Association of Wearable Activity Monitors and Digital Drainage Device With Daily Ambulation and Length of Stay Among Pulmonary Resection Patients: A Prospective, Randomized Controlled Study
Funding: This work was supported by Chang Gung Memorial Hospital Grant (CMRPG5H0031).
ABSTRACT
Background
With advancements in medical devices, more hospitals are incorporating the digital chest drainage (DCD) system into postoperative care. Although some studies have suggested that the DCD system provides accurate information and shortens hospital stays compared with the traditional chest drainage (TCD) system, the effect of the DCD system on quality of life remains unclear. This study investigated whether the digital chest drainage system improves postoperative outcomes and quality of life.
Methods
This single-center, prospective, randomized controlled trial initially included 362 patients. After exclusion and randomization, 128 and 125 patients were included in the DCD and TCD groups, respectively. Wearable devices were used to measure sleep duration and walking distance after surgery. Primary outcomes included postoperative recovery and quality of sleep and rehabilitation.
Results
Both groups had similar baseline characteristics. In terms of postoperative outcomes, the DCG group had shorter durations of chest tube insertion and hospital stays than the TCD group did. We noted no significant differences in postoperative pulmonary complications or extended hospitalizations exceeding 1 week between the groups. Regarding physiological changes, the DCD group had a longer sleep duration during the first 2 days after surgery. Furthermore, the number of walking steps after surgery was higher in the DCD group.
Conclusion
The DCD system provides precise information that can help surgeons in decision-making, potentially shortening the postoperative course and reducing the need for postoperative chest x-rays. Furthermore, the DCD system can enhance postoperative recovery by improving sleep quality and ambulation.
1 Introduction
With the advancement of surgical techniques, minimally invasive approaches, such as video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery, have been demonstrated to more effectively reduce morbidity and enhance recovery compared with thoracotomy [1, 2]. However, surgical outcomes depend on not only the surgical method used but also postoperative care. An increasing number of hospitals are replacing the traditional chest drainage (TCD) system with the digital chest drainage (DCD) system, because the DCD system provides more objective and real-time data. This enables clinicians to more accurately determine the appropriate timing for chest tube removal in patients with an uneventful postoperative course. Over the past decades, many studies have demonstrated that the DCD system leads to shorter durations of chest tube placement and hospital stays and higher patient satisfaction scores on questionnaires [3-5]. However, little information is available regarding ambulation and quality of life during the postoperative course, which are crucial factors for early recovery after surgery [6]. In the present prospective randomized study, we compared objective perioperative and postoperative clinical data obtained from a portable electronic chest drainage system (Thopaz plus, Medela Healthcare, USA) and a wearable device equipped with step tracking and global positioning system functionality (Amazfit, China). This study used a new digital device to investigate the effect of the DCD system in pulmonary resection from various clinical perspectives.
2 Materials and Methods
2.1 Study Design
This study is an open-label, single-center, prospective, randomized controlled trial that compared the effects of the DCD system with those of the TCD system in managing chest tube placement after lung resection. This study was approved by the Clinical Research Ethics Committee of Chang Gung Memorial Hospital (approval number: 201701495A3) and conducted in accordance with the Declaration of Helsinki. Data were collected from Chang Gung Memorial Hospital, and the trial was registered on ClinicalTrials.gov (NCT03791437). The primary endpoints of the study was the duration of drain placement and length of postoperative hospitalization. The secondary endpoint was postoperative biochemical markers and rehabilitation data.
2.2 Patient Selection
Patients scheduled for unilateral lung resection (wedge resection, segmentectomy, or lobectomy, excluding pneumonectomy, bilobectomy, and sleeve lobectomy) were eligible for inclusion in the study. Additional eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 or 1, indicating adequate organ function. Written informed consent was obtained from all patients prior to surgery. Exclusion criteria included a history of thoracotomy, anticipated postoperative mobilization problems, current systemic steroid use (either intravenous or oral), presence of a psychiatric disorder or psychological symptoms, insertion of multiple chest drains, and presence of any condition deemed by the investigator as rendering the patient unsuitable for study participation.
2.3 Sample Size Calculation
To achieve a 20% reduction in the duration of chest tube insertion (with a standard deviation of 20%), a significance level (α) of 0.05, and a power (β) of 0.9, we calculated that 108 participants would be needed in each group. Considering a potential dropout rate of 10%, we set a target enrollment of 120 participants per group to ensure adequate statistical power.
