Peripheral nervous system and neuromuscular disorders in the emergency department: A review
Supervising Editor: Latha Ganti
Abstract
Introduction
Acute presentations and emergencies in neuromuscular disorders (NMDs) often challenge clinical acumen. The objective of this review is to refine the reader's approach to history taking, clinical localization and early diagnosis, as well as emergency management of neuromuscular emergencies.
Methods
An extensive literature search was performed to identify relevant studies. We prioritized meta-analysis, systematic reviews, and position statements where possible to inform any recommendations.
Summary
The spectrum of clinical presentations and etiologies ranges from neurotoxic envenomation or infection to autoimmune disease such as Guillain–Barré Syndrome (GBS) and myasthenia gravis (MG). Delayed diagnosis is not uncommon when presentations occur “de novo,” respiratory failure is dominant or isolated, or in the case of atypical scenarios such as GBS variants, severe autonomic dysfunction, or rhabdomyolysis. Diseases of the central nervous system, systemic and musculoskeletal disorders can mimic presentations in neuromuscular disorders.
Conclusions
Fortunately, early diagnosis and management can improve prognosis. This article provides a comprehensive review of acute presentations in neuromuscular disorders relevant for the emergency physician.
INTRODUCTION
The spectrum of neuromuscular disorders is broad, and misdiagnosis may occur when presenting “de novo” in the emergency department (ED). The diagnosis is based on the temporal progression and appropriate localization. Neuromuscular disorders may be immune mediated, infectious, toxic, and less commonly due to an acute decompensation of an underlying degenerative disease.1 Not infrequently, disorders affecting the central nervous system (CNS), musculoskeletal system or even functional “nonorganic” disorders can mimic focal or generalized weakness (Table 1). Neuromuscular disorders can evolve into life-threatening respiratory or metabolic emergencies requiring prompt recognition and treatment in the ED.
| Disease | Presentation | Mimic |
|---|---|---|
| Cortical stroke, subdural collections | “Pseudoneuritic” syndrome of isolated distal limb weakness | Nerve palsies/peripheral neuropathies (e.g., cortical hand syndrome) |
| Bilateral medial medullary syndrome | Acute quadriparesis with bulbar and respiratory weakness | GBS |
| Chiari I malformation with or without syrinx | Type 2 respiratory failure, fatigue, dropped head | Lower motor neuron disorders |
| High cervical myelopathy, longitudinally extensive transverse myelitis, epidural abscess | Flaccid paraparesis or quadriparesis during the stage of spinal shock | Demyelinating disorders, polyradiculopathies |
| Electrolyte disturbances: hyponatremia/hypernatremia, hypokalemia/hyperkalemia, hypermagnesemia, hypophosphatemia |
Acute weakness Thyrotoxicosis can present with muscular and respiratory weakness |
Channelopathies, myopathies, GBS, neuromuscular junction disorders |
| Musculoskeletal causes such as tendon rupture | Distal limb weakness | Peripheral neuropathies and myopathies |
- Abbreviation: GBS, Guillain–Barré syndrome.
The following review aims to discuss the different neuromuscular conditions that can present to the ED and require appropriate identification to guide management including directed initial treatment and consultation. The review is aimed at physicians and clinicians who may encounter these conditions in the emergency setting and focuses on an approach depending on presentation. In addition, conditions in which the initial presentation is neuromuscular in nature are prioritized over patients with established chronic neuromuscular diagnoses requiring end-stage medical management as this was felt to be of less value. The article is not meant to be an exhaustive review of the pathophysiology, mechanisms, diagnostics, and comprehensive management of all neuromuscular conditions, but instead provides the salient diagnostic and management features of conditions that can be stabilized and triaged appropriately if the principles identified are followed. For a complete and up-to-date review of neuromuscular disorders, the reader is redirected to the most recent continuum series published by the American Academy of Neurology.2
METHODS
A panel of four physicians coauthored this paper consisting of a board-eligible Royal College emergency medicine physician (MACL) and three neurologists (ACL, AS, and HK)—two who have subspecialty training in neuromuscular disorders. All authors conceptualized the project together and agreed on its structure. We searched PubMed, World of Science, Google Scholar, and academic textbooks. References were reviewed to further identify relevant studies. We prioritized meta-analysis, systematic reviews, and position statements where possible to inform any recommendations.
TERMINOLOGY AND APPROACH TO LOCALIZATION
Localization of clinical findings is an important first step in the prompt and accurate diagnosis of neuromuscular disorders, and an approach depending on the pattern and distribution of symptoms according to clinical signs is outlined in Figure 1. In patients presenting with sensorimotor symptoms, identifying the pattern of signs and symptoms as well as the presence of either upper- or lower-motor neuron signs is critical in localization. CNS disorders are predicted by hyperreflexia, hypertonia, hemibody findings, and predominant weakness of the extensor muscles in the upper extremities and flexor muscles in the lower extremities. Spinal cord disorders should be suspected in those with sharp sensory levels, bilateral upgoing plantar reflexes, and weakness with contralateral pinprick sensory loss. Clinical features helping in localizing the disorder to the peripheral nervous system (PNS) include flaccid weakness (hypotonia), hyporeflexia, muscle atrophy, and fasciculations. Axial or limb pain, hyporeflexia, and truncal-sparing distal upper- and lower-extremity sensory loss are features suggestive of peripheral neuropathies.2 One should remember that disorders of the CNS could have hypotonia and hyporeflexia in the acute stage attributable to spinal shock.
