/NOP receptor agonist is a desirable target as a novel anti-addiction therapy (McCann 2008). The reduction in cocaine use observed in the studies carried out by Rothman et al. (2000) and Gerra et al. 2006) could be via antagonism of mu-opioid receptors attenuating the positive reinforcing effects of cocaine (Bilsky et al. 1992), via antagonism of kappa-opioid receptors conferring stress resilience (Redila & Chavkin 2008), or via more generalized anti-addictive effects of agonism of the NOP receptor (Shoblock et al. 2005; Kuzmin et al. 2007). Alternatively, it could simply be a consequence of reduction in heroin use (these two drugs of abuse are often used by addicts to ‘complement’ each other). Therefore, the effect of buprenorphine/naltrexone on the effects of cocaine in a controlled experiment has been assessed here. The aims of this study were to determine the ratio of buprenorphine/naltrexone, that is neither rewarding nor aversive, and then to evaluate how this drug combination can inhibit drug-primed reinstatement of both morphine- and cocaine-induced CPP.
Materials and Methods
Subjects
All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act of 1986 and the University of Bath's ethical review documents. Male Sprague Dawley rats (Charles River, Kent, UK) were used; 260–420 g (7–11 weeks old) for tail-withdrawal and rat vas deferens experiments, 250–320 g (7–9 weeks old) for CPP experiments. All rats were housed four per cage with ad libitum access to food and water and maintained on a 12:12 hours light–dark cycle (lights on 7:00 am, lights off 7:00 pm).
Tail-withdrawal assay
A water bath (Grant Instruments, Cambridgeshire, UK) was maintained at 52°C. The rats were held firmly in a vertical position and lowered until the distal third of the tail was in the water. The time taken for the rat to withdraw the tail was recorded. A 20-second cut-off was imposed to avoid tissue damage. All rats were opioid naïve and were not reused.
Measurement of receptor affinity—rat vas deferens
Rats were killed using CO2 and vasa deferentia were excised and suspended in a siliconized tissue bath (3 ml volume) under 0.5 g tension in Krebs’ bicarbonate solution [composition (mM): NaCl 118, KCl 4.74, CaCl2 2.50, KH2PO4 1.19, MgSO4 1.20, NaHCO3 25, glucose 11, bubbled with 95% O2/5% CO2 and maintained at 37°C]. Nerve-evoked muscle contractions were induced with single square pulses (0.1 millisecond duration, 0.1 Hz, supramaximal voltage) and measured isometrically with ‘LabChart’ software (AD Instruments, Oxford, UK). Cumulative concentration response curves to the selective mu-opioid receptor agonist, [D-Ala2,NMe-Phe4,Gly-ol5]-enkephalin (DAMGO; concentration increased at 5-minute intervals) were constructed in the absence then presence of buprenorphine or naltrexone.
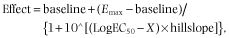
For naltrexone, a Schild plot was constructed, deriving a pA2 value (equivalent to the log KB value). As buprenorphine has been shown to be pseudo-irreversible, KB values were obtained using a single concentration of buprenorphine (1 nM) and the Schild equation.
Blood and brain tissue concentrations
Plasma sample preparation
Blood samples (30-minute post-injection) were centrifuged (3300 rpm, 10 minutes) and plasma recovered. Plasma samples were basified using ammonium hydroxide solution (pH 10) and loaded onto a solid phase extraction (SPE) cartridge (Waters Oasis HLB, Hertfordshire, UK) previously conditioned with 1 ml methanol and 1 ml water. The cartridge was washed with 1 ml 2% methanol in ammonium hydroxide solution (pH 10), rinsed with water and then the analyte was eluted with 440 μl 60% methanol containing 2% acetic acid. The sample was filtered (nylon 0.45-μm syringe filter) before injection into the liquid chromatography–mass spectrometer (LC–MS). Samples were injected undiluted to analyze buprenorphine, then diluted 1:1 with water to analyze naltrexone.
Brain tissue sample preparation
The brain tissue (30-minute post-injection) was prepared using the same SPE process as the plasma samples. Prior to the SPE process, water was added (1.8 ml/g of brain tissue) to facilitate homogenization (Tissue Master 240, OMNI International, Kennesaw, GA, USA), the sample was centrifuged and the supernatant fluid was collected.