2.4 Randomization
Patients were enrolled in the study by thoracic surgeons 1 week prior to surgery and were randomized using computer-generated random numbers in sequential order. Randomization was performed using a 1:1 ratio and a block size of 6. The results were sealed in envelopes and stored by the study staff. At the end of the surgery, once surgeons confirmed the absence of intraoperative exclusion criteria (e.g., bilobectomy and sleeve lobectomy), the research nurse opened the sealed envelope and informed the surgeon of the group assignment. For patients in the experimental group, the drainage tube was connected to an electronic drainage system, Thopaz (Medela AG, Baar, Switzerland), with the pressure set at-10 cmH2O. For patients in the control group, the drainage tube was connected to a traditional water seal chest bottle. After the patient returned to the recovery unit and ward, the same external pressure of 10-cm H2O was applied to the water seal chest bottle. For patients in the TTD group who wished to mobilize within the ward, the negative pressure was temporarily discontinued until they returned to bed.
2.5 Surgical Technique
The surgical procedure was standardized and performed by three thoracic surgeons. We used the same approach to single-port VATS as described previously. Surgery was performed under general anesthesia, with the patient in the lateral decubitus position and using single-lung ventilation. An incision was made in the fifth intercostal space without rib spreading. Visualization was achieved using a 30°, 10-mm thoracoscopic scope (Endoeye, Olympus, Japan). Articulated instruments (Scanlan, USA) were used for the dissection of vessels and the bronchus, which were then severed using articulated endostaples. The specimen was removed via a plastic bag or surgical glove. A water immersion test was conducted before closing the wound to detect any intraoperative air leaks, identified by the presence of air bubbles during the test. Any leaks were repaired with 4–0 prolene suture or a polyglycolic acid sheet depending on the surgeon's decision. After the air leak test, a standard chest tube (Fr. 16) was routinely placed at the end of the procedure.
2.6 Device Management
After surgery, each participant was fitted with a wearable device (Amazfit, China) on their wrist. Participants were encouraged to wear the device continuously throughout their hospital stay, including during bathroom visits. The device recorded daily step counts and sleep duration. Before placement, a study team member inspected the wearable device for proper functioning and recharged it if necessary. Data from the wearable device were securely uploaded through Bluetooth at the time of patient discharge. Before the experiment commenced, researchers calibrated all wearable devices to ensure accuracy and to prevent measurement errors.
Patients adhered to a conventional postoperative regimen. After surgery, they were initially transferred to a recovery unit for observation and then sent to their ward. Nursing staff encouraged early ambulation as the patient's condition allowed. The nursing staff on duty monitored the chest drain at least once in every shift (three shifts of 8 h each per 24 h). A research nurse was responsible for implementing postoperative rehabilitation programs and data collected by nursing staff in the ward. Chest drain removal criteria for both groups were strictly followed: cessation of airflow for 16 consecutive hours on the electronic drainage device (with pressure set at −10 cm H2O) without significant oscillations (spikes of air leak) on the graph or no visible air bubbles in the traditional chest bottle for 16 consecutive hours and when fluid drainage was less than 300 mL/day. For postoperative pain management, patients in both groups received a continuous intercostal nerve block, oxycodone 5 mg every 6 h, and intravenous morphine as needed.
Data were collected preoperatively, perioperatively, and postoperatively. Research nursing staff recorded daily activity levels and sleep duration by using the wearable device during patient visits. Electronic chest drainage device data were collected upon removal of the chest tube. All postoperative complications within 30 days were also recorded.
2.7 Statistical Analysis
All statistical analyses were conducted using SPSS Version 27 (IBM, USA). Data are presented as the prevalence, mean (standard deviation), or median (range). Continuous data were compared using the Mann–Whitney U test. Ordinal data were compared using the χ2 test or Fisher's exact test as appropriate. All statistical tests were two-sided. A p < 0.05 indicated statistical significance.
3 Results
Figure 1 presents the flowchart of patient selection. Initially, 362 patients scheduled for pulmonary surgery were enrolled. After patient selection and randomization, 128 and 125 patients were assigned to the DCD and TCD groups, respectively.
