Heart rate variability monitoring for the detection of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage
Funding information
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-720511, ALFGBG-722181), the Healthcare Board, Region VästraGötaland (VGFOUREG-856851), and the Gothenburg Medical Society (GLS-779521).
Abstract
Background
Delayed cerebral ischemia (DCI) is a major cause of impaired outcome after aneurysmal subarachnoidal hemorrhage (aSAH). In this observational cohort study we investigated whether changes in heart rate variability (HRV) that precede DCI could be detected.
Methods
Sixty-four patients with aSAH were included. HRV data were collected for up to 10 days and analyzed offline. Correlation with clinical status and/or radiologic findings was investigated. A linear mixed model was used for the evaluation of HRV parameters over time in patients with and without DCI. Extended Glasgow outcome scale score was assessed after 1 year.
Results
In 55 patients HRV data could be analyzed. Fifteen patients developed DCI. No changes in HRV parameters were observed 24 hours before onset of DCI. Mean of the HRV parameters in the first 48 hours did not correlate with the development of DCI. Low/high frequency (LF/HF) ratio increased more in patients developing DCI (β −0.07 (95% confidence interval, 0.12-0.01); P = .012). Lower STDRR (standard deviation of RR intervals), RMSSD (root mean square of the successive differences between adjacent RR intervals), and total power (P = .003, P = .007 and P = .004 respectively) in the first 48 hours were seen in patients who died within 1 year.
Conclusion
Impaired HRV correlated with 1-year mortality and LF/HF ratio increased more in patients developing DCI. Even though DCI could not be detected by the intermittent analysis of HRV used in this study, continuous HRV monitoring may have potential in the detection of DCI after aSAH using different methods of analysis.
Editorial Comment
In this observational study, patterns of heart rate variability, frequently used to assess autonomic tone in various clinical states, were compared between patients with a delayed ischemia event complicating a subarachnoid haemorrhage and those who did not. The authors found an overall increase in the LF/HF ratio with time in patients who developed delayed ischemia, no pattern was found to reliably precede the ischemic event. While the study was likely underpowered to detect a subtle change in heart rate variability patterns, the authors highlight several lessons from these findings to further the development of this technique to predict outcomes in critical care.
1 INTRODUCTION
Delayed cerebral ischemia (DCI) is a strong determinant of poor neurological outcome as well as high mortality and morbidity after aneurysmal subarachnoid hemorrhage (aSAH). DCI develops in up to 30% of all patients with aSAH and usually occurs 4-14 days after the ictus, where patients with a more severe aSAH are at a higher risk.1, 2 DCI has been suggested to have a multifactorial background, including microvascular dysfunction, coagulation activation, neuroinflammation, and cortical spreading depolarization.1
While early detection of DCI symptoms is critical for treatment initiation, timely DCI diagnosis is challenging because of limitations in monitoring capabilities. The most powerful and accurate diagnostic tool for DCI detection is still repeated neurological examination.3 The value, however, is limited in patients with impaired consciousness or sedation.2, 3
Ideally, a monitoring tool for the early detection of incipient cerebral ischemia should provide continuous bedside information, irrespective of the patients’ neurological state.3 This would provide an early warning system that could alert clinicians to imminent brain ischemia, allowing the initiation of interventions before cerebral infarction develops.
Brain injury affects the autonomic nervous system (ANS) and changes in activity and sympathovagal balance could be detected as changes in heart rate variability (HRV). Thus, HRV, as a physiological biomarker, is also interesting in patients with aSAH. HRV is defined as the variability in the length of the RR interval in the ECG and provides an indirect measurement of the ANS.4-6 Changes in the balance between the sympathetic and the parasympathetic nervous systems detected with HRV have been reported as predictors of poor outcomes in terms of mortality and risk of complications after traumatic brain injury7 and after stroke,8, 9 including aSAH.10-14 However, the potential of HRV changes as an indicator of incipient cerebral ischemia has not been thoroughly evaluated.