Buprenorphine and norbuprenorphine LC–MS method
Separation was performed using a GeminiNX column (3 μm C18 110A 50 × 2 mm) from Phenomenex, Chesire, UK, maintained at 25°C, on a Shimadzu LC-2010AHT high-performance liquid chromatography (HPLC) (Shimadzu, Milton Keynes, UK). The mobile phase was 18:82 acetonitrile: 0.1% acetic acid at a flow rate of 0.2 ml/minute. Thirty microliters of sample was injected.
The retention times for buprenorphine and norbuprenorphine were 9 and 3 minutes; masses per charge were 468 and 414. Standards were prepared in SPE eluent. Limit of quantitation was 0.18 ng/ml for buprenorphine and 0.8 ng/ml for norbuprenorphine.
Naltrexone and 6β-naltrexol LC–MS method
The method for analysis of naltrexone and 6β-naltrexol was adapted from Valiveti et al. 2004). Separation was performed using a Symmetry column (5 μm C18 110A 150 × 2.1 mm) from Waters, maintained at 23°C, on a Shimadzu LC-2010AHT HPLC. The mobile phase was 12:88 acetonitrile: 0.1% ammonium acetate at a flow rate of 0.25 ml/minute. Thirty microliters of sample was injected.
The retention times for naltrexone and 6β-naltrexol were 4 and 3 minutes; masses per charge were 342 and 344. Standards were prepared in SPE eluent. Limit of quantitation was 3.8 ng/ml for naltrexone and 0.28 ng/ml for 6β-naltrexol.
CPP apparatus
CPP boxes (Tracksys, Nottingham, UK) were three-chambered shuttle boxes comprising a small central compartment (10 × 10 cm) where rats were placed at the start of a test session, and two larger compartments (40 × 40 cm), one with horizontal black and white stripes and one with vertical black and white stripes. Floors were made of stainless steel sheeting with punched-out shapes (circles, 12 mm hole and squares, 10 mm hole) resulting in distinct textures (Novametals, Manchester, UK). Removable partitions allowed the boxes to be used either to restrict the rats to a particular compartment for conditioning or to allow the rats to be ‘free-to-explore’ during a test session. Experiments were performed between 8:00 am and 5:00 pm under dim white light (light intensity approximately 15 lux). During all test sessions, the time each rat spent in each compartment was recorded using EthoVision XT (Tracksys) tracking software.
Rats were assigned to treatment groups randomly, and the pairing was counterbalanced (i.e. within each cohort, equal numbers of rats were always drug-paired to each compartment type). Groups were organized such that mean baseline % preferences were close to zero.
CPP
Data throughout are presented after multiplying by a correction factor. The correction factor was calculated by dividing duration of test (seconds) by total time spent (seconds) in the two large compartments. This factor is used to proportionally divide the time spent in the neutral central compartment between the two conditioning compartments.
Percent preference was calculated using the corrected data such that if a rat spent equal time in the drug-paired compartment and the saline-paired compartment, the preference score would be 0%. If a rat spent no time in the saline-paired compartment, the preference score would be 100%.
Individual rats whose baseline preference was > 16.7 or < −16.7 were excluded.
Drugs and chemicals
Naltrexone hydrochloride dihydrate was from Sigma (Dorset, UK). Saline (sodium chloride 0.9%) was from Dechra, Shropshire, UK. Buprenorphine hydrochloride was prepared in-house. Cocaine hydrochloride and morphine sulphate were from MacFarlan Smith, Edinburgh, UK. All in vivo injections were intraperitoneal (1 ml/kg).
DAMGO was from Bachem, Merseyside, UK. Sigmacote®, buffer components and mobile phase components were from Sigma.
Procedures
Using naltrexone to block the rewarding effects of buprenorphine
Two separate assays, tail withdrawal and CPP, were used to establish the dose of naltrexone required to block the mu-opioid receptor agonism of buprenorphine.
Tail-withdrawal assay
Five baseline measurements were taken, one immediately after another, for each rat. In combination with either 0, 0.3 or 1.0 mg/kg naltrexone (n = 5, 4, 7), 0.3 mg/kg buprenorphine was then administered. Following injection of the drug, measurements were taken once every 7.5 minutes, up to 60 minutes. Data collected at the 60-minute time-point were used in subsequent analyses. Baseline tail-withdrawal time was taken as the mean of the last two baseline measurements. For each rat, analgesia was quantified as tail-withdrawal time post-drug treatment minus baseline measurement. Data were analyzed using one-way analysis of variance with the Bonferroni post-test.