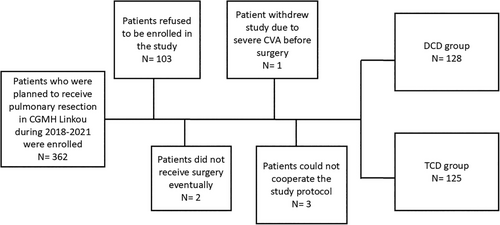
Table 1 displays the following baseline characteristics of the two groups: age, sex, body mass index (BMI), heart diseases, obstructive lung diseases, renal diseases, additional malignancies, smoking history, surgical method, and final diagnosis. Approximately one-third of the patients underwent each of the following procedures: wedge resection, segmentectomy, and lobectomy. Furthermore, approximately 60%, 30%, and 10% of the patients were diagnosed with primary malignancies, benign lesions, and metastatic tumors, respectively.
| Digital chest drainage | Traditional chest drainage | p | |
|---|---|---|---|
| n = 128 | n = 125 | ||
| Age (years) | 59.62 ± 13.54 | 62.77 ± 12.85 | 0.059 |
| Sex | 0.222 | ||
| Male | 66 (51.6) | 74 (59.2) | |
| Female | 62 (48.4) | 51 (40.8) | |
| BMI | 25.43 ± 9.23 | 26.02 ± 16.43 | 0.725 |
| Heart disease | 7 (5.5) | 10 (8.0) | 0.421 |
| Obstructive lung disease | 18 (14.1) | 21 (16.8) | 0.547 |
| Renal disease | 4 (3.1) | 9 (7.2) | 0.142 |
| Additional malignancy | 43 (33.6) | 52 (41.6) | 0.189 |
| Smoking history | 38 (29.7) | 44 (35.2) | 0.349 |
| Surgical method | 0.964 | ||
| Wedge resection | 43 (33.6) | 41 (32.8) | |
| Segmentectomy | 42 (32.8) | 40 (32.0) | |
| Lobectomy | 43 (33.6) | 44 (35.2) | |
| Final diagnosis | 0.226 | ||
| Primary malignancy | 77 (60.2) | 79 (63.2) | |
| Benign lesion | 38 (29.7) | 27 (21.6) | |
| Metastatic malignancy | 13 (10.2) | 19 (15.2) |
3.1 Differences in Perioperative and Postoperative Outcomes Between Two Groups
Table 2 lists the surgical outcomes for the two groups. The DCD group had shorter durations of chest tube insertion (77.55 ± 32.89 h vs. 73.80 ± 115.90 h, p = 0.028) and shorter hospital stays (77.55 ± 32.89 h vs. 107.02 ± 124.87 h, p = 0.012). Furthermore, the DCD group required fewer chest x-rays after surgery (2.08 ± 0.35 vs. 2.60 ± 0.86, p < 0.001). However, no significant differences were observed in postoperative pulmonary complications, such as pneumonia, chylothorax, and hemothorax, between the groups. Similarly, the proportion of patients using hypnotic drugs after surgery did not differ significantly between the groups (10.9% vs. 16.0%, p = 0.238).
| Digital chest drainage | Traditional chest drainage | p | |
|---|---|---|---|
| Overall | n = 128 | n = 125 | |
| Operation time | 164.70 ± 62.05 | 150.84 ± 63.77 | 0.081 |
| Length of post-op hospital stay (hour) | 77.55 ± 32.89 | 107.02 ± 124.87 | 0.012 |
| Length of chest tube insertion (hour) | 50.52 ± 28.73 | 73.80 ± 115.90 | 0.028 |
| Number of CXR after surgery | 2.08 ± 0.35 | 2.60 ± 0.86 | < 0.001 |
| Use of hypnotic drugs after surgery | 14 (10.9) | 20 (16.0) | 0.238 |
| Post-operative pulmonary complications | 10 (7.8) | 5 (4.0) | 0.157 |
| Wedge rection | n = 43 | n = 41 | |
| Operation time | 125.35 ± 42.75 | 104.05 ± 38.53 | 0.019 |
