Understanding and extending the Starling principle
Abstract
The Starling Principle states that fluid movements between blood and tissues are determined by differences in hydrostatic and colloid osmotic (oncotic) pressures between plasma inside microvessels and fluid outside them. The Revised Starling Principle recognizes that, because microvessels are permeable to macromolecules, a balance of pressures cannot halt fluid exchange. In most tissues, steady oncotic pressure differences between plasma and interstitial fluid depend on low levels of steady filtration from plasma to tissues for which the Revised Principle provides the theory. Plasma volume is normally maintained by fluid losses from filtration being matched by fluid gains from lymph. Steady state fluid uptake into plasma only occurs in tissues such as intestinal mucosa and renal peri-tubular capillaries where a protein-free secretion of adjacent epithelia contributes significantly to interstitial fluid volume and keeps interstitial oncotic pressure low. Steady filtration rates in different tissues are disturbed locally by reflex changes in capillary pressure and perfusion. The rapid overall decline in capillary pressure after acute blood loss initiates rapid fluid uptake from tissue to plasma, that is, autotransfusion. Fluid uptake is transient, being rapid at first then attenuating but low levels may continue for more than an hour. The Revised Principle highlights the role of oncotic pressure of small volumes of interstitial fluid within a sub-compartment surrounding the microvessels rather than the tissue's mean interstitial fluid oncotic pressure. This maximizes oncotic pressure differences when capillary pressure are high and enhances initial absorption rates when pressures are low, accelerating short-term regulation of plasma volume.
Editorial Comment
In this special article, which is one of a pair of invited narrative reviews, the author group focuses on review and reassessment of the model for fluid movement between capillary plasma and tissue, including recent developments in thinking.
1 INTRODUCTION
When Woodcock and Woodcock1 suggested that more recent work on the physiology of blood-tissue fluid exchange might be relevant in the management of intravenous fluids in clinical situations, they referred to the newer work as the “Revised Starling Principle.” Here they were merely adopting the phrase used by Levick & Michel,2 originated by others3 to describe these ideas. The name “Revised Starling Principle” or RSP might imply that the original version of the Starling Principle was wrong and required revision. It should be emphasized that Starling's fundamental idea, that movements of fluid between the circulating plasma and the tissues are determined by the differences between hydrostatic pressures and colloid osmotic (or oncotic) pressures across microvascular walls, remains the central idea of the RSP. This essential point seems to have been overlooked by some who may have been confused by the word “Revised” in the title.4, 5
To emphasize that the RSP builds on and extends Starling's original proposal,6 we first summarize its key elements, which describe how the oncotic pressures of the plasma proteins oppose hydrostatic pressure driven filtration of fluid from blood to tissues, when microvascular walls have low but finite permeabilities to plasma protein. We then briefly review the train of logic on which these developments of Starling's classical principle are based.
2 OVERVIEW
Far from challenging the traditional Starling Equation4 the RSP uses it as a starting point and consequently as a keystone of its structure. The equation describes both transient and steady state of fluid exchange. The RSP, however, points out that because microvascular walls are permeable to plasma proteins, there is never a true Starling equilibrium. An equilibrium would mean that fluid exchange could be halted by a balance of the opposing forces. While hydrostatic and oncotic pressures might balance momentarily, the leakage of protein from plasma to interstitial fluid (ISF) will reduce oncotic pressure differences across microvascular walls creating an imbalance. In tissues where ISF is generated entirely as capillary filtrate, a low level of filtration is necessary to maintain a steady difference in oncotic pressure across microvascular walls. This has been widely recognized in the past as an excess of filtration over absorption to account for lymph flow. The RSP shows how its magnitude can be calculated from the permeability properties of the microvascular walls.
A second development emphasizes that differences in oncotic pressure across microvascular walls in a tissue can exceed that between plasma and mean ISF oncotic pressure of that tissue. This is even possible in steady states because macromolecules are effectively restricted to a “large pore” pathway through endothelia and are nearly all excluded for the route taken by most of the fluid and small solutes. The ultrafilter of microvascular walls is recognized as the glycocalyx on the luminal surface of the endothelia and it is across the glycocalyx that the oncotic pressure difference is exerted.
A third point is the importance of local hydrostatic pressure differences across microvascular walls, ΔP. Autonomic reflexes can rapidly alter pre-capillary and post-capillary resistances of the microcirculation, which determine ΔP. Unfortunately, there is no way of estimating ΔP at present. This is not a new development but is often forgotten.