CPP assay
Rats were conditioned with buprenorphine (0.3 mg/kg) administered in combination with either 0, 0.3, 1 or 3 mg/kg naltrexone (n = 16, 8, 8, 16). On day 1, rats had a 15-minute exploratory session; on day 4, they had a 15-minute baseline preference test. A % preference was obtained. On days 5–8 and 11–14, the rats received drug or saline on alternate days, thus, each rat had four drug injections and four saline injections. Injections were administered at least 24 hours apart to ensure that the effects of buprenorphine had dissipated before subsequent saline injections. Following injections, the rats were immediately confined to a compartment for 40 minutes. On day 15, % preference was obtained exactly as for baseline preference; i.e. the preference for each drug treatment was measured by recording the time spent in the drug-paired chamber in a free-to-explore test lasting for 15 minutes. To assess conditioning, a one-tailed Wilcoxon matched pairs signed-rank test was used (each group's % preference after drug treatment compared with its baseline).
Measuring mu-opioid receptor antagonism of a buprenorphine/naltrexone combination
Five baseline measurements were taken. Latency to withdrawal was measured following administration of 10 mg/kg morphine only (n = 4), and when both 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone (n = 5) or 1.0 mg/kg naltrexone alone (n = 7) were administered 30 minutes prior to the morphine. Following injection of the morphine, measurements were taken once every 5 minutes, up to 30 minutes. Data collected at the 30-minute time point were used in subsequent analyses.
Effects of buprenorphine/naltrexone on reinstatement of cocaine and morphine CPP
To test the ability of a buprenorphine/naltrexone combination to block drug-primed reinstatement, a CPP extinction and reinstatement method was established (Fig. 1).

Schematic of the time course of the conditioned place preference extinction and reinstatement method. Filled-in circles represent cocaine or morphine injections and empty circles represent saline injections (it can be seen that during conditioning, the order of injections alternated daily). This schematic shows the reconditioning style of extinction, where each rat received two saline injections per day. The arrow indicates that buprenorphine/naltrexone treatment (or saline, in the control groups) was administered 10 minutes prior to the priming dose
Rats had one 15-minute exploratory session and one 15-minute baseline preference test. Animals were conditioned using either 3 mg/kg cocaine or 5 mg/kg morphine, receiving drug and saline on the same day (at least 4 hours apart) for 3 consecutive days. Immediately after injection, rats were confined to a particular compartment (drug-paired or saline-paired) in the CPP box (for 20 or 40 minutes, cocaine or morphine, respectively). Following conditioning, % preference was obtained exactly as for baseline preference. Individual rats, which showed less than a 30-second increase for drug-paired side over their baseline preference during the post-conditioning test were excluded from extinction and reinstatement. To assess conditioning before exclusions, a one-tailed Wilcoxon matched pairs signed-rank test was used (each group's % preference after drug treatment compared with its baseline).
Four cohorts of rats were used: cocaine-conditioned control (n = 20), morphine-conditioned control (n = 24), cocaine-conditioned buprenorphine/naltrexone treatment (n = 16) and morphine-conditioned buprenorphine/naltrexone treatment (n = 16).
For all cocaine-conditioned rats, extinction was achieved by reconditioning. This involved injection of saline followed by confinement to a compartment for 20 minutes, twice a day for 4 days (4 hours apart). The rats were alternated daily as to whether they were placed in the previously drug-paired compartment or the previously saline-paired compartment morning or afternoon. Extinction was confirmed by a 15-minute free-to-explore test (day 15, see Fig. 1).
For the morphine-conditioned rats, two styles of extinction were used: a reconditioning style of extinction (saline injection with 15 minutes confinement in each compartment, as shown earlier) and a retesting style of extinction (daily retesting, 15 minutes per test). Of the animals subsequently tested for reinstatement, five rats in the control group underwent extinction training using the reconditioning style and nine underwent extinction using the retesting style. All rats in the buprenorphine/naltrexone treatment group underwent extinction training using the reconditioning style.