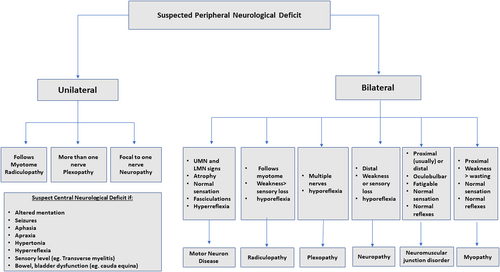
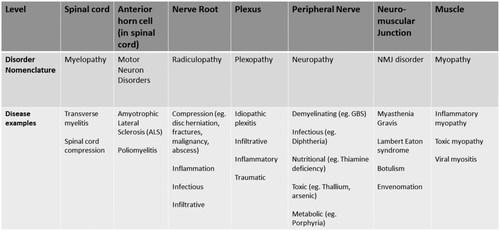
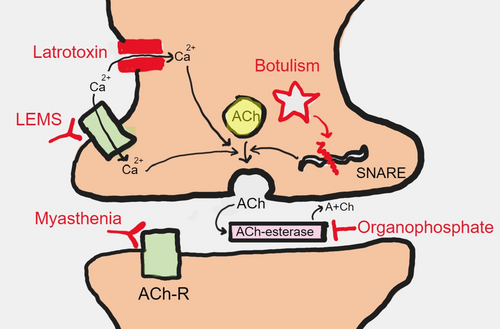
Neuromuscular disorders can be categorized as diseases affecting the anterior horn cells (neuronopathies), roots (radiculopathies), plexuses (plexopathies), peripheral nerves (neuropathies), neuromuscular junctions (neuromuscular junction disorders), and/or muscles (myopathies). A list of neuromuscular diagnoses that can present to the ED is shown in Figure 2 according to localization in the peripheral neuroaxis. A brief description of the pathophysiology of diseases of neuromuscular junction has been depicted in Figure 3.
The spectrum of presentations in neuromuscular emergencies includes (a) rapidly progressive weakness, either generalized or focal; (b) neuromuscular respiratory failure (with/without generalized weakness); (c) rhabdomyolysis and malignant hyperthermia; and (d) severe autonomic dysfunction. There is a fair degree of overlap between these presentations as will be described in the sections below.
ACUTE NEUROMUSCULAR PARALYSIS
Acute neuromuscular paralysis is characterized by rapidly progressive muscle weakness evolving to a nadir within a few days to weeks. An accurate clinical history and focused physical examination, supported by key clinical tests, helps to reach a correct diagnosis.
Guillain-Barré syndrome (GBS) and variants
The classic form of GBS, also called acute inflammatory demyelinating polyneuropathy (AIDP), presents with rapidly progressive quadriparesis or paraparesis and areflexia, usually within the setting of an antecedent infection or other physiological stressor.3 The immune response generates antibodies that cross-react with gangliosides in the nerve membrane, causing functional blockade or nerve damage. The nadir is often reached by the second or third week. Autonomic dysfunction can be seen in nearly 65% of patients. Severe pain is frequently reported at the time of onset of symptoms and could be attributed to radicular, neuropathic, or muscular pain. Neuropathic pain mimicking bilateral sciatica has been described at the onset of GBS. The diagnosis of GBS can be initially missed in cases with atypical presentations such as GBS variants, pure sensory complaints, or severe neuropathic pain. Moreover, symptoms can be more prominent than objective findings early in the course of the disease; for instance, areflexia may not be apparent in the first several days.
The spectrum of presentation of GBS is broad (Table 2). GBS variants include (a) lower-extremity paraparetic variant, (b) pharyngocervicobrachial variant, (c) pure motor variant, (d) Miller Fisher syndrome (ataxia, areflexia, ophthalmoplegia), (e) sensory ataxic variant, (f) pandysautonomia, and (g) bifacial palsy with distal paresthesias.4 AIDP is the most common subtype of GBS.