3 The Starling equation
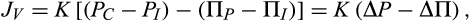 ()
()| ISF | Interstitial fluid. |
|---|---|
| JV | Fluid filtration (+) or absorption (−) rates per unit area through microvascular walls. |
| K | Microvascular filtration coefficient: product of hydraulic permeability and microvascular surface area |
| P | Hydrostatic pressure. |
| PC | Microvascular hydrostatic pressure. |
| PC(0) | Microvascular pressure when JV = 0 |
| PI | Interstitial fluid hydrostatic pressure. |
| PV | Venous pressure |
| Π | Oncotic pressure (colloid osmotic pressure) |
| ΠP | Microvascular plasma oncotic pressure. |
| ΠI | Interstitial fluid oncotic pressure. |
| ΠG | Interstitial fluid oncotic pressure on tissue side of endothelial glycocalyx. |
| ΔP, ΔΠ | Hydrostatic and oncotic pressure differences across microvascular walls. |
| RSP | Revised Starling Principle. |
| σ | Membrane or osmotic reflection coefficient to a solute. |
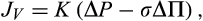 ()
()4 TRANSIENT AND STEADY STATE FLUID EXCHANGE
Starling6 believed that a constant plasma volume reflected an equilibrium between the opposing hydrostatic and oncotic pressures when JV would be zero. We have already noted that because microvascular walls are finitely permeable to proteins, a true equilibrium cannot be achieved, ΔP cannot equal σΔΠ (in Equation 2) for more than a fraction of a second. A steady state, however, can be reached where, at constant ΔP, and constant values of ΔΠ are maintained by low and constant filtration rates. Transients of JV change ΔΠ to new steady state values.
The molecular ultrafilter of microvascular walls is the glycocalyx on the luminal surface of the endothelial cells. Steady state concentration differences of macromolecules across the glycocalyx, resulting from ultrafiltration, involve a race between the few macromolecules carried through the endothelial luminal glycocalyx with the filtered fluid and the majority of macromolecules attempting to catch them up by diffusion. Because the reflection coefficients of the glycocalyx to most plasma proteins is high (σ ≥ 0.9) and its diffusional permeability to these macromolecules is low, steady state concentration differences can be maintained by very low rates of filtration. The steady state concentration of macromolecules in the ultrafiltrate can be calculated and in those few microvessels where all permeability coefficients have been estimated, it has been possible to confirm predictions of the steady state theory.13, 14
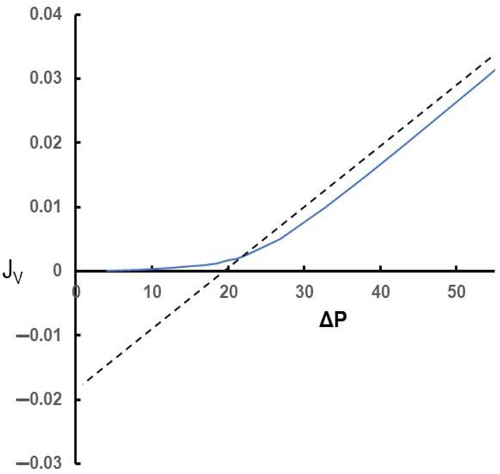
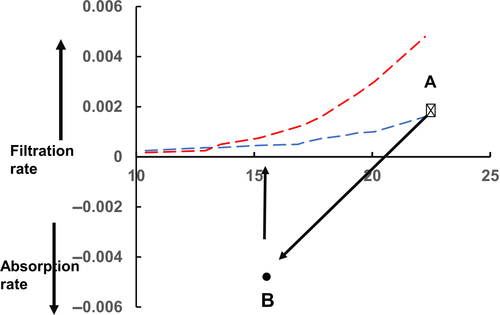
Figure 1 shows the relationship between steady state values of the filtration rate per unit area of microvascular wall (JV) to ΔP (blue curve). Also shown as a dashed linear relation are the transient changes in JV with changes in ΔP when initially ΔP lies on the steady state curve with a value which approximates that of ΠC. However, the transient relations show how JV varies linearly with ΔP at constant σΔΠ, the hockey stick shaped curve represents the steady state relations where σΔΠ is determined by JV at a given value of ΔP. Woodcock & Woodcock1 called the hockey stick curve the “J curve”. The differences between the transient and steady state values of JV are increasingly large as ΔP falls below Πp. By contrast, transient and steady state relations approach each other more and more closely as ΔP exceeds σΔΠ with high values of JV.