Extinction training was deemed complete if group mean preference was < 5%. For the retesting style of extinction, this was taken from the average of 2 consecutive days. Following extinction, rats were administered a priming dose of 3 mg/kg cocaine or 1.25 mg/kg morphine. The control groups received a saline injection 10 minutes prior to drug priming and the treatment groups received a buprenorphine/naltrexone injection (0.3 and 1 mg/kg, respectively) 10 minutes prior to drug priming. Following administration of the priming dose, rats were placed immediately into the CPP boxes and were free-to-explore during a 30-minute test.
Conditioning and reinstatement were assessed using the Friedman's test followed by Dunn's multiple comparison test (each group's % preference compared with its baseline). A Mann–Whitney U-test was used to compare the % preference during the reinstatement test for rats that had undergone a retesting style of extinction and for rats that had undergone a reconditioning style of extinction.
It was observed that in the control groups, CPP behavior emerged at 13–15 minutes of the reinstatement test period in cocaine-conditioned rats; however, in the morphine-conditioned rats, drug-seeking was evident from the start of the reinstatement test period and then diminished somewhat after 15 minutes (data not shown). The data from 0 to 30 minutes of the reinstatement test were used for the cocaine-conditioned rats, whereas, the data from 0 to 15 minutes of the test were used for the morphine-conditioned rats. It is interesting that these findings are in contrast to Mueller & Stewart (2000) and Mueller, Perdikaris & Stewart (2002) who observed gradual emergence of drug-seeking over the course of the reinstatement test in both cocaine- and morphine-conditioned rats.
Results
Using naltrexone to block the rewarding effects of buprenorphine
Tail-withdrawal assay
Tail withdrawal is a commonly used assay of analgesia, and was used here as an indirect measure of mu-opioid receptor agonism. A dose of 0.3 mg/kg buprenorphine was selected because previous studies in rats have indicated that this dose is rewarding in the CPP assay (Suzuki et al. 1992; Rowlett, Gibson & Bardo 1994; Tzschentke 2004) and can elicit measurable mu-opioid receptor-mediated analgesia (Lutfy et al. 2003). Figure 2a shows that 0.3 mg/kg buprenorphine elicited marked analgesia in this experimental setup. This can be seen by the increase in time taken to withdraw the tail compared with baseline. A clear dose-dependent effect of naltrexone countering buprenorphine-induced analgesia was observed.
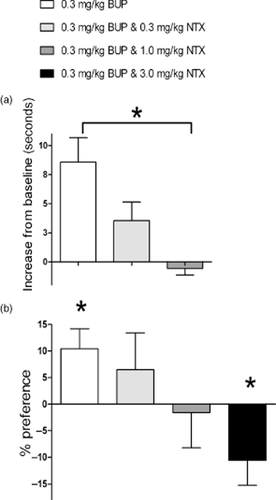
The tail-withdrawal assay and the conditioned place preference (CPP) assay show how naltrexone (NTX) blocks the mu-opioid receptor agonism of buprenorphine (BUP) in a dose-dependent fashion. (a) Increase from baseline (seconds) in tail-withdrawal assay at 60 minutes time point (n = 5, 4, 7), mean + SEM. Baseline values were in the range 4–6 seconds; *Indicates that first and third columns are significantly different from one another P < 0.05. (b) The % preference in CPP assay (n = 15, 7, 8, 16), mean + standard error of the mean (SEM); *Indicates significantly different from baseline P < 0.05. From left to right: 0.3 mg/kg BUP, 0.3 mg/kg BUP and 0.3 mg/kg NTX, 0.3 mg/kg BUP and 1.0 mg/kg NTX, and (CPP only) 0.3 mg/kg BUP and 3.0 mg/kg NTX
CPP assay of rewarding effects of buprenorphine
Group mean baseline preferences were 0 ± 2, 2 ± 5, 2 ± 2 and 0 ± 2% [mean ± standard error of the mean (SEM) ]. One rat in the 0.3 mg/kg buprenorphine group and one rat in the 0.3 mg/kg buprenorphine and 0.3 mg/kg naltrexone group were excluded for having a preference at baseline.
In this setup, 0.3 mg/kg buprenorphine elicited drug-seeking behavior (Fig. 2b). This can be seen as a significant increase in time spent in the drug-paired compartment compared with baseline. As observed in the tail-withdrawal assay, a clear dose-dependent effect of naltrexone was observed; naltrexone countered the rewarding effects of buprenorphine to the extent that co-administration of 3.0 mg/kg naltrexone actually elicited aversion (rats spent significantly more time in the saline–paired compartment compared with baseline test).