| GBS variant | Core features |
|---|---|
| AIDP | Ascending quadriparesis with sensory symptoms, bulbar, respiratory and autonomic involvement, absent reflexes |
|
Axonal forms Acute motor axonal neuropathy Acute motor sensory axonal neuropathy |
Clinical features similar to AIDP Acute motor axonal neuropathy can have early nadir, finger drop, and preserved reflexes Spectrum in acute motor axonal neuropathy varies from acute motor conduction block neuropathy with reversible conduction block to axonal degeneration Acute motor sensory axonal neuropathy has more severe disability and slow, protracted recovery |
|
Miller Fisher syndrome Bickerstaff encephalitis |
Ophthalmoplegia, ataxia, areflexia Can overlap with typical GBS GQ1b positive in 85% of cases Bickerstaff encephalitis with additional encephalopathy, hypersomnolence, and hyperreflexia |
| Paraparetic variant | Weakness restricted to the lower limbs |
| Pharyngocervicalbrachial variant | Weakness of the bulbar, neck, and upper limbs without lower limb weakness |
| Sensory variant | Pure sensory involvement with pain, numbness, and/or sensory ataxia without weakness |
| Bifacial palsy with paresthesias | Bifacial weakness with paresthesias and hyporeflexia |
| Pandysautonomia variant | Multiple domains of autonomic nervous system involvement without weakness |
- Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; GBS, Guillain–Barré syndrome.
Respiratory weakness occurs in 12%–51% of patients with GBS.5 The Erasmus GBS Respiratory Insufficiency Score (EGRIS) is a clinical prediction model for the possibility of mechanical ventilation within the first week of admission based on: (a) days between onset and hospitalization, (b) presence of facial and/or bulbar weakness, and (c) severity of weakness.6
Electrodiagnostic tests help with localization, subtype differentiation, identification of atypical variants, and prognostication, although they may appear normal in the first week of GBS.7 Referral for nerve conduction studies/electromyography is required; however, these are often performed after hospitalization from the ED and are unlikely to influence treatment decisions in the acute setting.
Clinical features that should cast doubt on the diagnosis of GBS include fever at onset, persistent and marked asymmetry of weakness, sharp sensory level, hyperacute nadir, marked abnormalities in mentation, or the presence of cerebrospinal fluid (CSF) pleocytosis, which indicate an infectious process or vascular insult affecting different parts of the neuraxis.3, 7, 8 Similarly, systemic features such as abdominal pain, vomiting, eosinophilia, anemia, renal failure, and weight loss usually point to an alternate etiology such as porphyria, systemic vasculitis, or heavy metal intoxication. HIV seroconversion may present with a GBS-like syndrome. Other mimics of GBS include brainstem stroke, vitamin B1 (thiamine) deficiency, nerve root infiltration, infection, neurotoxic envenomation, electrolyte disturbances or autoimmune disorders (including myasthenia gravis [MG] or inflammatory myopathies).
Optimal supportive care including intensive care unit (ICU) monitoring, respiratory support, and management of autonomic dysfunction are crucial for improving outcomes. Immunotherapy is the mainstay of treatment, and it is indicated when independent ambulation for 10 m is difficult and when patients have rapidly progressing weakness, autonomic dysfunction, or bulbar or respiratory involvement.7 Both plasmapheresis and intravenous immunoglobulin (IVIG) have been shown to be beneficial, especially when initiated within 4 weeks (plasmapheresis) or 2 weeks (IVIG) of the onset of illness.9 These have been shown to shorten the time to independent ambulation and to weaning from ventilation.10, 11 Corticosteroids are unhelpful in GBS and have been associated with delayed recovery.12 Poor prognosis has been described in the elderly and those with preceding diarrhea or severe disease at onset.13
Infectious and toxic acute neuropathies
West Nile virus
Most infections with West Nile virus are asymptomatic. The spectrum of neurological involvement includes meningoencephalitis and acute flaccid paralysis.14 Weakness may be proximal and asymmetric and accompanied by encephalopathy.5, 15, 16 Bulbar and respiratory involvement may occur, though rare. Sensation is typically normal. The CSF is typically consistent with an aseptic meningitis and the diagnosis is confirmed by CSF PCR or IgM serologies. MRI may show signal changes involving the anterior horn cells. Inflammation of the muscles, peripheral nerves, and ganglia may also be seen.
Poliomyelitis
Poliomyelitis presents similarly, with flaccid paralysis. Poliomyelitis had been successfully eradicated by widespread vaccination protocols; however, new cases are being reported as vaccination rates have declined.17 Respiratory involvement occurs due to medullary or phrenic involvement or intercostal muscle weakness.18 Supportive care forms the mainstay of treatment for West Nile and polio viruses.
Diphtheria
Diphtheria, due to toxin produced by Corynebacterium diphtheria, presents as a biphasic illness initially characterized by fever, throat congestion, neck swelling, and palatal weakness, followed by an acute demyelinating polyneuropathy in 20% of patients. Classically, there is descending weakness with loss of accommodation, convergence, pupillary reflexes, and extraocular movements. Diaphragmatic weakness and autonomic dysfunction may occur.19, 20 The antitoxin ideally needs to be administered within 1–2 days of the onset of symptoms. Death may occur due to cardiac arrhythmias, myocarditis, respiratory failure, or autonomic dysfunction.