The primary aim of this study was to investigate the possible potential of HRV monitoring for the detection of incipient cerebral ischemia. Secondary aims were to analyze if there are differences in HRV parameters between patients developing DCI and those who did not, whether HRV at arrival could predict DCI and whether differences in HRV in the acute phase were related to outcome. We hypothesized that significant HRV changes precede the development of DCI, that DCI could be predicted by early registration of HRV, and that differences would be related to outcome.
2 METHODS
This observational prospective study adheres to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.15
2.1 Patients
Patients with aneurysmal aSAH admitted to Sahlgrenska University Hospital, Gothenburg Sweden between May 2015 and October 2016 were enrolled in this study. The inclusion criteria were age ≥18 years and aSAH verified by digital subtraction angiography. The exclusion criteria were the use of pacemaker and previous aSAH, stroke, or brain injury.
Patients were primarily treated at the neurointensive care unit (NICU), in accordance with a local protocol, whose main parts are consistent with the American Heart Association/American Stroke Association guidelines.16 The aneurysms were secured as early as possible (usually within 24 hours after admission). Nimodipin (Nimotop®, Bayer) 0.2 mg/mL was administered intravenously as a prophylactic treatment for cerebral vasospasm. Patients developing hydrocephalus received an external ventricular drain. Normovolemia was maintained using albumin 20% or Ringer-Acetat, and norepinephrine was used as a vasopressor, if needed, to titrate the mean arterial blood pressure. Patients with mechanical ventilation were normoventilated with pO2 >12 kPa. If sedation was needed, infusion of propofol and fentanyl was used. In patients who developed clinical or radiological signs of DCI, hypertension was induced, and the patients underwent angiography with the possibility of intra-arterial nimodipine administration or balloon angioplasty.
2.2 Data collection
Demographics, past medical history, baseline clinical status, imaging results, and treatment and complications during hospitalization were recorded.
The day of admission to the NICU was defined as day 0. On admission, the clinical condition was scored by the attending neurosurgeon, according to the World Federation of Neurological Surgeons scale,17 and the amount of blood in the subarachnoid space was evaluated by modified Fisher's scale.18 The Glasgow coma scale (GCS) score used was the one recorded at the emergency room at the hospital to which the patient first was admitted.
All patients had invasive monitoring of arterial blood pressure. Physiological parameters and medications were recorded every 5 minutes on the NICU chart. All nursing interventions were recorded on the same chart. Neurological assessment was performed by specially trained nurses at least three times a day and the results were recorded. Transcranial Doppler (TCD) measurement of blood flow velocities in the cerebral arteries was performed by a trained examinator once a day on weekdays. Computed tomography (CT) or magnetic resonance imaging (MRI) scans were performed at the discretion of the attending physician. DCI was defined according to current guidelines.19 Clinical deterioration due to DCI was defined as focal neurological impairment or a two-point decrease in GCS score, which lasts for at least 1 hour and is not attributed to other causes. Cerebral infarction from DCI was defined as the presence of cerebral infarction on CT or MRI within 6 weeks after aSAH that is not attributed to aneurysmal surgery.
Clinical outcome was assessed by telephone interview 1 year after the aSAH using the Extended Glasgow Outcome Scale (GOSe).20
2.3 Heart rate variability
Upon arrival to the NICU, a device for HRV monitoring (eMotionLAB Mega Electronics Ltd., Kupio, Finland) was attached to the patient's chest. Data were continuously collected at a sampling rate of 1000 Hz up to 10 days or until discharge. HRV data were extracted and analyzed offline in time (RMSSD, STDRR) and frequency (nLF, nHF, LF/HF) domains using Kubios HRV analysis software and in accordance with The Task Force of European Society of Cardiology and the North American Society of Pacing and Electrophysiology standards for the use of HRV.4, 21 Time domain parameters quantify the variability of the normal RR interval in the ECG. Frequency domain parameters quantify the power within different frequency bands of the RR interval tachogram in ms2 (Table 1).