There was good agreement between the results of the tail-withdrawal and the CPP assays; taken together, it was clear that, in these rats, 1.0 mg/kg naltrexone blocked the mu-opioid receptor agonism of 0.3 mg/kg buprenorphine. This finding fits with earlier data (Walker et al. 1994), which showed that 1.0 mg/kg naltrexone blocked the subjective effects of 0.3 mg/kg buprenorphine in a discrimination assay in rats. A combination of 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone was therefore selected for use in subsequent behavioral experiments.
Measuring mu-opioid receptor antagonism of a buprenorphine/naltrexone combination
After establishing that 1.0 mg/kg naltrexone was sufficient to block the mu-opioid receptor agonism of 0.3 mg/kg buprenorphine, we tested whether this combination would show mu-opioid receptor antagonism; 10 mg/kg morphine elicited a measurable analgesia (Fig. 3).
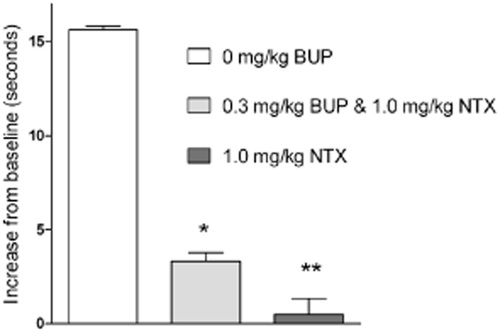
The tail-withdrawal assay shows antagonism of morphine by naltrexone (NTX), with and without buprenorphine (BUP). Data shows measurements taken 30 minutes after administration of 10 mg/kg morphine, and 60 minutes after administration of BUP and/or NTX. From left to right, rats received 0 mg/kg BUP (n = 5), 0.3 mg/kg BUP and 1.0 mg/kg NTX (n = 4), and 1.0 mg/kg NTX (n = 7). Baseline values were in the range 4–5 seconds. Mean + standard error of the mean (SEM); *Indicates significantly different (P < 0.05) versus morphine alone group; **Indicates significantly different (P < 0.05) versus morphine alone group and versus 0.3 mg/kg BUP and 1.0 mg/kg NTX group
Clear mu-opioid receptor antagonism was observed following administration of buprenorphine/naltrexone (morphine-induced analgesia decreased from 16 ± 0 to 3 ± 1 seconds, mean ± SEM). Administration of naltrexone alone blocked morphine-induced analgesia to a greater extent than did the combination.
CPP extinction and reinstatement model
The CPP extinction and reinstatement method is frequently used to study the effects of potential relapse prevention treatments (reviewed by Aguilar, Rodriguez-Arias & Minarro 2009). This method was therefore used to observe drug-primed reinstatement in cocaine- and morphine-conditioned rats, and the effects thereon of pretreatment with a buprenorphine/naltrexone combination. Table 1 shows the number of rats used in the experiments, the number of rats excluded and the time taken to reach extinction. Conditioning was statistically significant in each cohort before individual rats were excluded for insufficient conditioning.
| Cocaine control cohort | Morphine control cohort | Cocaine treated cohort | Morphine treated cohort | |
|---|---|---|---|---|
| At start | 20 | 24 | 16 | 16 |
| Excluded for preference | 0 | 2 | 2 | 1 |
| Excluded for insufficient conditioning | 8 | 8 | 5 | 7 |
| Time taken to reach extinction | 1 week | 1 week or 12 days | 1 week | 1 week |
Control groups
Figure 4 shows the data for the control groups (panels a and b). The % preference post-conditioning and following a drug prime was significantly different from baseline for both drugs. In other words, reinstatement of drug-seeking was successfully attained in both control groups. As there was no significant difference in % preference during the reinstatement test between the morphine-conditioned rats, which underwent the two styles of extinction training used (reconditioning 11 ± 7% n = 5, retesting 19 ± 9% n = 9, mean ± SEM), pooled data are shown.