Lyme disease
Lyme disease occurs due to infection with the spirochete Borrelia burgdorferi transmitted by the ixodes tick. Manifestations include fever, headache, and skin rash (erythema migrans) followed by neurological signs such as meningoencephalitis and peripheral or cranial nerve palsies. Lyme disease has been reported to mimic GBS by presenting as a demyelinating polyneuropathy.21, 22 Doxycycline or ceftriaxone can be given as treatment.23
Tick paralysis
Tick paralysis can be seen in North America or Australia and is characterized by ascending symmetric weakness that can progress to involve the bulbar and respiratory muscles.24 Tick paralysis can be distinguished from GBS by its prodromal ataxia, restlessness and irritability, a history of tick bite in an endemic area during spring or summer, and a more rapid progression of disease. The disease may be more severe in children compared to adults.25 The Rocky Mountain wood tick, Dermacentor andersoni, produces a toxin that interferes with release of acetylcholine from the presynaptic terminal. Removing the tick is essential for early recovery. Ticks should be removed carefully with clean, fine-tipped tweezers that are used to grasp the tick as close to skin as possible and then pull it upward with a steady pressure, without twisting or jerking motions.26 Care should be taken to ensure that all the mouthparts have been removed, as these contain salivary glands that may continue to secrete toxins even after the body of the tick has been removed.
Toxic neuropathies
Arsenic and thallium toxicities can present with acute neuropathies associated with gastrointestinal symptoms (both toxins) and alopecia (thallium). Buckthorn poisoning can cause a rapidly progressive axonal neuropathy mimicking GBS.15 Neurotoxic marine food poisoning (ciguatera) causes gastrointestinal symptoms associated with perioral paresthesias (with cold induced exacerbations) followed by a progressive neuropathy.27 The toxicity occurs due to the blockade of sodium channels. The management strategies include (a) identification of toxin; (b) supportive care; (c) where applicable, prevention of further absorption (e.g., using activated charcoal); and (d) administration of antidotes (dimercaptosuccinic acid for arsenic, prussian blue for thallium).
Acute intermittent porphyria (AIP)
AIP is characterized by attacks of severe abdominal pain and a motor axonal neuropathy affecting mainly the proximal muscles of the upper limb, although it can also involve the bulbar and respiratory muscles.28, 29 The pathogenesis is attributed to the neurotoxicity of the heme pathway intermediates, which accumulate due to an enzymatic defect in the heme biosynthetic pathway. Autonomic disturbances can be prominent and lead to cardiac arrhythmias and sudden death. Neuropsychiatric manifestations, seizures, and hypertensive encephalopathy may also occur. Urine can turn red on standing due to formation of porphobilin. Urine testing for porphobilinogen and delta-aminolevulinic acid help in achieving an early diagnosis. AIP is treated with high glucose supplementation, hematin, givosiran, and avoiding precipitants.30
Autoimmune neuromuscular junction dysfunction: MG
MG is a neuromuscular junction disorder characterized by episodic and fluctuating weakness. Although it can result in significant limb weakness, most commonly it presents with oculobulbar weakness with ptosis, diplopia, chewing difficulty, and/or dysphagia.31
The icepack test is a practical bedside test that can raise suspicion for MG in the ED. To perform an ice pack test, the palpebral opening is measured before and after an ice pack is applied for 2 min; an increase of at least 2 mm in the palpebral opening is considered positive (sensitivity 76%, specificity of 98%).32
Myasthenic crisis is the most feared complication of MG, defined as neuromuscular weakness causing respiratory failure. Clinically, predictors of requiring intubation for airway protection include forced vital capacity (FVC) < 20% predicted, maximum inspiratory pressure (MIP) < −30 cm H2O, maximum expiratory pressure (MEP) < 40 cm H2O, low single-breath count, and hypercarbia (pCO2 > 40–50).33, 34 Myasthenic crisis can be precipitated by infection, drugs, surgery, pregnancy, and other stressors. The most common drug triggers resulting in a myasthenic crisis are summarized in Table 3.35 In patients with impending respiratory compromise, noninvasive ventilation (NIV) has been shown to decrease the number of intubations, ventilation days, hospital days, and decrease the time in ICU.33 In those requiring intubation, rocuronium is preferred to succinylcholine. Patients with myasthenic crisis can be treated with either IVIG or plasma exchange depending on local practice patterns and availability. High-dose corticosteroids should be used with extreme caution with ICU monitoring or avoided as they can cause paradoxical worsening of MG especially in patients with bulbar weakness.
| Class and name of drug | |
|---|---|
| Drugs to be avoided in view of risk of exacerbating or unmasking myasthenia gravis | Antibiotics: aminoglycosides, fluoroquinolones, macrolides |
| Magnesium (parenteral) | |
| Class Ia antiarrhythmia drugs: quinidine, procainamide | |
| Drugs to be used with caution after ensuring that MG is stable | Beta blockers, calcium channel blockers, lithium, corticosteroids especially in high doses |
| Drugs capable of causing myasthenia gravis | Immune check point inhibitors, penicillamine, tyrosine kinase inhibitors |
- Abbreviation: MG, myasthenia gravis.