| HRV parameters | Unit | Description | Physiologic correlates |
|---|---|---|---|
| Time domain | |||
| STDRR | ms | Standard deviation of the RR intervals | Reflects components responsible for the heart rate variability |
| RMSSD | The square root of the mean squared difference of successive RR intervals | Reflects mainly the vagally mediated changes in the heart rate variability | |
| Frequency domain | |||
| LF | ms2 | The power within the low-frequency (LF; 0.04-0.15 Hz) band of the RR interval tachogram | Reflects both the sympathetic and parasympathetic parts of the ANS |
| HF | ms2 | The power within the high-frequency (HF; 0.15-0.40 Hz) band of the RR interval tachogram | Reflects the parasympathetic parts of the ANS |
| VLF | ms2 | The power within the very low-frequency (VLF; 0-0.04 Hz) band of the RR interval tachogram | The physiological characteristics are still subjected to discussion |
| LF/HF | Ratio | The LF/HF quota | Reflects the balance between the sympathetic and the parasympathetic parts of the ANS |
| Total power | VLF + HF + LF | ||
| nLF | nu, normalized units | LF/(LF + HF + VLF) | Reflects both the sympathetic and parasympathetic parts of the ANS |
| nHF | nu, normalized units | HF/(LF + HF + VLF) | Reflects the parasympathetic parts of the ANS |
Note
- Modified from Kamath et al21
- Abbreviation: ANS, autonomic nervous system.
We defined three periods evenly distributed during the day, ie, 6 am–2 pm, 2 pm–10 pm, and 10 pm–6 am, when the patient was at rest and there was limited amount of background noise (related to nursing, physiotherapy etc). By visual inspection a 5-minute sequence, without artefacts or ectopic beats, within each defined time period was captured for analysis. HRV during these three daily periods was correlated to clinical neurological findings and the physiological data noted on the NICU chart. Baseline HRV values were defined as mean values in the first 48 hours in the NICU.6
2.4 Ethics
This study was approved by the regional ethics review board in Gothenburg, Sweden (no. 053-15). Written informed consent was obtained from the patients or patients’ next of kin before study inclusion. The study adheres to the 1964 Declaration of Helsinki.
2.5 Statistics
Continuous variables were checked for normal distribution. Normally distributed variables are presented as mean ± standard deviation; non-normally distributed variables, as median with interquartile range (IQR). For the comparison of means of normally distributed variables, T-test was used. For the comparison of median of non-normally distributed variables, Mann-Whitney U-test was employed. For the evaluation of HRV variables over time, a linear mixed model with an autoregressive co-variance matrix and random intercept was used. For the comparison of binary variables between two groups, Fisher's exact test was performed. IBM SPSS 24.0 was used for statistical calculations. A p value < 0.05 was considered statistically significant.
3 RESULTS
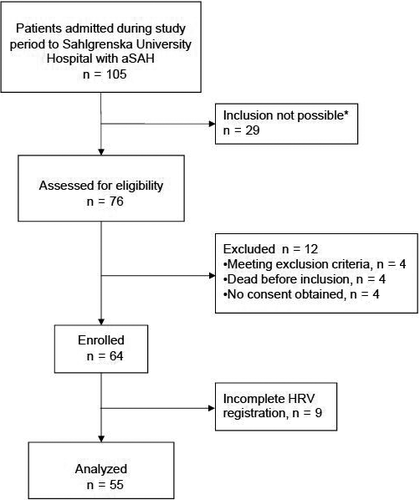
During the study period, 105 patients with aSAH were admitted to the NICU. For logistic reasons and lack of staff, it was not possible to include patients during holiday seasons. Thus, only 76 patients were screened for eligibility, of which four met any exclusion criteria, four died shortly after admission and were not included because of futile prognosis, and four did not admit consent. Hence, 64 patients were included in the study. Nine patients were excluded because of incomplete HRV recordings; thus, data from 55 patients were analyzed. (Figure 1). Median age was 58 (IQR 49-66, range 27-78) years, and 73% were women. Fifteen patients developed DCI and eight of those could be assessed for clinical deterioration where time of onset of DCI could be specified. One-year mortality was 9% (5/55). Patient characteristics are presented in Table 2.