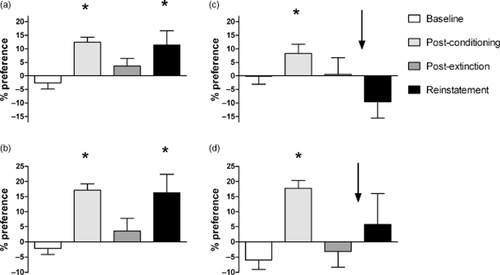
The conditioned place preference (CPP) assay shows the effect of buprenorphine/naltrexone treatment on reinstatement to drug-seeking. Percent preference of rats (left to right in each panel: baseline, post-conditioning, post-extinction and drug-primed reinstatement). Drug prime was 3 mg/kg cocaine or 1.25 mg/kg morphine. (a) Cocaine-conditioned control group (n = 12); (b) morphine-conditioned control group (n = 14). (c) Cocaine-conditioned buprenorphine/naltrexone treated group (n = 9); (d) morphine-conditioned buprenorphine/naltrexone treated group (n = 8). Mean + standard error of the mean (SEM); *Indicates significantly different from baseline P < 0.05. Arrows indicate that buprenorphine/naltrexone treatment (0.3 mg/kg/1.0 mg/kg) was administered 10 minutes prior to the priming dose
Buprenorphine/naltrexone treatment groups
Figure 4 shows the data for the buprenorphine/naltrexone treated groups (panels c and d); the effect of the combination on drug-primed reinstatement in cocaine- and morphine-conditioned rats is clear. Preference during reinstatement test was not significantly different from baseline for either drug. In the cocaine-conditioned rats, the buprenorphine/naltrexone treatment completely blocked reinstatement (preference score of −9 ± 5% mean ± SEM compared with 11 ± 5 in the control group). In the morphine-conditioned rats, the buprenorphine/naltrexone treatment attenuated the preference observed following administration of a drug prime (preference score of 6 ± 10%, mean ± SEM compared with 16 ± 6 in the control group).
Receptor occupancy of buprenorphine and naltrexone
Having demonstrated that a combination of 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone is neither rewarding nor aversive, and is capable of blocking drug-primed reinstatement to cocaine- and morphine-seeking, we next estimated the relative receptor occupancies of both drugs.
Receptor affinity values were determined in isolated tissue (rat vas deferens) and logKB values were −9.38 ± 0.12 for buprenorphine (i.e. KB = 0.41 nM) and −8.90 ± 0.12 for naltrexone (i.e. KB = 1.26 nM) (Fig. 5). Plasma and brain concentrations were measured 30 minutes after administration of buprenorphine (0.3 mg/kg) and naltrexone (1.0 mg/kg) (Table 2). From these data, receptor occupancy levels of each could be estimated.
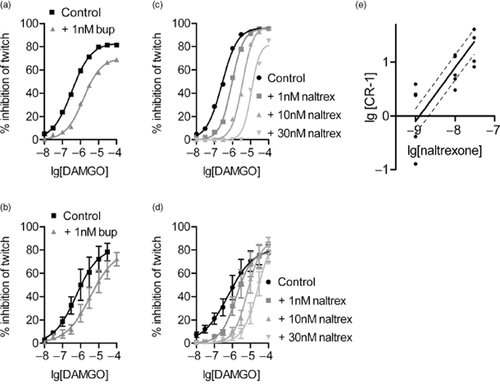
Inhibition of electrically-evoked twitch in rat vas deferens by[D-Ala2,NMe-Phe4,Gly-ol5]-enkephalin (DAMGO). Effects of DAMGO are inhibited by buprenorphine (bup), (a) data from a single tissue; (b) pooled data from four tissues. Effects of DAMGO are inhibited by naltrexone (naltrex), (c) data from a single tissue; (d) pooled data from five tissues; (e) Schild plot from data shown in (d). Solid line: line of best-fit when slope constrained to 1, dashed line: 95% confidence limits
| Buprenorphine 0.3 mg/kg | Naltrexone 1.0 mg/kg | |||
|---|---|---|---|---|
| Observed concentration (nM) | Predicted occupancy of mu receptor (%) | Observed concentration (nM) | Predicted occupancy of mu receptor (%) | |
| Plasma | 56 ± 4 | 38 | 285 ± 19 | 62 |
| Brain | 83 ± 14 | 45 | 313 ± 19 | 55 |
- Buprenorphine and naltrexone were administered simultaneously intraperitoneally. Plasma and brain samples were taken 30 minutes later and buprenorphine and naltrexone levels measured. The primary metabolites of buprenorphine and naltrexone (norbuprenorphine and 6β-naltrexol) were also assayed but were below the limit of quantitation in plasma and brain samples. Using the plasma and brain concentrations, and empirically determined KB values of both buprenorphine and naltrexone, receptor occupancy levels were determined. All data show as mean ± standard error of the mean.