Other acute neuromuscular junction disorders: neurotoxic envenomation
Botulism
Botulism results from exposure to Clostridium botulinum causing a blockage of presynaptic release of acetylcholine at the neuromuscular junction. Infection occurs due to ingestion of contaminated canned food with preformed toxin or infection of local wounds. At onset, symptoms can be nonspecific gastrointestinal disturbances. This can be followed by blurred vision (due to loss of accommodation), diplopia, dysarthria, and dysphagia followed by rapidly progressive descending weakness over a few hours to days. Autonomic involvement in the form of anhidrosis, loss of pupillary reflexes, constipation, and urinary retention can occur. In addition to supportive treatment, trivalent antitoxin may be administered to neutralize the circulating antitoxin in serum.5, 20
Snake envenomation
Exposure to venom of elapid snakes, e.g., cobra and krait, can trigger acute paralysis.36 Venom can cause neuromuscular blockade by either binding acetylcholine receptors in the motor end plate causing a fasciculating paralysis or interfering with release of acetylcholine from the presynaptic nerve terminal.36 A “pseudomyasthenic” presentation with extraocular, bulbar, limb, and respiratory weakness has been seen.37 Autonomic dysfunction, and “locked-in” states simulating brain death, have been reported.38, 39 Prompt diagnosis and initiation of supportive treatment is essential. Early administration of snake antivenom may reduce severity and prevent or reverse neurotoxicity.36
Latrotoxin (black widow spider)
Black widow spider bite can result in severe pain, board-like rigidity, and progressive weakness. Weakness from latrotoxin is due to the toxin causing a massive presynaptic calcium influx resulting in a depletion of neurotransmitters at the motor endplate.40 Management in severe cases include intravenous calcium, antivenom, muscle relaxation, and pain relief with opiods.41
Organophosphorous poisoning
Organophosphorous poisoning can occur from accidental exposures or deliberate self-harm. Its presentation is characterized initially by a cholinergic crisis for which the deadliest features are bradycardia, bronchorrhea, bronchospasms, and seizures. Atropine (an antimuscarinic agent) and pralidoxime (an acetylcholinesterase reactivating oxime drug) have been used as antidotes in addition to supportive treatment. An “intermediate syndrome” may occur 24–96 h after exposure due to downregulation of nicotinic receptors. This presents with proximal weakness involving the neck flexors and respiratory weakness.42 A delayed neuropathy can occur 2–6 weeks after the initial exposure.
Inflammatory myopathies
Polymyositis, dermatomyositis, and necrotising myopathy can all present with rapidly progressive weakness more profound in the proximal muscles compared to the distal ones.43 Dermatomyositis can present with myalgias as well as skin changes such as Gottron's papules (erythematous, scaly indurations over the knuckles, elbows, and knees), heliotrope rash (purple discoloration in a butterfly distribution over the periorbital areas and cheek), and a “V” sign over the neck and chest.44 Creatine kinase (CK) is usually elevated (though can be normal in dermatomyositis). Judicious use of immunotherapy is associated with favorable outcomes. Necrotizing myopathies carry a risk of rhabdomyolysis and renal failure.
Channelopathies
Periodic paralysis from potassium channelopathies can present with dramatic paralysis. Hypokalemic periodic paralysis can be inherited or secondary to thyrotoxicosis, renal tubular acidosis, or hyperaldosteronism.45, 46 Hypokalemic periodic paralysis is often precipitated by periods of exercise followed by rest, high carbohydrate intake, or sudden changes in temperature. Treatment for hypokalemic periodic paralysis is appropriate potassium replacement. Hyperkalemic periodic paralysis, on the other hand, is often precipitated by rest after strenuous exercise, cold, and fasting and is usually self-limited. Rarely does it require aggressive treatment unless there is critical hyperkalemia.47
RESPIRATORY FAILURE IN NEUROMUSCULAR DISORDERS
Respiratory muscle weakness and respiratory failure can occur in neuromuscular disorders, GBS and its variants, MG, inflammatory myopathies, muscular dystrophies, and motor neuron disease (MND). Sometimes, patients may require intubation before the etiology of the underlying disease is established.
Patients with neuromuscular disease and diaphragmatic weakness rely on their accessory respiratory muscles to maintain respiration. Triggers that exacerbate neuromuscular weakness such as sedation and anesthesia can result in imminent respiratory failure once the accessory muscles are no longer able to compensate. Hence, identifying the etiology of the respiratory compromise as being secondary to a neuromuscular disease is paramount for optimal management as well as preventing inadvertent use of medicines or procedures that may precipitate respiratory crisis.
Identification of impending respiratory failure
Respiratory muscle weakness usually correlates with the severity of generalized weakness, and the strength of neck flexors correlates with diaphragmatic power.48 Signs of impending respiratory failure may include tachypnea, use of accessory muscles of respiration, paradoxical breathing, inability to swallow or clear secretions, ineffective cough, inability to lift the head off the bed, and drowsiness/deteriorating sensorium.5 Importantly, patients with neuromuscular respiratory failure may not appear to be in overt respiratory distress, given hypoventilation (inability to increase respiratory rate or tidal volume)—breathlessness is often a late finding.49 A single breath count less than 20 correlates with a markedly reduced vital capacity.48 A weak cough is characteristic of neurogenic respiratory muscle weakness. Immediate onset of orthopnea on lying down is usually consistent with a neurogenic cause.5
Dysphagia in neuromuscular disorders may precede the overt manifestation of respiratory weakness and occurs in the oropharyngeal phase, characterized by symptoms that occur immediately upon swallowing.50 Patients may complain of nasal regurgitation, cough while swallowing, or choking.