| Category | Variable | All patients | DCI | P value | |
|---|---|---|---|---|---|
| No (n = 40) | Yes (n = 15) | ||||
| Demographic | Sex, n (%) women | 40 (73) | 31 (78) | 9 (60) | .307 |
| Age, y | 58 (49-66) | 60 (50-68) | 55 (48-59) | .192 | |
| Medical history | Cardiovascular disease | 6 (11) | 5 (13) | 1 (7) | >.999 |
| Hypertension | 15 (27) | 11 (28) | 4 (27) | >.999 | |
| Diabetes | 2 (4) | 2 (5) | 0 (0) | >.999 | |
| Smoking | 8 (15) | 5 (13) | 3 (20) | >.999 | |
| WFNS | WFNS 1-3 | 35 (64) | 27 (67) | 8 (53) | .361 |
| WFNS 4-5 | 20 (36) | 13 (33) | 7 (47) | ||
| GCS | GCS 13-15 | 35 (69) | 27 (68) | 8 (53) | .292 |
| GCS 9-12 | 8 (15) | 4 (10) | 4 (27) | ||
| GCS 3-8 | 12 (22) | 9 (23) | 3 (20) | ||
| Modified Fisher | Grade 1-2 | 6 (11) | 6 (15) | 0 (0) | .173 |
| Grade 3-4 | 49 (89) | 34 (85) | 15 (100) | ||
| Aneurysm location | Anterior circulation | 49 (89) | 35 (86) | 14 (93) | >.999 |
| Posterior circulation | 6 (11) | 5 (14) | 1 (7) | ||
| Intervention | Ventricular drainage | 29 (53) | 21 (53) | 8 (53) | >.999 |
| Embolization | 41 (75) | 28 (70) | 13 (87) | .304 | |
| Surgery | 14 (25) | 12 (30) | 2 (13) | .304 | |
| NICU-days | 14 (8-19) | 10 (7-17) | 18 (17-24) | .001 | |
- Abbreviations: DCI, delayed cerebral ischemia; GCS, Glasgow coma scale; NICU, neurointensive care unit; SAH, subarachnoid hemorrhage; WFNS, World Federation of Neurosurgery Scale for grading SAH.
3.1 Patterns of HRV over time
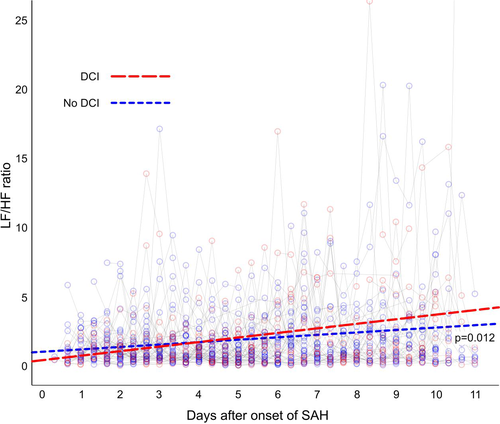
For the whole patient cohort, STDRR, RMSSD, and nHF significantly decreased over time, whereas nLF and LF/HF ratio significantly increased over time (Table 3). Patients with DCI had a significantly higher LF/HF ratio over time compared to patients without DCI, P = .012 (Table 4). Other HRV variables did not significantly differ over time between patients with and those without DCI (Table 4 and Figure 2).
| Intercept | Beta | 95% CI for beta | P value | |
|---|---|---|---|---|
| STDRR | 31.8 | −0.17 | −0.33 to −0.005 | .044 |
| RMSSD | 29.1 | −0.31 | −0.50 to −0.14 | .001 |
| nLF | 47.0 | 0.29 | 0.12 to 0.46 | .001 |
| nHF | 52.9 | −0.29 | −0.46 to −0.12 | .001 |
| Total power | 1363 | −13.5 | −28.0 to 1.21 | .072 |
| LF/HF | 0.93 | 0.07 | 0.04 to 0.09 | <.001 |
Note
- A linear mixed model was used for the evaluation of HRV variables over time.