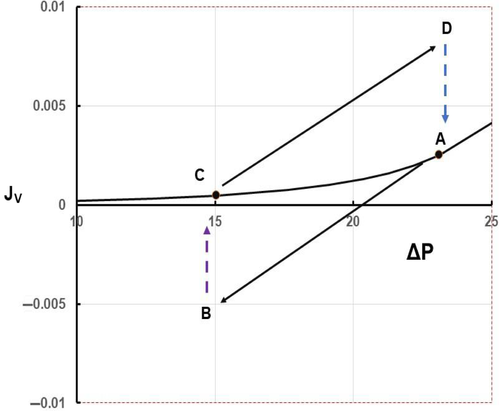
If ΔP is lowered by pre-capillary vasoconstriction in a tissue and held at its low value, fluid moves briskly from tissue into plasma (Equation 2). Even though ΔP is constant, fluid uptake falls with time as JV approaches its new steady state value. This is shown in Figure 2. Also shown here are the transient responses of JV when ΔP is restored to its initial value. These changes are typical of those in a tissue where a period of vasoconstriction is followed by vasodilatation with negligible changes in plasma volume and consequently ΠP.
More generalized vasoconstriction after haemorrhage involves a reduction in ΠC and a shift of the steady state curve (see Figure 3).
4.1 Steady state fluid uptake into the microcirculation
The steady state relations shown in Figures 1-3 apply in the tissues, which make up most of the body mass (eg, muscle, skin, connective tissues etc) where the great majority of microvessels have continuous (non-fenestrated) endothelia and the ISF is generated entirely by filtration from the microvessels.
In some tissues (kidney, gastro-intestinal mucosae), the ISF receives a major contribution of protein-free fluid secreted by adjacent epithelial cells. Here steady state fluid uptake into the fenestrated microvessels can be sustained if the rate of fluid uptake into the plasma does not exceed the rate of secretion of protein-free fluid into the ISF. If the epithelial secretion ceases, however, fluid uptake diminishes as ΠI increases and JV reverts to a steady state of low filtration.
5 ISF volumes and sub-compartments in fluid exchange
In 1969 Lunde & Waaler,15 reported that, in isolated perfused rabbit lungs, small but sustained increases and decreases in the PC led to small gains and losses in tissue fluid volume which rapidly attenuated. They suggested a small compartment of ISF was present immediately outside pulmonary capillaries so that the addition or subtraction of small fluid volumes rapidly altered its Π and hence σΔΠ. This hypothesis was consistent with Chinard's demonstration16 that during a single transit through the lung capillaries, small highly diffusible molecules and ions are distributed in a volume only slightly greater than that of the plasma.
A similar phenomenon was observed during experiments on perfused mesenteric capillaries.13 Following a step fall in ΔP, fluid absorption attenuated rapidly to its new steady state value. Since the macromolecular concentration of the solutions perfusing the vessels and ΔP were constant, it suggested that the oncotic pressure of fluid immediately outside the vessel must have risen sufficiently to bring σΔΠ close to ΔP. The rate of attenuation indicated that the volume of ISF in which Π had changed was a small fraction of the ISF in the surrounding tissue.
It seems likely that in the lung, rapid attenuation of fluid filtration is also related to changes in PI in addition to ΔΠ17 but the close correlation between the inflexion point of the steady state relation and ΠP indicates that changes in ΔΠ are also important.18 Changes in ΔP, however, cannot account for the rapid attenuation of JV in exposed super-fused mesenteries where PI is independent of varying JV.19 These observations emphasize that the ΔΠ term in Equation 2 is ΔΠ across microvascular walls and not the difference between ΠP and the mean value of ΠI for the tissue's ISF.
5.1 Levick's paradox of Starling pressures and lymph flow
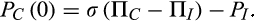 ()
()Assuming that σ = 1, Levick compared his estimates of PC(0) with the values of PV. In 11 out of the 14 tissues, PC(0) lay between 2 and 10 mm Hg below direct measurements of PV. Three tissues that were exceptions were the post-glomerular tissue of the renal cortex and medulla and intestinal mucosa during fluid uptake by the gut where PC(0) exceeded PV by 6-10 mmHg. Interestingly, PV rose to 4 mmHg above PC(0) in the mucosal capillaries when the intestine was not absorbing fluid, consistent with steady state fluid uptake being dependent on protein-free secretions into its ISF. In the 11 tissues, where PV was greater than PC(0), microvascular filtration was the only source of the ISF and their values suggested that lymph flows were many times greater than the highest measured values. To resolve the paradox, a hypothesis was suggested based on known differences between the permeability pathways for macromolecules and those for water and small solutes.