Once established, respiratory weakness can worsen rapidly, with bulbar weakness, pooling of secretions, and microaspirations further contributing to respiratory function decline. In GBS, diaphragmatic fatigue and fulminant respiratory failure can occur when the limb power remains static.5 While there should be a low threshold for intubation when acute respiratory failure is suspected, clinicians must also balance the risk of prolonged ventilation or low likelihood of extubation in patients with advanced disease course or other poor prognostic features.
Blood gas analysis, bedside spirometry, and end-tidal CO2 can provide useful clues about the respiratory status. Subtle changes in blood gas analysis that may be overlooked include (a) minor declines in oxygenation due to atelectasis, (b) relative lack of hypocapnia despite tachypnea, and (c) elevated pH and bicarbonate indicating chronic hypoventilation.48 It is important to note that hypoxia and hypercarbia may be late manifestations once respiratory failure is already well established. The presence of hypoxia may suggest atelectasis, aspiration, or other cardiopulmonary processes. Hypercarbia occurs due to muscle fatigue and reduction in tidal volumes.
A supine drop in the FVC by more than 20% indicates diaphragmatic weakness. Trends of change or decline in vital capacity may be more important than the absolute values.5 A drop in vital capacity by 50%–60% or more, to less than 15–20 mL/kg or to less than 1 L, is usually indicative of impending respiratory failure.48 MIP may be underestimated when a weak seal is present due to facial weakness. Other parameters that may indicate a need for mechanical ventilatory support include a MIP less than −30 cm H2O or a MEP less than 40 cm H2O5, 51 (Table 4). The “20-30-40 rule” refers to these thresholds for FVC/MIP/MEP that can predict respiratory failure.51 Functional residual capacity (determined by the elastic recoil of chest wall and lung) is relatively preserved in pure muscle weakness and can help differentiate neurogenic respiratory weakness from restrictive lung disease.
| What are the signs of (impending) neuromuscular respiratory failure? |
Weak, shallow breathing with tachypnea Paradoxical breathing Slurred speech and weak voice Pooling of secretions Ineffective cough Use of accessory muscles of respiration Neck flexor and shoulder weakness Abnormal mentation May not appear to be in overt respiratory distress due to inability to increase respiratory rate or tidal volume |
| When to suspect that a patient has a neuromuscular cause for respiratory failure? |
Lower motor neuron signs like fasciculations, wasting Presence of limb and/or neck weakness Fluctuations and fatigability Bulbar weakness, weak cough Ptosis, ophthalmoparesis Pes cavus, kyphoscoliosis, contractures Previous history of sleep disordered breathing, orthopnea, nocturnal hypoventilation Exposure to neurotoxins |
| What are the parameters to monitor in patients with neuromuscular respiratory failure? |
Worsening of bulbar weakness Single breath count FVC Maximal Inspiratory Pressure (MIP) Maximal Expiratory pressure (MEP) End tidal CO2 |
| What are the objective parameters to help decide need for intubation in a patient with a neuromuscular disorder? |
Inability to protect airway SBC < 5–10 “20-30-40 rule”:
Serial trends showing decline in trends along with evidence of worsening neurological status |
| What are the cautions to be exercised when assessing patients with neuromuscular respiratory failure? |
Hypoxia, hypercarbia may be late features Avoid NIV when there is bulbar weakness Caution with high-flow oxygen |
- Abbreviations: FVC, forced vital capacity; MEP, maximum expiratory pressure; MIP, maximum inspiratory pressure; NIV, noninvasive ventilation.
In summary, clinicians should be wary of relying on hypoxia or respiratory symptoms to predict respiratory distress in the setting of neuromuscular weakness; instead, impending respiratory failure should be suspected in patients with bulbar or facial weakness, head-drop, poor performance on bedside spirometry, low single breath counts, autonomic dysfunction, rapid or deteriorating course, or substantial global weakness.7, 8, 52-54
Oxygenation, neuromuscular blockade, and ventilation
NIV strategies are useful in situations with preserved bulbar function and lack of secretions and can help by improving gas exchange and reducing respiratory muscle fatigue. The optimal target of oxygenation in patients presenting with suspected acute exacerbation of chronic hypercapnic respiratory failure in a neuromuscular disorder remains to be ascertained. Up-titration of the oxygen fraction can result in worsening hypercarbia and acidemia should hypoventilation not be addressed.55 This can compromise the respiratory drive, precipitating respiratory arrest. Studies support the use of oxygen administered through high-flow nasal cannula to improve oxygenation, reduce work of breathing, and improve dyspnea in patients with COPD with mild to moderate hypercapnia56; however high-quality evidence for its use specifically in patients with neuromuscular respiratory failure is limited.