- Abbreviations: CI, confidence interval; LF/HF, ratio of low-frequency power/high-frequency power; nHF, high-frequency power in normalized units; nLF, low-frequency power in normalized units; RMSSD, root mean square of the successive differences between adjacent RR intervals; STDRR, standard deviation of all RR intervals.
| Intercept | Beta | 95% CI for Beta | P value | |
|---|---|---|---|---|
| STDRR | 28.1 | 0.21 | −0.14 to 0.56 | .232 |
| RMSSD | 26.3 | 0.27 | −0.11 to 0.65 | .166 |
| nLF | 44.4 | −0.15 | −0.52 to 0.21 | .409 |
| nHF | 55.4 | 0.14 | −0.22 to 0.51 | .435 |
| Total power | 1125 | 11.4 | −20.1 to 42.9 | .477 |
| LF/HF | 0.42 | −0.07 | −0.12 to −0.01 | .012 |
Note
- A linear mixed model was used for the evaluation of HRV variables over time. Patients without DCI are classified as the reference group. Data presented are the temporal changes in HRV in DCI patients compared to those in patients without DCI.
- Abbreviations: DCI, delayed cerebral ischemia; CI, confidence interval; DCI, delayed cerebral ischemia; nHF, high-frequency power in normalized units; nLF, low-frequency power in normalized units; LF/HF, ratio of low-frequency power/high-frequency power; RMSSD, root mean square of the successive differences between adjacent RR intervals; STDRR, standard deviation of all RR intervals.
3.2 HRV on admission as a predictor of DCI
The mean values of all HRV variables obtained three times daily during the first 48 hours after admission, were calculated as baseline values. The mean HRV baseline values did not differ between patients who developed DCI and those who did not (Table 5).
| No DCI | DCI | P value | |
|---|---|---|---|
| STDRR | 28.3 (21.1-41.6) | 23.8 (12.1-31.9) | .217 |
| RMSSD | 23.9 (17.5-40.3) | 17.1 (8.7-36.4) | .364 |
| nLF | 50.8 ± 16.2 | 48.1 ± 12.5 | .610 |
| nHF | 49.1 ± 16.1 | 51.8 ± 12.5 | .610 |
| Total power | 906 (457-1560) | 638 (112-1230) | .189 |
| LF/HF | 1.3 (0.71-1.74) | 1.0 (0.87-1.58) | .655 |
- Abbreviations: DCI, delayed cerebral ischemia; nHF, high-frequency power in normalized units; nLF, low-frequency power in normalized units; LF/HF, ratio of low-frequency power/high-frequency power; RMSSD, root mean square of the successive differences between adjacent RR intervals; STDRR, standard deviation of all RR intervals.
3.3 HRV for detection of DCI (24 hours before clinical onset of DCI)
In the subgroup of patients with clinically assessable DCI, where a specific time-point of clinical deterioration attributed to DCI could be specified, HRV temporal pattern was analyzed 24 hours before onset of clinical symptoms. None of the HRV variables analyzed three times daily had a visually recognizable specific temporal pattern before DCI onset (supplement 1). Similarly, in a linear mixed model, none of the HRV variables had a specific temporal pattern 24 hours before DCI onset (Table 6).
| Intercept | Beta | 95% CI for Beta | P value | |
|---|---|---|---|---|
| STDRR | 36.9 | 1.97 | −2.83 to 6.76 | .339 |
| RMSSD | 27.4 | 0.69 | −5.14 to 6.52 | .758 |
| nLF | 52.5 | 5.2 | −2.92 to 13.39 | .195 |
| nHF | 47.5 | −5.2 | −13.3 to 2.88 | .193 |
| Total power | 1890 | 223 | −307 to 754 | .326 |
| LF/HF | 1.4 | 0.01 | −0.48 to 0.51 | .953 |
Note
- A linear mixed model was used for the evaluation of HRV variables over time.