| Length of post-op hospital stay (hour) | 65.84 ± 18.85 | 83.83 ± 27.36 | 0.001 |
| Length of chest tube insertion (hour) | 38.88 ± 15.37 | 55.39 ± 25.23 | < 0.001 |
| Number of CXR after surgery | 2.02 ± 0.15 | 2.51 ± 0.71 | < 0.001 |
| Use of hypnotic drugs after surgery | 5 (11.6) | 4 (9.8) | 0.782 |
| Post-operative pulmonary complications | 1 (2.3) | 0 (0) | 0.326 |
| Segmentectomy | n = 42 | n = 40 | |
| Operation time | 167.90 ± 53.50 | 166.38 ± 47.56 | 0.892 |
| Length of post-op hospital stay (hour) | 74.48 ± 28.66 | 91.15 ± 40.41 | 0.034 |
| Length of chest tube (hour) | 46.90 ± 23.82 | 59.70 ± 22.53 | 0.015 |
| Number of CXR after surgery | 2.07 ± 0.34 | 2.48 ± 0.85 | 0.005 |
| Use of hypnotic drugs after surgery | 8 (19.4) | 10 (25.0) | 0.827 |
| Post-operative pulmonary complications | 3 (7.1) | 2 (5.0) | 0.685 |
| Lobectomy | n = 43 | n = 44 | |
| Operation time | 200.91 ± 63.91 | 180.32 ± 71.36 | 0.160 |
| Length of post-op hospital stay (hour) | 92.26 ± 41.75 | 143.07 ± 201.76 | 0.109 |
| Length of chest tube (hour) | 65.67 ± 36.33 | 103.77 ± 190.42 | 0.115 |
| Number of CXR after surgery | 2.14 ± 0.47 | 2.80 ± 0.98 | < 0.001 |
| Use of hypnotic drugs after surgery | 6 (14.0) | 6 (13.6) | 0.966 |
| Post-operative pulmonary complications | 6 (14.0) | 3 (6.8) | 0.275 |
3.2 Subgroup Analysis Based on Different Operation Methods
After subgrouping the patients by surgical method, similar results were observed in those undergoing wedge resection and segmentectomy. However, in patients receiving lobectomy, both the length of hospital stay and duration of chest tube insertion were comparable between the groups. Only the number of chest x-rays after surgery significantly differed between the groups (2.14 ± 0.47 vs. 2.80 ± 0.98, p < 0.001).
3.3 Analysis of Biochemical Markers and Rehabilitation Data From Wearable Device
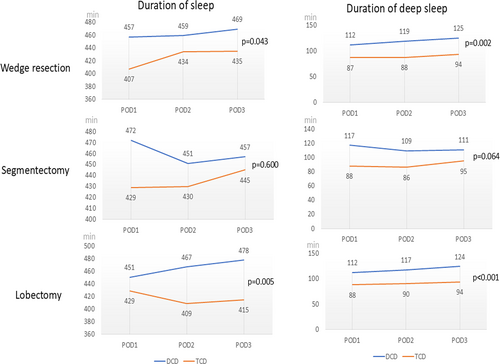
In addition to surgical outcomes, perioperative biochemical and physiological data were analyzed, and the results are presented in Table 3. In terms of changes in perioperative biochemical data, no significant differences in white blood cell (WBC) counts and C-reactive protein levels were observed between the groups. However, significant differences in physiological changes were noted between the groups. Specifically, the DCD group had longer durations of total and deep sleep from postoperative day (POD) 1 to POD 3. The same trend was noted in all three surgical subgroups (Figure 2). For patients undergoing wedge resection or lobectomy, both total and deep sleep durations were longer in the DCD group than in the TCD group. Although total and deep sleep durations did not significantly differ between the DCD and TCD groups among the patients undergoing segmentectomy, the DCD group still had a longer sleeping duration than did the TCD group.