These data suggest that >95% of all available mu-opioid receptors are occupied either by buprenorphine or naltrexone when administered at these doses, which, as Fig. 3 shows, is sufficient to block morphine-induced analgesia. Furthermore, when buprenorphine (0.3 mg/kg) is co-applied with naltrexone (1.0 mg/kg), buprenorphine only occupies ∼40% of available receptors, and at this receptor occupancy level, there is not sufficient mu-opioid receptor activation to produce either analgesic or rewarding effects (see Fig. 2). In addition, similar experiments (data not shown) were performed to determine the affinity of buprenorphine at kappa-opioid receptors. The KB value of buprenorphine at kappa-opioid receptors (0.6 nM) means that at the observed brain concentrations, > 95% of all available kappa-opioid receptors would be occupied by buprenorphine or naltrexone.
Discussion
Data from both the tail-withdrawal and the CPP assays showed that 0.3 mg/kg buprenorphine alone displayed marked mu-opioid receptor agonism and that naltrexone dose-dependently blocked the mu-opioid receptor agonism in both assays. A critical feature for a potential anti-addiction therapy is that the therapy itself is neither rewarding (and therefore would itself have an abuse liability) nor aversive [and could reduce compliance or effectiveness (Greenwald et al. 2007; Manlandro 2007; Nutt 2010) ]. In our study, although 0.3 mg/kg buprenorphine alone was rewarding, as seen by CPP, when buprenorphine was administered in combination with 1.0 mg/kg naltrexone, the combination was neither rewarding nor aversive. If a higher dose of naltrexone (3.0 mg/kg) was used, the combination became aversive. Previous studies have shown that mu-opioid receptor antagonism can induce conditioned place aversion, an effect attributed to blockade of endogenous opioid activity (Mucha et al. 1985).
We generated estimates of relative receptor occupancies following buprenorphine/naltrexone combination (0.3/1 mg/kg); >95% of available mu-opioid receptors were estimated to be occupied, 40% of these by buprenorphine. One caveat is that we could only measure absolute brain levels rather than free concentrations of each drug. A high proportion of the compounds may be in the lipid compartment and unavailable for receptor binding (buprenorphine being particularly lipophilic). In vivo autoradiography studies have found in vivo KD values for buprenorphine and naltrexone at mu-opioid receptors to be approximately 23 and 30 μg/kg, respectively (Höllt et al. 1975; Richards & Sadee 1985). Although brain and plasma concentrations were not measured in those studies, if we extrapolate from our empirical brain concentrations, this suggests brain concentrations for both drugs in those studies would be approximately 10× higher than in vitro KB values. Hence, approximately 10% of both buprenorphine and naltrexone in the brain would be ‘free.’ If this were the case, this would have a negligible impact on both relative and total receptor occupancy by buprenorphine and naltrexone at the doses used in this study, based on the fact that the doses used here resulted in brain levels of both drugs far in excess (> 100-fold higher) of their KB values.
The clinical trials carried out by Rothman et al. (2000) and Gerra et al. (2006) administered naltrexone orally (50 mg daily) and buprenorphine sublingually (4 mg daily). Although plasma drug concentrations were not performed in those studies, they would likely have been of the order of 4 nM buprenorphine and 60 nM naltrexone (peak levels) declining to 0.4 nM buprenorphine and 3 nM naltrexone (at 24 hours) (Verebey et al. 1976; Everhart et al. 1997), compared with 56 nM buprenorphine and 285 nM naltrexone in this study. While there are limits to the extent to which our studies in rat can be compared with clinical studies, our data suggest two things for future clinical studies. Firstly, that the ideal buprenorphine : naltrexone plasma concentration ratio is around 1:5, theoretically meaning that relatively higher buprenorphine doses would be more clinically effective. Secondly, higher doses of both buprenorphine and naltrexone than those used by Rothman et al. (2000) and Gerra et al. (2006) may be even more effective clinically, as the combination would result in greater mu- and kappa-opioid receptor occupancies.