Nondepolarizing neuromuscular agents (e.g., rocuronium) are the preferred paralytic when intubating patients with neuromuscular respiratory failure. In patients with GBS, depolarizing agents (e.g., succinylcholine) carry a risk of hyperkalemic respiratory arrest,57 and in patients with MG, rocuronium has a more predictable dose response. The rocuronium dose should be adjusted to 0.5 mg/kg for those with MG as there are fewer acetylcholine receptors to block to cause nondepolarizing paralysis.33, 54 Mechanical ventilation when required in addition to supportive therapy helps in tiding over the acute period of crisis in most disorders (apart from MND and muscular dystrophies, which eventually have poor long-term outcomes).
New-onset weakness or worsening of weakness while in the ICU can be due to critical illness myopathy (due to loss of myosin and myoglobin-related proteins in the limb and trunk muscles) or critical illness polyneuropathy (due to axonal degeneration). These disorders can occur independently or in combination, often in the setting of sepsis and concomitant steroid use, and manifest as difficulty in weaning from ventilation in addition to limb weakness.58
Atypical presentations with neuromuscular respiratory failure
Hypercapnic respiratory failure can present as a dominant or isolated manifestation (without overt neuromuscular weakness) presenting a diagnostic and management challenge.59 Moreover, symptoms like fatigue or orthopnea can be falsely attributed to cardiopulmonary illness in these patients.
A small proportion of patients with MG (approximately 14%) or MND (approximately 3%) have respiratory involvement as a dominant presenting feature.60 Isolated respiratory involvement in MG, though rare, has been reported in a few case series.61, 62 Associated features described in cases of MND with respiratory onset include male predominance, neck weakness, fasciculations, and cachexia.63, 64 Mean survival up to 27 months has been reported with timely initiation of NIV.65 Importantly, there are often ethical considerations regarding long-term ventilation in these patients; decisions regarding endotracheal intubation in the emergency setting may be especially challenging when advanced care directives have not been previously established.
Isolated phrenic neuropathy with diaphragmatic weakness has been associated with GBS, neuralgic amyotrophy, pneumonia, herpes zoster, drugs (e.g., amiodarone and chemotherapy), and malignancy and in the postoperative setting.66, 67 Patients with unilateral phrenic palsies are usually asymptomatic and often diagnosed due to an incidental radiological finding of an elevated hemidiaphragm. However, they can become symptomatic with coexistent cardiopulmonary disorders, abdominal distention, and pregnancy. A sizeable proportion of the patients have good functional recovery at 1-year follow-up.68, 69
OTHER PRESENTATIONS AND COMPLICATIONS OF NEUROMUSCULAR DISORDERS
Approach to focal weakness: Radiculopathies, plexopathies, and neuropathies
The localization of focal weakness can be challenging. Musculoskeletal injuries and cortical lesions should be considered in the differential diagnosis for the patient presenting with weakness.70, 71 Radiculopathies need to be considered in the context of radicular pain, weakness involving specific myotomes with or without sensory involvement in the same dermatome, and focal areflexia. Focal cervical and lumbar radiculopathies are often secondary to compression due to disc herniation and root compression.72 Polyradiculopathies can occur due to nerve root inflammation (radiculitis/arachnoiditis) secondary to infections and meningeal inflammation. Leptomeningeal infiltration can be seen in malignancies and lymphoproliferative disorders. Diagnosis is usually confirmed by MRI and CSF analysis. Patients with suspected polyradiculopathies should be promptly referred to neurology.
Cauda equina syndrome presents with acute or subacute onset of lower limb pain, numbness, myotomal weakness, and/or bladder retention as well as bladder/bowel incontinence. A central lumbar disc is often the culprit and most cases occur due to trauma or injury. Cauda equina syndrome is a surgical emergency and timely intervention is required to prevent morbidity.73
Motor and sensory deficits in a limb involving more than two nerves should raise the possibility of plexopathy. Causes of acute/subacute plexopathies include trauma, hemorrhage, inflammation, and infiltration. Brachial plexitis (also called neuralgic amyotrophy) is characterized by severe pain followed by weakness in the distribution of multiple nerves in the upper limbs. Brachial plexitis has restricted forms involving the phrenic, axillary, anterior and posterior interosseus nerves.74 Herpetic plexitis or neuropathies may present with weakness and sensory loss or pain in the areas adjacent to the rash.75
Focal neuropathy can occur from trauma, nerve compression, inflammation, and nerve ischemia secondary to vasculitis or atherosclerosis. Acute compressive neuropathy can occur in the setting of soft tissue edema, compartment syndrome, or hematoma. It may also occur as a surgical complication. The neuropathies of the upper limb can involve the median, ulnar, radial, anterior interosseus, posterior interosseus, axillary, suprascapular, and musculocutaneous nerves. The neuropathies of the lower limb can involve the sciatic, femoral, and peroneal nerves.