| Digital chest drainage | Traditional chest drainage | p | |
|---|---|---|---|
| Overall | n = 128 | n = 125 | |
| WBC (*1000) | |||
| Pre-operative | 5.48 ± 1.85 | 5.85 ± 2.23 | 0.154 |
| OP day | 9.38 ± 4.44 | 9.62 ± 4.27 | 0.664 |
| POD 3 | 10.22 ± 3.14 | 9.97 ± 3.28 | 0.540 |
| OPD | 7.92 ± 2.20 | 8.14 ± 2.71 | 0.490 |
| CRP | |||
| Pre-operative | 2.76 ± 7.39 | 5.33 ± 14.07 | 0.073 |
| OP day | 3.14 ± 7.13 | 8.66 ± 27.34 | 0.031 |
| POD 3 | 117.74 ± 64.92 | 104.57 ± 55.83 | 0.099 |
| OPD | 25.08 ± 34.28 | 23.71 ± 36.41 | 0.759 |
| Duration of sleep | |||
| POD 1 | 460.52 ± 101.74 | 422.08 ± 146.73 | 0.016 |
| POD 2 | 459.58 ± 70.66 | 424.40 ± 87.97 | 0.001 |
| POD 3 | 469.44 ± 80.47 | 430.76 ± 84.70 | 0.001 |
| Duration of deep sleep | |||
| POD 1 | 114.09 ± 39.63 | 88.13 ± 38.30 | < 0.001 |
| POD 2 | 115.35 ± 38.60 | 87.97 ± 27.78 | < 0.001 |
| POD 3 | 120.78 ± 34.60 | 92.45 ± 33.35 | < 0.001 |
| Walking steps | |||
| POD 1 | 220.30 ± 476.81 | 145.91 ± 304.43 | 0.141 |
| POD 2 | 1399.32 ± 1396.73 | 978.01 ± 1580.36 | 0.006 |
| POD 3 | 2750.77 ± 2359.95 | 2082.36 ± 2267.92 | 0.038 |
- Abbreviations: OP, operation; OPD, outpatient departmen; POD, post-operative day.
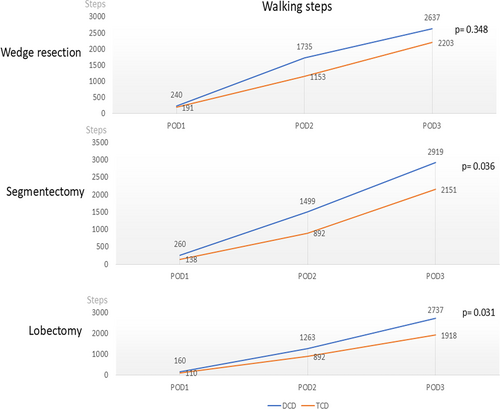
Ambulation significantly differed between the groups. The overall number of walking steps was higher in the DCD group than in the TCD group, especially on POD 2 and 3 (Table 3). The subgroup analysis revealed that the number of walking steps was higher in the DCD group than in the TCD group, especially in the patients undergoing segmentectomy (2919 vs. 2151, p = 0.036) and lobectomy (2737 vs. 1918, p = 0.031; Figure 3).
4 Discussion
Early postoperative recovery without substantial complications is a crucial indicator of surgical effectiveness [7]. Increasing attention has been paid to advancements in this field. Preoperative assessment, surgical refinement, and objective postoperative care assessment are essential elements. The DCD system can objectively quantify the volume of air leaks and provide real-time information, both of which are integral to enhanced recovery after surgery [8]. Moreover, digital drainage devices can improve the accuracy of monitoring and managing drainage fluids following lung resection surgery, thereby helping medical personnel in addressing potential drainage problems promptly and thus reducing the duration of hospitalization and risk of complications [9].
Our study presents several key findings. First, the durations of hospital stay and chest tube insertion were shorter in the DCD group than in the TCD group. This finding suggests that the DCD system accelerates the postoperative recovery process. Second, the use of the DCD system resulted in fewer chest x-rays. Finally, the DCD system might enhance postoperative quality of life, improving both the quality and duration of sleep and ambulation. The use of the DCD system enables the more precise measurement of fluid drainage and air leaks. Unlike the TCD system, which relies on surgeons' subjective judgments for chest tube removal, the DCD system provides objective quantitative assessments, enabling streamlining the process for trained, nonsurgeon staff [10]. Thus, surgeons can make decisions regarding chest tube removal and discharge more confidently and quickly. Furthermore, the acceleration of postoperative recovery can reduce medical costs and alleviate the burden on surgical waiting lists [11]. However, these benefits were less pronounced in patients undergoing lobectomy, possibly because these patients require a longer time for lung expansion after surgery [12, 13]. Alternatively, our criteria for chest tube removal, which differ from those in other studies, may affect outcomes. We waited until the DCD's leak test value reached zero, and this level was maintained for 16 h before chest tube removal [14]. The advantage of this approach is that it prevented reintubation or inadequate lung expansion in our series. However, a potential drawback is that it obscures the observed reduction in hospitalization days for DCD patients undergoing lobectomy [15]. In addition, lobectomy typically involves a larger resection area and more extensive lymph node dissection than segmentectomy. This naturally results in greater pleural fluid production and a higher chance of prolonged air leak, especially for patients without well-developed fissure [16, 17]. In the present study, 16 patients in the DCD group and 22 patients in the TCD group had a separated fissure (37.2% vs. 50.0%, p = 0.229). Although we noted no significant difference between the groups, a higher percentage of fused fissures in the DCD group might have caused a delay in the removal of the chest tube.