- Abbreviations: DCI, delayed cerebral ischemia; nHF, high-frequency power in normalized units; nLF, low-frequency power in normalized units; LF/HF, ratio of low-frequency power/high-frequency power; RMSSD, root mean square of the successive differences between adjacent RR intervals; STDRR, standard deviation of all RR intervals.
3.4 HRV and outcome
The mean values of STDRR, RMSSD, and total power in the first 48 hours after admission were significantly lower in patients who died within one year after admission. Neither nLF, nor nHF, nor LF/HF ratio differed between patients who died and those who lived 1 year after admission (Table 7).
| Alive | Dead | P value | |
|---|---|---|---|
| STDRR | 27.6 (20.7-41.6) | 8.17 (8.11-9.13) | .003 |
| RMSSD | 23.5 (13.5-40.3) | 7.98 (6.61-11.5) | .007 |
| nLF | 50.7 ± 14.9 | 43.6 ± 23.1 | .343 |
| nHF | 49.2 ± 14.9 | 53.0 ± 24.3 | .613 |
| LF/HF | 1.16 (0.87-1.74) | 1.11 (0.71-1.91) | .933 |
| Total power | 867 (387-1560) | 68.3 (57.7-118.5) | .004 |
- Abbreviations: nHF, high-frequency power in normalized units; nLF, low-frequency power in normalized units; LF/HF, ratio of low-frequency power/high-frequency power; RMSSD, root mean square of the successive differences between adjacent RR intervals; STDRR, standard deviation of all RR intervals.
4 DISCUSSION
In this prospective observational cohort study on DCI after aSAH, HRV was analyzed intermittently (three times/24 hours).We found no significant changes in HRV preceding the development of DCI in the eight patients who could be clinically assessed. HRV in the first 48 hours after admission could not predict the development of DCI in the acute phase. However, HRV temporal pattern, which was analyzed as LF/HF ratio, increased more during the study period in patients developing DCI, possibly indicating increased sympathetic tone. Furthermore, attenuated HRV in the first 48 hours after admission correlated significantly with worse outcome, in terms of one-year mortality.
While overall mortality after aSAH has decreased over the past decades and the proportion of survivors returning to independent life is approximately 55%, the single factor with the greatest influence on outcome is the development of DCI.2 Thus, to further improve patient outcomes, a key factor would be to reduce the incidence of DCI. This would require improved monitoring that enables early detection of imminent cerebral ischemia, which could in turn intensify treatment efforts and possibly prevent cerebral infarction. Commonly used diagnostic approaches, such as TCD ultrasonography and different imaging techniques, are insufficient for the detection of incipient ischemia.1, 3, 22
In the search of a more suitable monitoring technique, analysis of HRV, which reflects the balance between the sympathetic and parasympathetic nervous systems, has gained interest.
Dysfunction of the autonomic nervous system, with a preponderance of sympathetic activity, has been observed after acute ischemic stroke and has been found to be associated with poor neurological outcome and increased mortality.23, 24
HRV monitoring as a clinical application is relatively new despite its long existence. Studies performed indicate that HRV may be used for detection of DCI, but substantially more research is required before HRV may be used clinically.13
Reduced HRV, as an indication of an increase in sympathetic activity, has been reported in patients with cerebral infarction.25
Changes in HRV have been correlated with poor prognosis following different types of stroke and traumatic brain injury.7-9, 26, 27 Studies in patients with SAH have shown that HRV analysis early after admission could predict poor outcome at hospital discharge,10, 11 which is consistent with our finding that attenuated HRV in the first 48 hours correlated with 1-year mortality. Moreover, changes in HRV have also been correlated with the development of complications in patients with SAH. Su et al reported a difference in HRV patterns (ie, LF/HF slope) between patients who developed different complications within a week after SAH and those who did not.14 These findings correspond to our results showing that patients developing DCI had a more pronounced increase in LF/HF ratio over the recorded period, indicating a shift towards increased sympathetic tone.
In a retrospective case-control study Schmidt et al found that HRV could predict occurrence of complications defined as DCI or infection 24 hours before onset.28 The study design is however not comparable to ours where we focused only on clinically assessable DCI and the 24 hours of HRV data preceding DCI development. With offline analysis of short-term, 5-minute periods of HRV data, we could not predict DCI 24 hours before the event.