Rhabdomyolysis
The classic presenting triad in rhabdomyolysis of myalgia, weakness, and myoglobinuria is present in only around 10% of cases.76 The biochemical hallmark of rhabdomyolysis is a marked elevation of serum CK, and its associated electrolyte disturbances include hyperkalemia, hyperphosphatemia, and hypocalcemia.77, 78 Rhabdomyolysis can result in renal failure, electrolyte derangements, compartment syndrome, metabolic acidosis, dysrhythmias, disseminated intravascular coagulopathy, hypovolemia, and liver dysfunction.79
Rhabdomyolysis can occur secondary to exertion, trauma, heat-related illness, seizures, compartment syndrome, toxins, envenomation, medications (e.g., statins), and endocrinologic or metabolic derangements.80 Underlying myopathy may be suggested by recurrent rhabdomyolysis, positive family history, or persistent muscle weakness along with elevations in CK beyond 8 weeks.80
The principal treatment of rhabdomyolysis is fluid resuscitation, electrolyte replacement, and dialysis in the event of fulminant renal failure or related complications.
Malignant hyperthermia is a life-threatening hypermetabolic crisis, for which its hallmark features are hyperthermia, tachypnea, tachycardia, acidosis, myoglobinuria, coagulopathies, arrhythmias, and electrolyte derangements.81 Increases in end-tidal CO2 can be an early warning of malignant hyperthermia. The usual precipitating factor is exposure to volatile anesthetic or succinylcholine in a susceptible individual. Mutations in RYR1, CACNA1S, and STAC3 have been associated with increased susceptibility for malignant hyperthermia.82
Severe autonomic dysfunction
The manifestations of autonomic dysfunction are varied. Autonomic hyperactivity syndromes are often associated with tachycardia/bradycardia, labile blood pressure, diaphoresis and sweating abnormalities, urinary retention, constipation, or diarrhea.83 There may be severe orthostatic intolerance due to postural hypotension. Cardiovascular autonomic dysfunction is associated with a higher risk of arrhythmias and sudden cardiac death. GBS can present with marked autonomic disturbances. Continuous monitoring of heart rate and blood pressure in an ICU setting may be warranted in severe cases of autonomic dysfunction.
FUNCTIONAL WEAKNESS
Functional or “nonorganic” weakness is an important differential diagnosis to consider in patients presenting with an acute onset of limb weakness.84 Some of the clinical signs that are useful in identifying functional weakness include Hoover's sign, hip abduction sign, drift without pronation, dragging a monoplegic limb, “give-way”/collapsing weakness, and motor inconsistencies.85 Importantly, any of these signs when present in isolation need not necessarily exclude a structural or organic cause for the weakness. Similarly, focal pain can lead to functional limitation and may mimic weakness with a few signs suggestive of functional weakness. A low threshold is needed to evaluate for and exclude organic causes. There has been remarkable progress with the advent of tailored approaches for the treatment and rehabilitation of patients with functional weakness, often involving multidisciplinary care.
CONCLUSIONS
Neuromuscular emergencies are common and have a varied spectrum of presentations and etiologies. Diagnosis of underlying neuromuscular disorders is often delayed, especially when patients present with de novo respiratory failure or atypical presentations. Respiratory failure in neuromuscular disease may not present with obvious distress, given hypoventilation—a low threshold for careful evaluation is paramount. Mimics of neuromuscular disorders include central nervous system processes or electrolyte disturbances. A systematic approach to eliciting a history and an appropriate examination helps to localize the site(s) of involvement as well and to reach an etiological diagnosis. Knowledge of local epidemiology helps to formulate a pretest hypothesis. In some cases, prompt diagnosis is paramount for optimal outcomes.
AUTHOR CONTRIBUTIONS
Ajith Sivadasan, Miguel A. Cortel-LeBlanc, Achelle Cortel-LeBlanc, and Hans Katzberg conceptualized the review article. All authors conducted the literature review. Hans Katzberg, Miguel A. Cortel-LeBlanc, and Achelle Cortel-LeBlanc provided expert counsel and oversight and edited the article. Ajith Sivadasan drafted the manuscript, and all authors contributed to its revisions. Ajith Sivadasan takes responsibility for the paper as a whole.
CONFLICT OF INTEREST STATEMENT
Miguel A. Cortel-LeBlanc has received honoraria from the Canadian Association of Emergency Physicians for educational events and from AbbVie for speaking engagements. He receives an honorarium from the Ontario Medical Association for serving as a member of the Physician Payment Committee. He has also received consulting fees for the provision of medico-legal expert opinions. Achelle Cortel-LeBlanc is a minority shareholder at 360 Concussion Care. She has received consulting fees for the provision of medicolegal expert opinions and independent medical evaluations. She receives an honorarium for serving as a member of the Ontario Medical Association Neurology Section Executive. She has also received honoraria from Pfizer and AbbVie for educational events. The other authors declare no conflicts of interest.