Although the durations of hospital stay and chest tube insertion did not significantly decrease in patients receiving, the DCD group required fewer chest x-rays than did the TCD group. This finding indicates that with the use of the DCD system, health-care providers can avoid unnecessary x-ray exposure and the associated waiting times for ambiguous air leak tests [5, 18]. Overall, the results indicate that the DCD system provides reliable and accurate information for postoperative care.
Several studies have reported the advantages of the DCD system [19-21]. The DCD system can help medical staff, both surgeons and nonsurgeons, to determine when to remove the chest tube. However, some studies have suggested that although the DCD system can provide accurate data, it cannot shorten the duration of postoperative care by itself. In the present study, we determined that the DCD system enhanced the quality of life after lung surgery, potentially reducing the duration of postoperative care both directly and indirectly. First, patients in the DCD group had longer overall and deep sleep durations. In contrast to the TCD system, which generates air bubble noise due to negative pressure, the DCD system operates silently. This quiet operation plays a crucial role in enhancing sleep quality and prolonging sleep duration (Video S1). The background volume of the TCD system (approximately 70 dB) is effectively a hundred times louder than that of the DCD system (approximately 50 dB). Reviewing the literature, noise was one of the factors affecting sleep hygiene [22, 23]. The frequent noise during sleeping was expected to cause worse sleep quality and even insomnia [24]. Therefore, the DCD system seemed to be related to a better sleeping environment for patients. Furthermore, the DCD system's lightweight and portable design, which does not require connection to low-pressure suction, offers greater convenience for patient mobility [6, 25]. Patients can carry the DCD system in a sling, enhancing comfort and mobility. By contrast, patients using the TCD system must manually carry the chest bottle, which can be cumbersome and discourage mobility and rehabilitation (Video S2). Multiple factors affect postoperative care after lung surgery, including chest care, pain control, and rehabilitation [26, 27]. Pain is a major factor affecting postoperative sleep and mobility. However, in the present study, we observed no difference in the use of hypnotic drugs after surgery between the DCD and TCD groups. This finding suggests that the DCD system is beneficial not only for improving sleep quality and duration but also for enhancing rehabilitation outcomes. To the best of our knowledge, this is the first study to demonstrate that the DCD system improves quality of life after pulmonary resection surgery.
This study has some limitations. First, although this is a prospective, randomized controlled trial, the small sample sizes limit its power to represent the general population effectively. Second, the current trackers are adept at identifying and recording step and movement data; however, they may not accurately detect the altered gait patterns, which are often more shuffled, that occur after major surgeries. Thus, these devices might underestimate postoperative activity levels. Third, we had no stringent daily requirement for walking steps for patients. The varying motivation of individuals could affect postoperative rehabilitation and potentially affect the duration of chest tube insertion [28]. Finally, we used a general-purpose health monitoring wearable device, which may not provide data as accurate as those from medical-grade wearable devices. However, by using the same device, we could more reliably identify inconsistencies in trends concerning the activity levels and sleep quality of patients in different groups. Future studies should explore these limitations and delve deeper into their implications.
5 Conclusion
This study demonstrated that the application of the DCD system not only assists medical staff in providing postoperative care but also enhances postoperative quality of life by improving both sleep and rehabilitation outcomes in patients.
Author Contributions
Tzu-Yi Yang: conceptualization, data collection, formal analysis, writing-original draft. Ching-Yang Wu: data collection, formal analysis, supervision. Ming-Ju Hsieh: data collection, supervision. Yin-Kai Chao: conceptualization. Ching-Feng Wu: conceptualization, data collection, formal analysis, methodology, data curation, writing - original draft.
Acknowledgments
The authors thank Su-Ying Lu for her technical assistance throughout this study.
Ethics Statement
This retrospective study was approved by the Clinical Research Ethical Committee of Chang Gung Memorial Hospital (approval number: 201701495A3) and registered on ClinicalTrials.gov (registration number: NCT03791437). The category in which the manuscript is being submitted: randomized clinical trial.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.