The ratio of naltrexone to buprenorphine dose in the current study (3:1) is very different to that used in a recent study examining cocaine self-administration in rats (Wee et al. 2012) where 0.3 mg/kg naltrexone and 3 mg/kg buprenorphine was predominantly used (1:10). Although different routes of administration were used (subcutaneous in Wee et al. 2012; intraperitoneal in the current study), our data would suggest that if more buprenorphine than naltrexone is administered, there would still be a significant mu-opioid receptor agonist response. Indeed, Wee et al. (2012) showed that although 0.3 mg/kg naltrexone reduced somatic withdrawal signs and analgesia following 3 mg/kg buprenorphine, there were still significant signs of residual mu-opioid receptor activation. Our study shows that when plasma and brain levels of buprenorphine are around four- to fivefold less than that of naltrexone, there is no measurable mu-opioid receptor agonist response.
The reduction in morphine-induced analgesia in the tail-withdrawal test following administration of the combination indicates that the combination has significant mu-opioid receptor antagonist properties. Indeed, when buprenorphine and naltrexone were administered in combination, >95% of available mu-opioid receptors were estimated to be occupied. However, the combination did not counter morphine to the same extent as naltrexone alone. This may be due to very low levels of mu-opioid receptor activation by the buprenorphine/naltrexone combination which, although below the level of functional effect, may be added to with a morphine challenge.
Data generated using the CPP extinction and reinstatement method showed clearly that buprenorphine-/naltrexone reduced reinstatement to drug seeking following a drug prime, in both cocaine- and morphine-conditioned rats. Unexpectedly, the effect was more dramatic in the cocaine-conditioned rats. While there is pre-clinical data showing that buprenorphine (Kosten, Marby & Nestler 1991; Suzuki et al. 1992; Comer et al. 1993) and naltrexone (Bilsky et al. 1992; Suzuki et al. 1992) can reduce the ability to acquire a CPP to cocaine, the research here goes further. First, the ability of buprenorphine and naltrexone to block reinstatement to cocaine seeking following extinction was demonstrated; and second, the two compounds were administered as a combination that by itself induces neither CPP nor aversion. Importantly, the observed reduction in cocaine seeking implies that the decrease in cocaine use reported in clinical trials (Rothman et al. 2000; Gerra et al. 2006) was probably not a simple consequence of reduced heroin use.
As well as blocking mu-opioid receptors, the combination of buprenorphine and naltrexone used here also acts as a functional kappa-opioid receptor antagonist (>95% estimated occupancy of available kappa-opioid receptors). However, kappa-opioid receptor antagonism is not thought to be effective in blocking drug-primed reinstatement to cocaine (Beardsley et al. 2005), only in blocking that which is stress-induced (Beardsley et al. 2005; Carey et al. 2007; Land et al. 2009). Therefore, it may be postulated that the successful blocking of reinstatement to cocaine seeking observed here was due, at least in part, to some ‘non-kappa’ effects, such as mu-opioid receptor antagonism. Certainly, the opioid system is heavily involved in the hedonic response and the subsequent reinforcement process of all drugs of abuse, including cocaine (Soderman & Unterwald 2008; Le Merrer et al. 2009). While NOP activation in mice has been shown to counteract cocaine reward (Bebawy et al. 2010) and morphine rewards, and antinociception (Lutfy et al. 2003; Marquez et al. 2008; Rutten et al. 2011), it is not yet known what dose of buprenorphine would be required for NOP agonism to become relevant to drug-seeking behavior in rats.
In summary, it has been shown that a combination of 1.0 mg/kg naltrexone and 0.3 mg/kg buprenorphine, administered i.p. in Sprague Dawley rats, is non-rewarding and non-aversive, but results in high occupancy levels of both mu- and kappa-opioid receptors, and so acts as a functional mu-/kappa-opioid receptor antagonist. It was then demonstrated that 0.3 mg/kg buprenorphine and 1.0 mg/kg naltrexone blocked drug-primed reinstatement to cocaine seeking, and attenuated drug-primed morphine-seeking. These data add to the growing evidence that a buprenorphine/naltrexone combination may be effective in a polydrug abuse situation.
Acknowledgements
This work was supported by grant G0802728 (to S.M.H) from the Medical Research Council. We thank Vicki Wright for assisting with the behavioral work.
Conflict of Interest
The authors declare no conflict of interest.
Authors Contribution
The study was conceived by SMH. CPB was involved in all experimental design. SFC and AT were responsible for the behavioral experiments, and the tissue concentration measurements. IER was responsible for the receptor affinity experiments. CPB and SFC drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content.