5.2 Microvascular permeability pathways for water and macromolecules
Macromolecules cross microvascular walls by a separate route from the main pathway for water and small solutes. Grotte21 first proposed these separate pathways to account for the transport of dextran polymers from plasma to lymph. Whereas, transport of smaller polymers (molecular radii < 4 nm) was strongly dependent upon molecular size, the much lower transport rates of larger polymers were only slightly size dependent. He proposed two populations of pores through microvascular walls: large numbers of small pores (radii ~ 4 nm) per unit area for water and small hydrophilic molecules; and a few large pores (radii ~ 10 to 20 nm) for macromolecules. Grotte's experimental findings have been widely confirmed22-24 and his description of the two pathways as small pores and large pores is generally accepted, though whether the “large pores” are fluid conducting pores or represent cellular mechanisms (eg, transcytosis) has been vigorously debated.24
The small pores are now believed to be the interstices of the endothelial glycocalyx.25, 26 To account for Levick's lymph paradox, Michel27 argued that if the ultrafiltrate emerging through the glycocalyx did not immediately mix with macromolecules, a larger difference in oncotic pressure could be sustained across it. All the vessels exhibiting the lymph paradox have continuous endothelia where the ultrafiltrate leaving the glycocalyx then crosses through intercellular clefts. Because the area of the entrances to the clefts are less than 1% of the endothelial surface and between 90% and 99% of the clefts are closed by the tight junctions, the velocity of fluid flow through the breaks in the tight junctions is 103-104 times greater than its average velocity through unit area of microvascular wall. These velocities are driven by pressure differences as little as 1-2 mm Hg. Protein molecules arriving in the ISF by a different route would have to diffuse against an increasing fluid velocity in the outer regions of the clefts to reach the fluid on the downstream side of the glycocalyx. Michel27 calculated this high fluid velocity would act as a barrier to diffusion of protein molecules. Consequently, the oncotic pressure of the ultrafiltrate, identified2 as ΠG, would be considerably less than the mean value for ISF, and ΔΠ across the glycocalyx would be considerably more than the values used in Levick's calculations. Quite independently, Weinbaum28 reached a similar conclusion and followed this up with a comprehensive mathematical model.3
Meanwhile, Curry had suggested how the theory might be tested. Curry, Weinbaum, and their colleagues14, 29 collaborated to show that even when ΠI in contact with the outer surface of mesenteric venules equaled ΠC within the vessel, fluid filtration through the vessel walls was dominated by PC and ΠP. These workers also proposed that a sub-compartment of the interstitial space of the mesentery was formed between microvessels’ basement membranes and a close-fitting sleeve of pericytes.30
A more detailed review of this work is given by Levick and Michel2 and Michel et al.31
6 GENERAL CONCLUSIONS
The Revised Starling Principle does not challenge4 but arises from the meaning of the traditional Starling equation for microvessels with low permeabilities to plasma proteins. It does not say that ΠP is less important than previously thought4 nor that there is no osmotic gradient between plasma and ISF.5It recognizes that Equation 2 describes both transients and steady states of fluid exchange. It emphasizes that the oncotic pressure difference (ΔΠ in Equation 2) is that between plasma and ISF on the tissue side of the endothelial ultrafilter (ΠG)which may be considerably less than the mean value of ΠI. In many tissues, ΠG behaves as if in an ISF sub-compartment.
Because microvascular walls are permeable to plasma proteins, there are no true Starling equilibria but steady states are maintained by low levels of filtration in most tissues. Steady state fluid uptake occurs only to specialized tissues when a sizeable fraction of ISF volume is contributed by a protein-free epithelial secretion. Steady state fluid uptake here is dependent on epithelial secretion rates keeping interstitial ΠG low.
In most tissues, however, where the ISF is entirely generated from the capillary filtrate, steady states require low levels of filtration to maintain a constant σΔΠ across microvascular walls. This steady flow of ultrafiltrate entering the tissue equals the lymph flow leaving it.
That filtration is necessary to maintain steady states in most tissues has been widely misunderstood. It is, however, implicit in the traditional picture as an excess of filtration over reabsorption to account for lymph flow and the steady state theory indicates how this is achieved throughout the microvascular bed.
While confirmation of the predictions of RSP has come from experiments on single mesenteric microvessels where all variables can be measured, the steady state theory was first used in a review of microvascular fluid exchange12 and found to account for many of the discrepancies between traditional thinking and observations (eg, in lungs and human limbs). It is in this context that the RSP should be judged as a guide in new scenarios.
As noted earlier “The Revised Starling Principle” may be too grandiose and misleading a title for these newer concepts of blood-tissue fluid exchange. The ideas are really a development of earlier concepts and might be more appropriately described as “The Extension of Starling's Principle”.
CONFLICT OF INTEREST
None.