An advantage of HRV is that this biomarker could be continuously and non-invasively recorded and is independent of the patient's neurological status. Continuous monitoring implies that huge amount of data will be generated. In our study, we only managed to analyze 1% of the collected HRV data. To be able to analyze all available data, competence in handling large data sets is vital. Another challenge using HRV is that it could be affected by numerous patient- and situation-specific factors. HRV could be influenced by age, sex, weight, and comorbidities as well as body position, temperature, sedation, ventilatory settings, volemic status, multiple drugs affecting the ANS, and nursing maneuvers.5, 29 It is a challenge to sort out the important signals from the noise and influence of ICU-related activities.
In recent publications, the use of computer-based analysis of larger dynamic data sets on the individual level has been suggested to improve prediction precision. Roederer et al combined automated data from electronic medical records with physiological data, which could improve DCI risk stratification.29 Megjhani et al managed to predict DCI better, using machine learning for the analysis of high-frequency sampled individual physiological data during the first 4 days after the SAH, compared to gold standard predictive models.30 Artificial neural networks31, 32 have also been used to improve prediction models based on patient data on admission. However, prediction models lack accuracy and precision when applied to individual patients. The question is whether artificial intelligence using machine learning applied on continuously collected physiological data, including HRV, combined with data from other easily available noninvasive monitoring tools could not only better predict but also foresee the development of cerebral ischemia. Computational analysis of HRV for the early detection of clinical events rather than for prediction was used in a small study, the results indicate that HRV could be useful for the early detection of complications.12
The strength of this study is its prospective design, where HRV, neurological examination, and physiological parameters were recorded continuously during 10 days. Furthermore, the studied patient cohort is well representative of the SAH population in terms of age, gender and incidence of DCI. However, the size of the study population is a limitation. In nine patients HRV equipment failed and these patients had to be excluded. Although 15 of the 55 patients analyzed developed clinical and/or radiological signs of DCI, only eight patients could be timely assessed for deterioration attributed to DCI so that HRV data could be coupled to the time of symptom onset. Due to the limited patient number finally included for analysis the results should be interpreted with caution.
The study design where we only analyzed HRV in 5-minute periods three times a day makes it possible that we could have missed the presence of signals or patterns in the rest of the data that could indicate cerebral ischemia. Using software that could identify patterns in continuously collected individual data may generate different results. Future studies should concentrate on continuously monitored HRV data with a focus on intra-individual changes.
A next step could be to analyze HRV data continuously and to correlate HRV patterns to sedation, pharmacological agents and external disturbances, and by doing so eventually different results could be revealed.
In this study a special HRV monitor equipment was used providing the HRV parameters via a computer-based software. For the future the best and easiest way of monitoring HRV would probably be from calculations made from the ordinary ECG signal, available in all patients. However, it will require development of software that can transfer the ECG signal for HRV calculations.
5 CONCLUSIONS
Intermittently analyzed HRV could not detect upcoming DCI in patients with aSAH. HRV in the first 48 hours after admission could not predict the development of DCI but was associated with one-year mortality. A more pronounced increase in LF/HF ratio over the study period was noted in DCI patients consistent with increased sympathetic tone. These results indicate that the HRV monitoring may have potential in the detection of DCI after aSAH using different methods of analysis. In future studies analyzing HRV data, techniques for big data management should be considered.
ACKNOWLEDGEMENT
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-720511, ALFGBG-722181), the Healthcare Board, Region VästraGötaland (VGFOUREG-856851), and the Gothenburg Medical Society (GLS-779521).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Study design: PL, JO, HOH, SN Data acquisition: SBW, PL, JO, JL, HOH Data analysis and interpretation of results: SBW, PL, JO, LJ, HOH, LB, SN Manuscript drafting: SBW, PL, HOH Manuscript revision and editing: JO, JL, LB, SN Manuscript approval: SBW, PL, JO, SN, LB, JL, HOH




